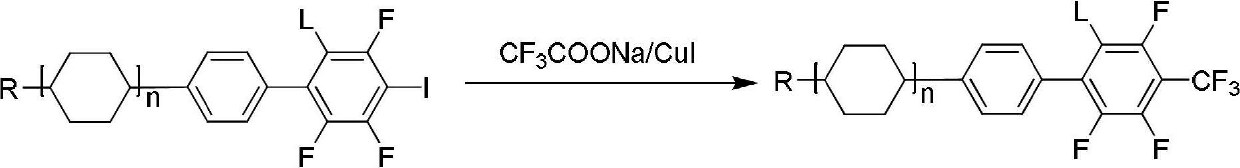Method for preparing trifluoromethyl-benzene-containing liquid crystals
A technology of trifluoromethylation and alkylation, applied in the field of catalytic synthesis, can solve problems such as low yield, and achieve the effects of improving yield and product quality, improving yield and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
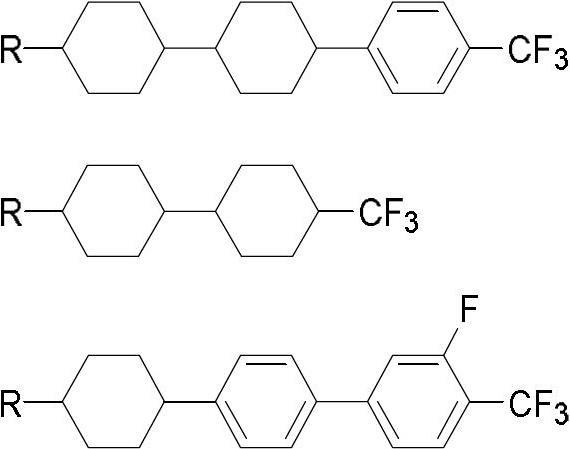
[0039] Example 1 Preparation of propylcyclohexylphenyl o-fluorotrifluoromethylbenzene
[0040]
[0041] Add 50.6g (0.12mol) iodopropylcyclohexyl m-fluorobiphenyl (reactant), 45.7g (0.24mol) (catalyst) of cuprous iodide, 45.6g (0.3mol) trifluoroacetic acid in a 1L three-necked flask Potassium (reactant), 506g N.N dimethylformamide (solvent), heated to 150°C for 5 hours, evaporated 400ml N.N dimethylformamide under reduced pressure, added 300ml toluene (solvent), poured out the supernatant, Add 200ml of water to the lower solid to filter out cuprous iodide, combine the filtrate and supernatant, separate the lower aqueous phase and extract it with 100ml×2 toluene, combine the organic phase, wash with 200ml×3 water, evaporate toluene to dryness, and detect the product in the reaction solution The content is 95.4%. The product is decolorized by silica gel column chromatography, and recrystallized three times with 3 times of petroleum ether and 0.5 times of ethanol to obtain the ...
Embodiment 2
[0046] Example 2 Preparation of propylcyclohexylphenyl-2,6-difluoro-4-trifluoromethylbenzene
[0047]
[0048] In a 2L three-necked flask, add propylcyclohexylphenyl-2,6-difluoroiodobenzene (reactant) 66g (0.15mol) potassium trifluoroacetate (reactant) 57g (0.375mol), cuprous iodide (catalyst) ) 57g (0.3mol), N.N dimethylformamide (solvent) 660g, heated to 150°C for 5 hours, distilled off 400ml of solvent, cooled to 80°C, added 500ml of toluene (solvent), 500ml of water, stirred for 20 minutes, The solid was filtered out, the filtrate was separated, the water was extracted, and the toluene was evaporated to dryness. The detected content of the reaction solution was 93% propylcyclohexylphenyl-2,6-difluoro-4-trifluoromethylbenzene, and the silica gel chromatography column Decolorize and recrystallize with 1.5 times of petroleum ether and 1 times of ethanol to obtain 28.5 g of the target compound with a yield of 60%. The experimental results are as follows:
[0049] (1) Gas ...
Embodiment 3
[0053] Example 3 Preparation of pentylcyclohexylphenyl o-fluorotrifluoromethylbenzene
[0054] Referring to Example 1 in this example, the reaction raw material in Example 1 was replaced with iodopropylcyclohexyl-m-fluorobiphenyl to prepare the following compounds with a yield of 60%;
[0055]
[0056] (1) GC-MS data analysis: 392 (M+46.4) 373 (6.2) 279 (27.8) 266 (100) 253 (58.7) 196 (13.4) 183 (14.4);
[0057] (2) DSC analysis, mp: 66.17-70.39°C.
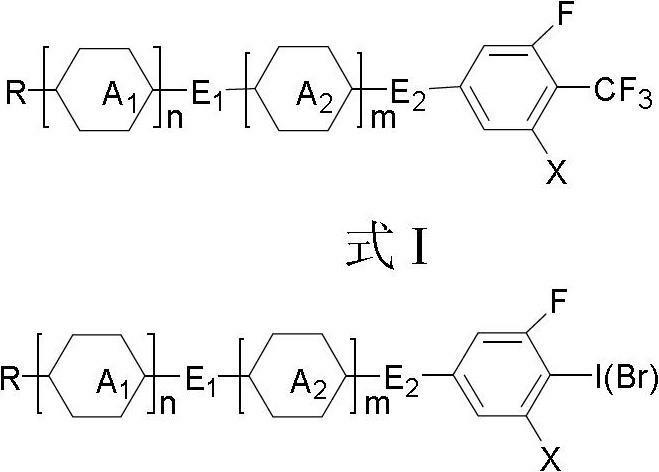
[0058] As can be seen from the above, the product has a correct structure and is the target compound shown in Formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com