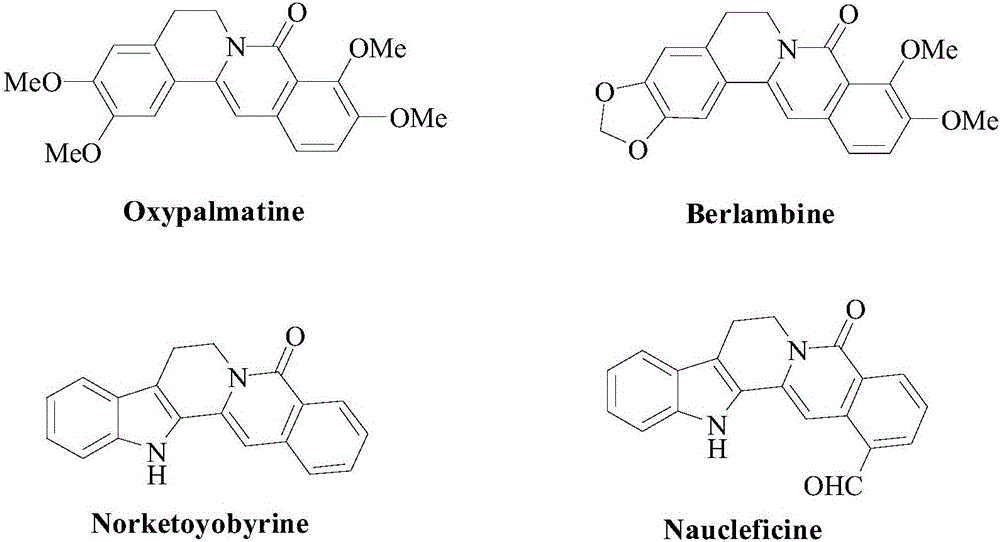
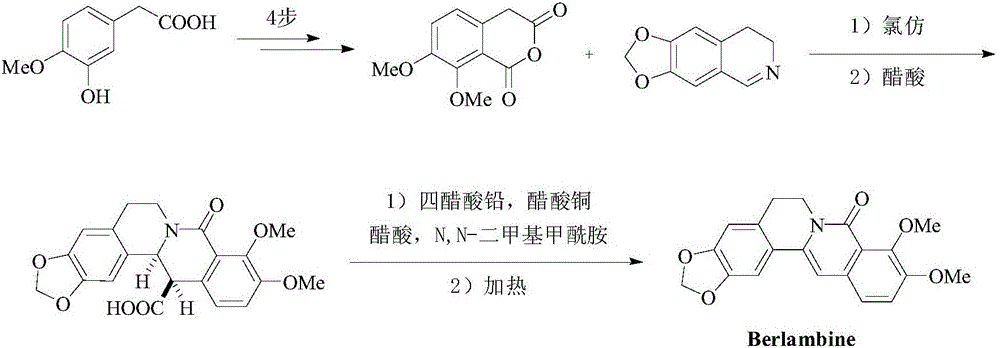
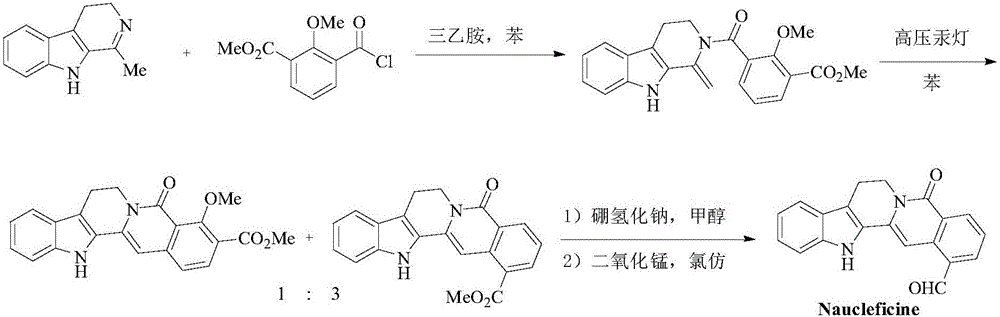
Method for synthesizing 6-6 fused ring structure in berberine and ebony natural product
A natural product, berberine technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems that hinder the development and application of the pharmaceutical field, such as harsh conditions and high costs, and achieve the promotion of extensive development and application, mild reaction conditions, and production low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: preparation of
[0038]
[0039] 1) Take methyl salicylate 1 (1.7mL, 13.1mmol) in a 100mL round bottom flask, add 60mL (0.2M) of dichloromethane, add 5.5mL triethylamine and 3.3mL trifluoromethane dropwise at 0°C Sulfonic anhydride, then reacted at 0°C for 2 hours, added water, extracted with ethyl acetate, combined the extracts, washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, spin-dried the solvent, column chromatography (3% ethyl acetate / petroleum ether) to obtain a light yellow liquid, namely: compound 2 (3.3 g, yield 89%), Rf=0.56 (20% ethyl acetate-petroleum ether).
