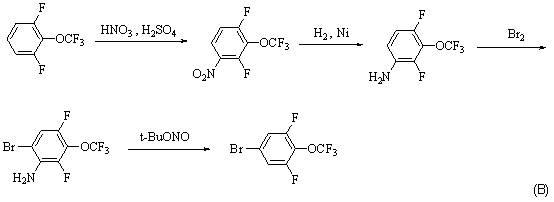
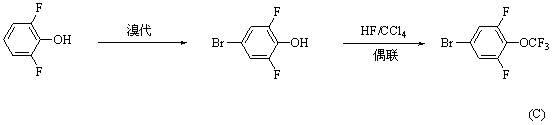
Synthesis method for 4-bromo-2, 6-difluoro-trifluoromethoxybenzene
A technology for the synthesis of trifluoromethoxybenzene and its synthesis method, which is applied to the synthesis of 4-bromo-2,6-difluoro-trifluoromethoxybenzene and the field of intermediate synthesis, and can solve the problems that are not suitable for industrial production and synthesis. Problems such as short routes and highly toxic hydrogen fluoride have achieved the effects of being conducive to industrial production, short synthesis routes, and reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 250 ml three-necked flask, add 0.2 mol (42.2 g) of 3,4,5-trifluorobromobenzene, stir, and start to add 57.9 g of 28% sodium methoxide (0.3 mol) methanol solution dropwise at room temperature. Reflux, reaction 6h. After the reaction is complete, filter off the solid, remove the solvent by rotary evaporation of the filtrate, add 50ml of water and 80ml of 1,2-dichloroethane to the residue, separate the layers, wash the organic phase with water until neutral, add 4.0g of anhydrous magnesium sulfate to dry, and filter with suction , the filtrate was rotary evaporated to remove the solvent, the residue was subjected to vacuum distillation, and 41.6 g of fractions at 92~93°C / 15mmHg were collected, with a yield of 93.3%.
Embodiment 2
[0026] In a 250 ml three-necked flask, add 0.2mol (42.2g) of 3,4,5-trifluorobromobenzene, 1.25mol (40.0g) of anhydrous methanol, stir, and start adding 0.3mol (12.5 g) 96% sodium hydroxide, after the addition, the temperature was raised to reflux, and the reaction was carried out for 7 hours. After the reaction is completed, filter, remove the solvent by rotary evaporation of the filtrate, add 50ml of water and 80ml of 1,2-dichloroethane to the residue to separate the liquid, wash the organic phase with water until neutral, add 4.0g of anhydrous magnesium sulfate to dry, filter with suction, and spin the filtrate The solvent was removed by evaporation, and the residue was rectified under reduced pressure to collect 42.4 g of fractions at 92-94°C / 15mmHg, with a yield of 95.1%.
[0027] 2. Synthesis of compound II 4-bromo-2,6-difluorophenetole
[0028] Example 1
[0029] In a 250 ml three-necked flask, add 0.28 mol (19.1 g) of sodium ethoxide and 80 ml of toluene, stir, and st...
Embodiment 3
[0038]In a 250 ml three-necked flask, add 0.1mol (23.7g) 4-bromo-2,6-difluorophenetole, 21.9g DMF, stir, add 0.15mol (6.4g) lithium chloride, after the addition is complete, heat up to reflux , Reaction 5h. After the reaction is complete, pour the reaction solution into a mixture of ice and water, extract with 50ml of toluene, separate the layers, wash the organic phase with water until neutral, add 2.0g of anhydrous magnesium sulfate to dry, filter with suction, remove the solvent by rotary evaporation of the filtrate, and depressurize the residue Rectification, collecting 18.3g fraction at 71~74℃ / 8mmHg, yield 87.6%, gas phase monitoring, purity 99.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com