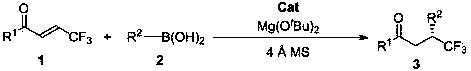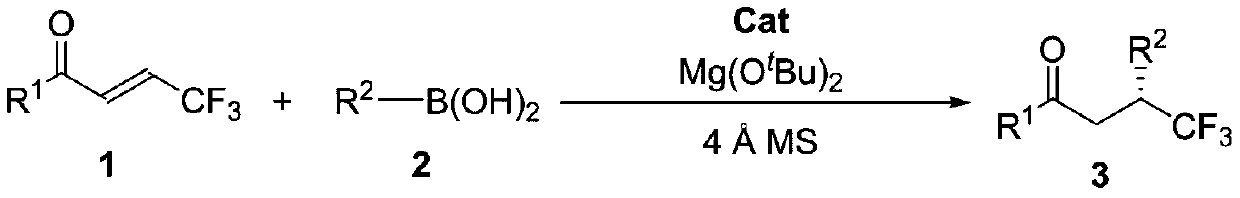Method for synthesizing optically active trifluoromethyl compound by asymmetric conjugate addition reaction of organic boronic acid and alpha, beta-unsaturated ketone
An organic boronic acid, conjugated addition technology, applied in the preparation of organic compounds, organic chemical methods, organic addition and other directions, can solve the problem that trifluoromethyl compounds are not reported, and achieve product yield and enantioselectivity Good properties, simple post-treatment and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023]
[0024]
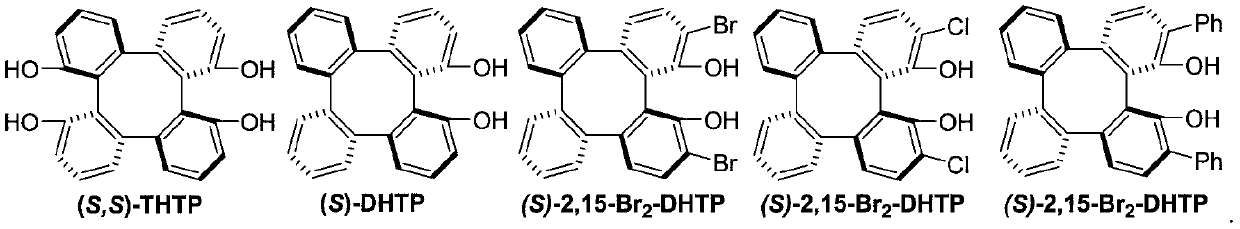
[0025] [a] Reaction condition: β-CF 3 -α,β-unsaturated ketone 1a (0.1mmol), trans-2-phenylvinylboronic acid 2a (0.12mmol), (S)-2,15-Br 2 -DHTP(0.01mmol),Mg(O t Bu) 2 (0.01mmol), Molecular sieves (50 mg), and 1.0 mL of anhydrous solvent in N 2 Stirring under atmosphere. [b] Isolated yield. [c] ee value obtained by HPLC chiral analysis. [d] 0.5mL DCE.
[0026] In the screening process of reaction conditions, the influence of different solvents on the reaction was first investigated (entries 1-6), and finally 1,2-dichloroethane (DCE) was selected as the solvent. When the reaction concentration increased, the product yield Both efficiency and enantioselectivity were improved (entry 7). Subsequently, the influence of different chiral catalysts on the reaction was investigated (entries 8-14), and Cat 1 was finally determined to be the best catalyst. At the same time, the effects of temperature and catalyst dosage on the reaction were invest...
Embodiment 2
[0031]
[0032] Under nitrogen protection, add 50mg Molecular sieves, chiral catalyst Cat1 (4.9mg, 0.01mmol, 10mol%), magnesium tert-butoxide (1.7mg, 0.01mmol, 10mol%), β-CF 3 -α,β-unsaturated ketone 1b (20.0mg, 0.1mmol) and organoboronic acid 2a (17.7mg, 0.12mmol, 1.2equiv), pumped and exchanged gas 3 times, then added dry 1,2-dichloroethane ( 0.5mL), stirred at 25°C for 48h. TLC spot plate tracking until the disappearance of raw material 1b, add 0.1mL water to quench the reaction, remove the solvent under reduced pressure and then directly separate and purify by fast silica gel column chromatography (the eluent is dichloromethane / petroleum ether volume ratio 1 / 5) to obtain the target product 3ba, 93% yield, 94% ee.
[0033] 3ba white solid (28.4mg, yield 93%); mp 41-43°C; HPLC (Daicel Chiralcel OD-H, n-hexane / isopropanol=90:10, flow rate 0.8mL / min, λ=254nm)t R (minor) = 6.68min,t R (major)=8.09min, ee=94%; [α] D 17 =-20.0(c1.0,CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl ...
Embodiment 3
[0035]
[0036] Under nitrogen protection, add 50mg Molecular sieves, chiral catalyst Cat1 (4.9mg, 0.01mmol, 10mol%), magnesium tert-butoxide (1.7mg, 0.01mmol, 10mol%), β-CF 3 -α,β-unsaturated ketone 1c (23.0mg, 0.1mmol) and organoboronic acid 2a (17.7mg, 0.12mmol, 1.2equiv), pumped and exchanged gas 3 times, then added dry 1,2-dichloroethane ( 0.5mL), stirred at 25°C for 48h. Tracked by TLC until the raw material 1c disappeared, the reaction was quenched by adding 0.1mL of water, and the solvent was removed under reduced pressure, followed by rapid silica gel column chromatography (eluent: dichloromethane / petroleum ether volume ratio 1 / 5) to separate and purify to obtain the target product 3ca, 98% yield, 94% ee.
[0037] 3ca white solid (33.0mg, yield 98%); mp 82-84°C; HPLC (Daicel Chiralcel OD-H, n-hexane / isopropanol=90:10, flow rate 0.8mL / min, λ=254nm)t R (minor)=9.61min,t R (major)=21.21min, ee=94%; [α] D 17 =-3.1(c2.0, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com