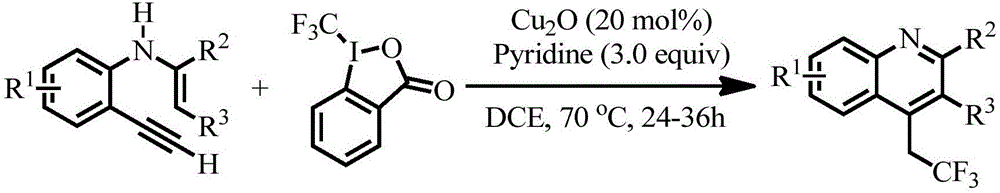
Preparation method of 4-(2',2'2'-trifluoro)ethylquinoline series
A technology of ethylquinoline and trifluoromethyl, applied in the field of preparation of 4-ethylquinoline derivatives, can solve the problems of low yield of target product, harsh conditions, poor reaction selectivity, etc., and achieve chemoselectivity Excellent, simple reaction operation, and good product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019]
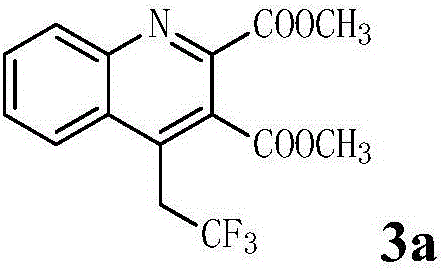
[0020] In a 25mL Schlenk reaction tube equipped with a magnetic stir bar, add N-(2-ethynylphenyl)enamine-2,3-dicarboxylic acid methyl ester (0.2mmol, 1.0eq.), 1-(trifluoro Methyl)-1,2-phenyliodide-3(1H)-one (Togni's reagent) (0.4mmol, 2.0eq.), cuprous oxide (0.04mmol, 20mol%), pyridine (0.6mmol, 3.0eq. ) and 1,2-dichloroethane (2 mL), the reaction tube was placed in a heating module at 70° C., heated and stirred for 24-36 h, and TLC detected that the reaction was complete. After the reaction solution was cooled to room temperature, it was washed with saturated sodium bicarbonate solution, extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, filtered, added a small amount of silica gel, spin-dried, and the crude product was subjected to flash column chromatography Separation and purification gave methyl 4-(2',2',2'-trifluoro)ethylquinoline-2,3-dicarboxylate 3a with a yield of 61%.
[0021] 1 H NMR (400MHz...
example 2
[0024]
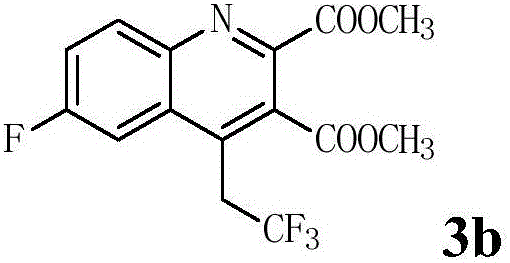
[0025]In a 25mL Schlenk reaction tube equipped with a magnetic stirrer, add methyl N-(2-ethynyl-4-fluoro-phenyl)enamine-2,3-dicarboxylate (0.2mmol, 1.0eq.), Togni's reagent (0.4mmol, 2.0eq.), cuprous oxide (0.04mmol, 20mol%), pyridine (0.6mmol, 3.0eq.) and 1,2-dichloroethane (2mL) put the reaction tube at 70°C In the heating module, react for 24-36h, and TLC detects that the reaction is complete. After the reaction solution was cooled to room temperature, it was washed with saturated sodium bicarbonate solution, extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, filtered, added a small amount of silica gel, spin-dried, and the crude product was subjected to flash column chromatography Separation and purification gave methyl 6-fluoro-4-(2',2',2'-trifluoro)ethylquinoline-2,3-dicarboxylate 3b with a yield of 45%.
[0026] 1 H NMR (400MHz, CDCl 3 )δ3.96(s,3H),4.04(s,3H),4.13(q,J=10.0Hz,2H),7.67-7.59(m,1H)...
example 3
[0029]
[0030] In a 25mL Schlenk reaction tube equipped with a magnetic stirrer, add methyl N-(2-ethynyl-4-isopropyl-phenyl)enamine-2,3-dicarboxylate (0.2mmol, 1.0eq. ), Togni's reagent (0.4mmol, 2.0eq.), cuprous oxide (0.04mmol, 20mol%), pyridine (0.6mmol, 3.0eq.) and 1,2-dichloroethane (2mL) put the reaction tube in In a heating module at 70°C, react for 24-36h, and the reaction is complete as detected by TLC. After the reaction solution was cooled to room temperature, it was washed with saturated sodium bicarbonate solution, extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, filtered, added a small amount of silica gel, spin-dried, and the crude product was subjected to flash column chromatography Separation and purification gave methyl 6-isopropyl-4-(2',2',2'-trifluoro)ethylquinoline-2,3-dicarboxylate 3c with a yield of 56%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ1.45-1.30(m,6H),3.15(s,3H),3.95(s,3H),4.04(s,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com