Synthesizing method of 2-trifluoromethyl-3-fluoropyridin
A technology of trifluoromethylation and synthesis method, which is applied in the field of synthesis of 2-trifluoromethyl-3-fluoropyridine, can solve the problems of trifluoromethylation reagent toxicity, complex reaction, expensive, etc., and achieve low cost of raw materials , simple and easy to operate, and the effect of low production equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
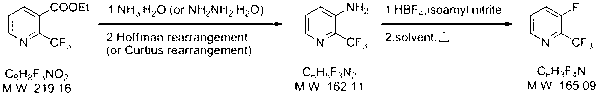
[0020] (1) Dissolve ethyl 2-trifluoromethylnicotinate (47.5 g, 0.217 mol) in 475 mL of methanol, add 85% hydrazine hydrate (128 g, 2.17 mol) and heat to 65°C, keep the reaction for 10 hours Afterwards, the reaction solution was concentrated to dryness (desolvation) to obtain 42 g of 2-trifluoromethylnicotinic acid hydrazide, with a yield of 94%.
[0021] 2-Trifluoromethylnicotinic acid hydrazide (42 g, 0.2 mol) was dissolved in 210 mL of 2 N HCl aqueous solution, and the reaction solution was cooled to -5°C. Slowly add sodium nitrite aqueous solution (16.87 g, 0.244 mol) dropwise to the reaction solution, keep the temperature of the reaction solution at -5°C, and keep it warm for 1 hour, then filter the reaction solution, and rinse the filter cake with 50 mL of water to obtain a white solid. For the intermediate, put the white solid into 450 mL of acetic acid aqueous solution (acetic acid: water = 1:1 (volume ratio)) to dissolve, heat to 90°C, and keep the reaction for 4 hours...
Embodiment 2
[0025] (1) Dissolve ethyl 2-trifluoromethylnicotinate (2.19 g, 10 mmol) in 22 mL of methanol, add ammonium chloride (0.53 g, 10 mmol) and 15 mL of 25% ammonia water (150 mmol) and tetrabutylammonium bromide (0.32 g, 1 mmol). The reaction solution was heated to 65~70°C for reflux reaction. After reflux reaction for 24 hours, the reaction solution was concentrated (precipitated) to obtain a crude product, which was then rinsed with 2 mL of water and methanol to obtain 0.95 g of 2-trifluoromethylnicotinamide. The yield was 50%.
[0026] Dissolve 9.8 g (240 mmol) of sodium hydroxide in 104 mL of water, cool down to 0°C and add 12.5 g (78 mmol) of liquid bromine, stir for 10 minutes and then dissolve 2-trifluoromethylnicotinamide (12.16 g, 64 mmol ) was added to the above reaction solution at one time, after stirring at 0°C for 15 minutes, the temperature of the reaction solution was raised to 75°C, and the reaction was kept for 90 minutes, then the reaction was cooled to room tem...
Embodiment 3
[0030] (1) Dissolve ethyl 2-trifluoromethylnicotinate (21.9 g, 0.1 mol) in 219 mL of tetrahydrofuran, add 80% hydrazine hydrate (50.1 g, 0.8 mol) and heat to 70°C, keep the reaction for 24 hours . After the reaction was completed, the reaction solution was concentrated to dryness (desolvation) to obtain 18.2 g of 2-trifluoromethylnicotinic acid hydrazide, with a yield of 88.7%.
[0031] Put 2-trifluoromethyl nicotinic acid hydrazide (18.2 g, 88.7 mmol) into 91 mL of 1 N HCl aqueous solution to dissolve, then cool the reaction solution to 0 °C, and slowly add sodium nitrite aqueous solution (6.12 g, 88.7 mmol), keep the temperature of the reaction solution at 0°C, keep the temperature for 2 hours, filter the reaction solution, rinse the filter cake with 20 mL of water to obtain a white solid intermediate, put the white solid into 200 mL volume concentration of 40 % of acetic acid aqueous solution, and heated to 100 ° C, heat preservation reaction for 6 hours. The reaction sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com