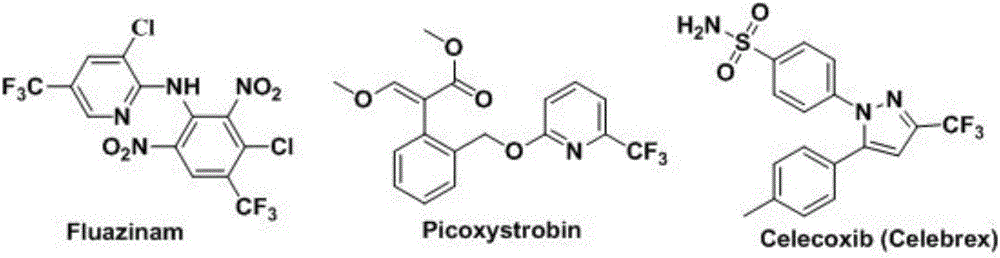
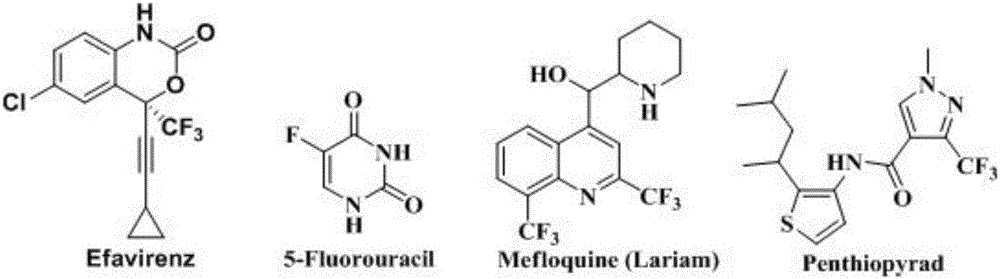
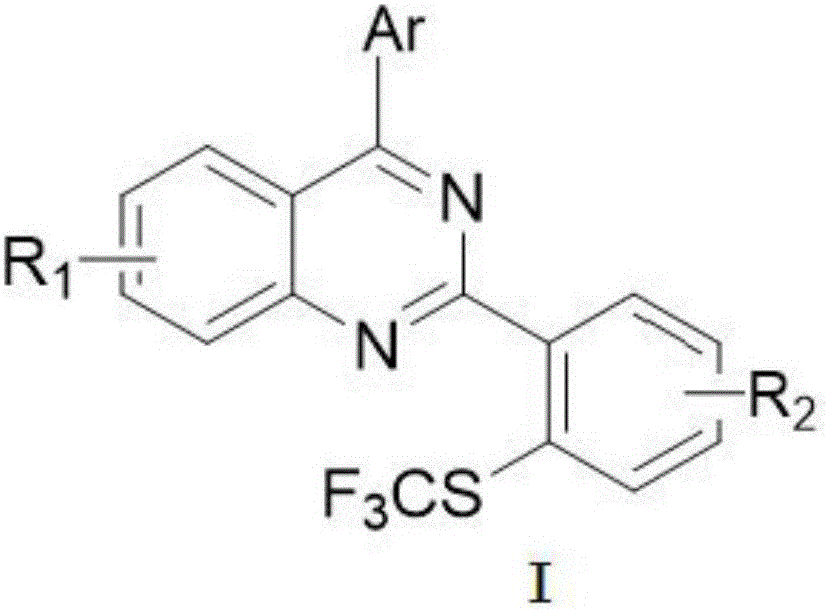
Method for preparing 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline
A technology of sulfur trifluoromethyl and phenylquinazoline, which is applied in the field of synthesis of fluorine-containing quinazoline compounds, can solve the problems of complex reaction and low nucleophilicity, and achieve high yield, clean and good spectrum The effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0052]2-o-tolyl-4-p-tolylquinazoline (0.3mmol), iodination reagent NIS (0.45mmol, 1.5equiv), catalyst dichloro(pentamethylcyclopentadienyl) rhodium (III) Dimer (0.006mmol, 0.02equiv) and silver hexafluoroantimonate (0.024mmol, 0.08equiv) were dissolved in the organic solvent 1,2-dichloroethane, stirred and reacted in air at 80°C for 1 to 4 hours After that, add AgSCF 3 (0.6mmol, 2.0equiv), CuI (0.03mmol, 10%equiv), reacted at 85°C for 3-4 hours, TLC detected that the reaction was complete. When processing, add a small amount of silica gel to absorb the crude product and spin the solvent to dry, and directly separate the pure product 2-(2-methyl-6-(sulfotrifluoromethyl)phenyl)-4-p-toluene through silica gel column chromatography Quinazolines (3a).
