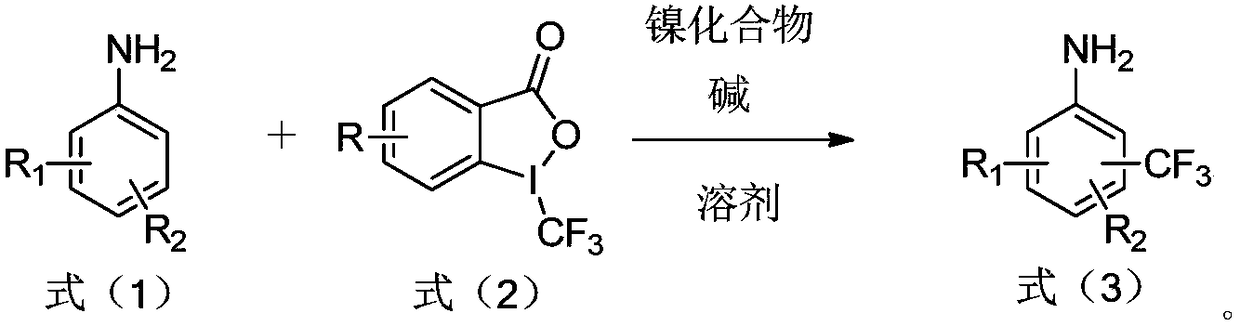
Preparation method of trifluoromethylamine
A technology of trifluoromethyl aromatic amines and trifluoromethylation, which is applied in the preparation of organic compounds, the preparation of amino hydroxyl compounds, the preparation of amino compounds from amines, etc., can solve the problems of not being suitable for industrial production and high synthesis costs, and achieve Conducive to large-scale industrial production, low cost of raw materials, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of the trifluoromethyl aromatic amine of the present embodiment adopts the following steps:
[0025] 1) Weigh aniline (0.25mmol, 1.0eq), 1-trifluoromethyl-1,2-phenyliodide-3(H)-one (0.3mmol, 1.2eq), base (potassium carbonate, 0.75mmol, 3.0eq) and nickel compounds (NiCl 2 ·6H 2 O, the molar weight is 10% (10 mol%) of the molar weight of 1-trifluoromethyl-1,2-phenyliodyl-3(H)-one) in a reaction tube, add 2 mL of dioxane as a solvent , Under the protection of argon, heated to 80 ° C and stirred for 12 h.
[0026] 2) The reaction solution obtained after the reaction was cooled to room temperature, and the reaction solution was diluted with 10 mL of dichloromethane, filtered, and the solid was washed with 5 mL of dichloromethane, and the washing