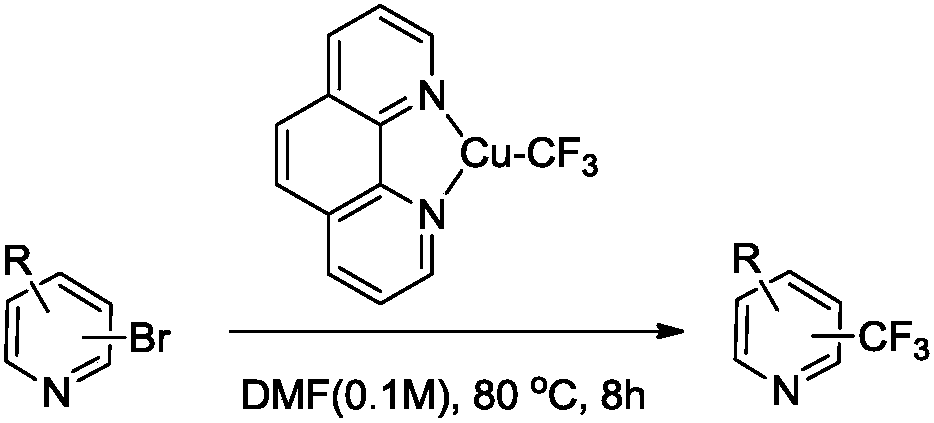
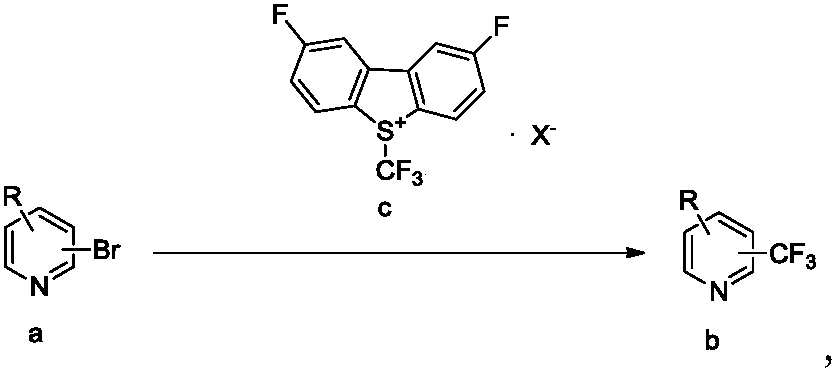
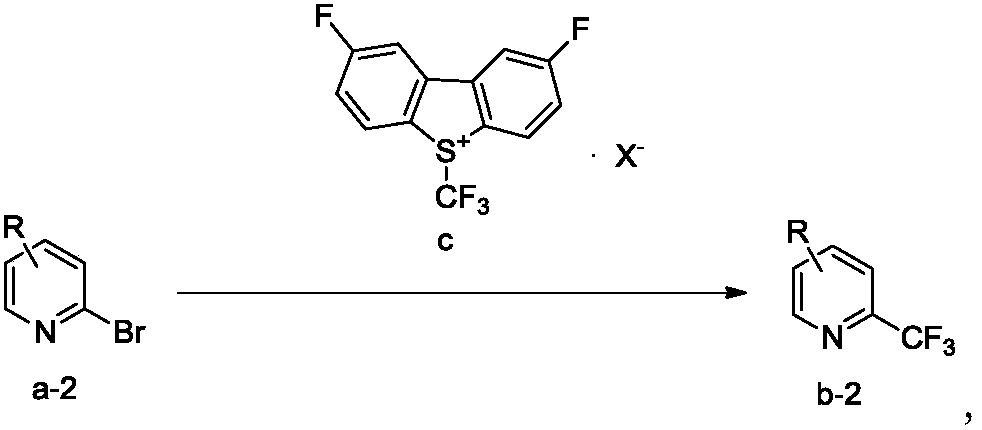
Trifluoromethylation process for bromo-pyridine and derivatives thereof
A technology of bromopyridine and trifluoromethyl, which is applied in the field of organic fluorine chemistry and organic chemistry, can solve the problems that the process cannot reach the yield, is suitable for large-scale production, and the reaction activity of trifluoromethylation is poor, and achieves high industrial efficiency. Application and economic value, suitable for large-scale production, and the effect of cheap bromination reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of 2-bromoisonicotyl tert-butyl ester
[0046]
[0047] Add 2-bromoisonicotinic acid (48.1g, 0.238mol), NMP (120ml) into a 1L three-necked flask, start stirring, then add di-tert-butyl dicarbonate (126ml, 0.547mol), DMAP (5.82g, 0.056mol ), reacted at 25°C for 16h. 1 HNMR monitoring showed that the starting material was completely converted. NaCl (25g) and KH 2 PO 4 (25g) was dissolved in 250ml of water and added dropwise to the reaction flask, the temperature did not change significantly. After the dropwise addition was completed, 250ml of methyl tert-butyl ether was added, stirred for 15min, and extracted. Wash with 100ml of water. After concentrating under reduced pressure, a white solid was obtained with a yield of 92.6%.
Embodiment 2
[0048] Embodiment 2: the preparation of 2-trifluoromethyl isonicotinic tert-butyl ester
[0049]
[0050] in N 2 Under protection, add 2-bromoisonicotinic acid tert-butyl ester (25.7g, 0.1mol), 250ml DMF, Cu powder (19.2g, 0.3mol) into a 1L three-necked flask, start stirring, and cool to 0-5°C in an ice-water bath . Umemoto's reagent (87.6 g, 0.2 mol) was added. After stirring for 1 h in an ice-water bath, the temperature was raised to 80° C., and the reaction was carried out for 3 h. Take the reaction solution 19 FNMR analysis, with OTf as the internal standard, the yield was 93%. Dilute the reaction solution with 500ml isopropyl acetate, add dropwise 40.4g KH 2 PO 4 500ml of aqueous solution. After dripping, stir for 1h. Suction filtration, rinse filter cake with 160ml isopropyl acetate. The filtrate was allowed to stand, and the upper organic phase was separated and dried over anhydrous magnesium sulfate. Suction filtration, concentration to dryness under reduc...
Embodiment 3
[0051] Embodiment 3: Preparation of 2-trifluoromethylisonicotinic acid
[0052]
[0053] 2-Trifluoromethylisonicotinic acid tert-butyl ester (14.82g, 0.06mol) was added to 150ml of 25% hydrochloric acid by mass fraction, and reacted at 80°C for 3h. Cool to 25°C and filter with suction to obtain off-white solid. 10.54 g after drying, the yield is 92.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com