[0053]
[0054] 2-(2-methyl-6(trifluoromethylthio)phenyl)-4-p-tolylquinazoline 3a
[0055] Yellow oil (91%)
[0056] 1 H NMR (400MHz, CDCl 3 )δ8.14(d, J=8.0Hz,1H),8.06(d,J=8.4Hz,1H),7.87–7.83(m,1H),7.65(d,J=8.0Hz,2H),7.61– ...
example 2
[0058] 2-(2-chlorophenyl)-6-methoxy-4-o-tolylquinazoline (0.3mmol), iodination reagent NIS (0.45mmol, 1.5equiv), catalyst dichloro(pentamethylcyclo Pentadienyl) rhodium (III) dimer (0.006mmol, 0.02equiv) and silver hexafluoroantimonate (0.024mmol, 0.08equiv) were dissolved in the organic solvent 1,2-dichloroethane, at 80°C After stirring the reaction in the air for 1 to 4 hours, add AgSCF 3 (0.6mmol, 2.0equiv), CuI (0.03mmol, 10%equiv), reacted at 85°C for 3-4 hours, TLC detected that the reaction was complete. When processing, add a small amount of silica gel to absorb the crude product and spin the solvent to dryness, and directly separate the pure product 2-(2-chloro-6-(sulfurtrifluoromethyl)phenyl)-6-methoxy through silica gel column chromatography -4-o-tolylquinazoline (3b).
[0059]
[0060] 2-(2-chloro-6-(trifluoromethylthio)phenyl)-6-methoxy-4-o-tolylquinazoline 3b
[0061] Yellow oil (88%)
[0062] 1 H NMR (400MHz, CDCl 3 )δ7.99(d,J=8.8Hz,1H),7.65(d,J=8.0Hz,1...
example 3
[0064] Combine 6-methoxy-2,4-two-o-tolylquinazoline (0.3mmol), iodination reagent NIS (0.45mmol, 1.5equiv), catalyst dichloro(pentamethylcyclopentadienyl) Rhodium(III) dimer (0.006mmol, 0.02equiv) and silver hexafluoroantimonate (0.024mmol, 0.08equiv) were dissolved in the organic solvent 1,2-dichloroethane, stirred and reacted in air at 80°C After 1-4 hours, add AgSCF 3 (0.6mmol, 2.0equiv), CuI (0.03mmol, 10%equiv), reacted at 85°C for 3-4 hours, and TLC detected that the reaction was complete. When processing, add a small amount of silica gel to absorb the crude product and spin the solvent to dry, and directly separate the pure product 6-methoxy-2-(2-methyl-6-(sulfurtrifluoromethyl)phenyl through silica gel column chromatography )-4-o-tolylquinazoline (3c).
[0065]
[0066] 6-methoxy-2-(2-methyl-6-(trifluoromethylthio)phenyl)-4-o-tolylquinazoline 3c
[0067] Yellow oil (69%)
[0068] 1 H NMR (400MHz, CDCl 3 )δ8.07(d,J=9.2Hz,1H),7.66(d,J=7.6Hz,1H),7.61(dd,J=9.2,2.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com