[0040] 2) Weigh known compound 2 (63mg, 0.22mmol) and compound 3 (100mg, 0.33mmol) in a 25mL round bottom flask, add 13mg tetraphenylphosphine palladium, 250mg tetrabutylammonium iodide, 2mg iodide Cuprous, under the protection of nitrogen, add 4.5mL (0.1M) deoxygenated triethylamine / acetonitrile (1 / 4), react at 80°C for 33 hours, filter w...
Embodiment 2
[0045] Example 2: preparation of
[0046]
[0047] 1) Weigh compound 6 (325mg, 1.33mmol) in a 100mL round bottom flask, add 6.6mL acetonitrile, 27mL silver nitrate aqueous solution (0.1N), 6.6mL10% sodium hydroxide aqueous solution, react at room temperature for 23 hours, diatoms Filter with soil, add 6N aqueous sodium hydroxide solution to adjust the pH value to less than 5, extract with ethyl acetate, combine the extracts, wash once with 1N aqueous hydrochloric acid solution, dry over anhydrous sodium sulfate, filter, and spin to dry the solvent;
[0048] The resulting crude product was directly dissolved in 6.6mL (0.2M) N,N-dimethylformamide, 110mg potassium carbonate and 0.15mL dimethyl sulfate were added, and reacted at room temperature for 2 hours. Extract the ester, combine the extracts, wash with saturated aqueous sodium chloride, dry over anhydrous sodium sulfate, filter, spin to dry the solvent, and column chromatography (20% ethyl acetate / petroleum ether) to ob...
Embodiment 3
[0054] Example 3: preparation of
[0055]
[0056] 1) Weigh commercially available raw material compound 9 (360mg, 2.3mmol) and dissolve it in 5.7mL (0.4M) of N,N-dimethylformamide, add 42mg of 4-dimethylaminopyridine and 0.5mL of ethanol, and slowly Add 1.1 g of dicyclohexylcarbodiimide, react at room temperature for 8 hours, filter through diatomaceous earth, spin to dry the solvent, and perform column chromatography (10-20% ethyl acetate / petroleum ether) to obtain a light yellow solid, namely: Compound 10 (208 mg, 49% yield), Rf=0.40 (30% ethyl acetate-petroleum ether).
[0057] 2) Weigh compound 10 (51mg, 0.28mmol) and compound 3 (80mg, 0.26mmol) used in Example 1 in a 10mL round bottom flask, add 10mg dichlorodibenzonitrile palladium, 15mg tri-tertiary tetrafluoroborate Butylphosphine, 3mg cuprous iodide, 290mg tetrabutylammonium iodide, add 2.6mL (0.1M) deoxygenated triethylamine / acetonitrile (1 / 4) under nitrogen protection, and react at 80°C for 5 hours , filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com