liquid was merged into the filtrate, and then the filtrate was washed twice with water (each time consumption 10mL), the organic phase after extraction is dried with anhydrous sodium sulfate, filtered, and t...
Embodiment 2-27
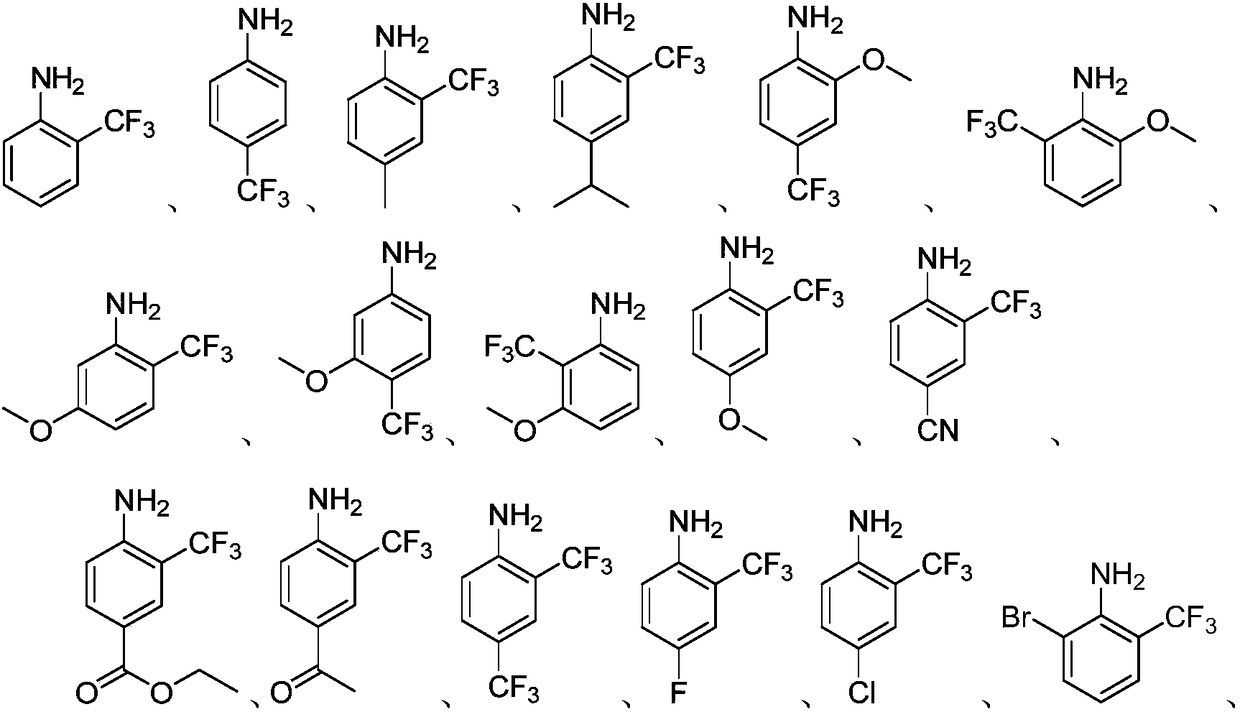
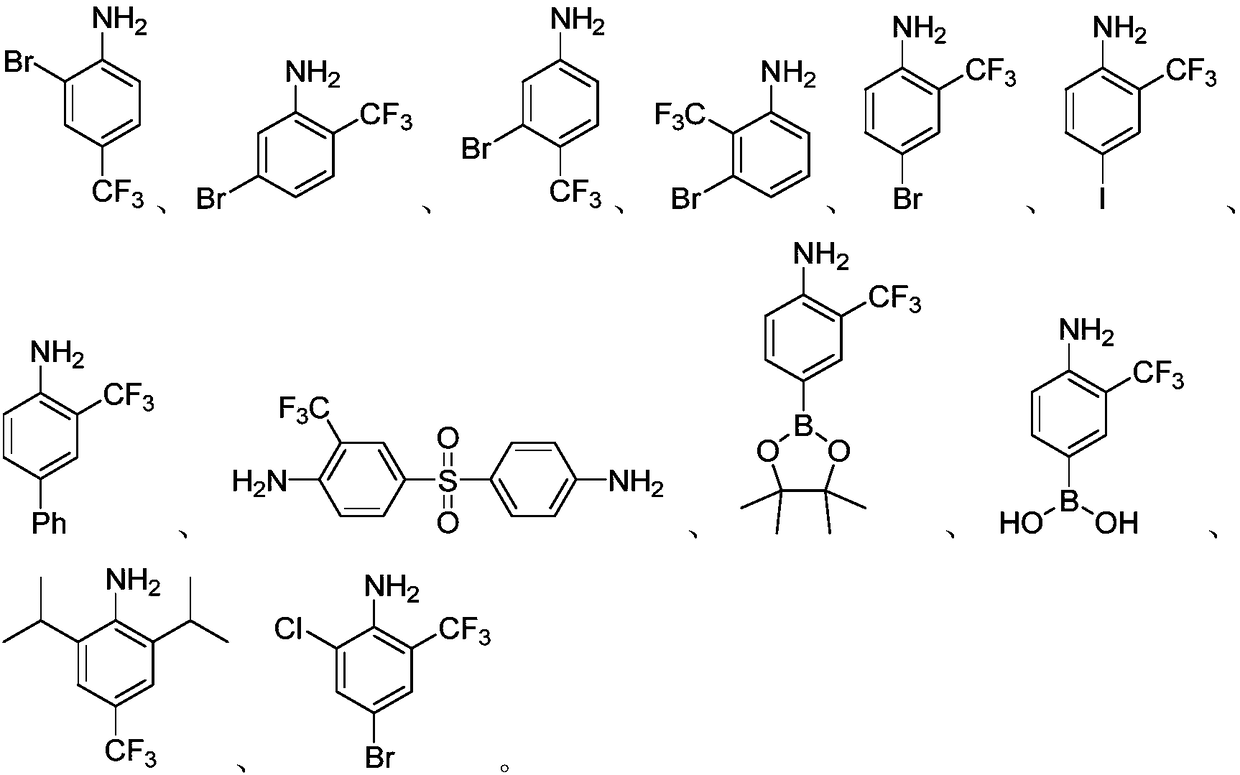
[0028] The preparation process parameters of the trifluoromethyl aromatic amines of Examples 2-27 are shown in Table 1.
[0029] The preparation process parameter of the trifluoromethyl aromatic amine of table 1 embodiment 2-27
[0030]
[0031]
[0032] The reaction process of embodiment 1-27 is as follows:
[0033]
[0034] The yield listed in Table 1 is the total yield of products 3a and 4a, which is determined by gas chromatography with dipentyl phthalate as the internal standard.
[0035] The yield of embodiment 1 is 38%, and the reaction of embodiment 2-7, only the kind of nickel compound is different, and other is identical with embodiment 1, as can be seen, the yield with nickel hydroxide as catalyzer is the highest.
Embodiment 8
[0036] In embodiment 8, the addition of nickel hydroxide is 5mol% (in terms of the molar weight of 1-trifluoromethyl-1,2-phenyliodyl-3(H)-ketone), and other conditions are the same as in embodiment 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com