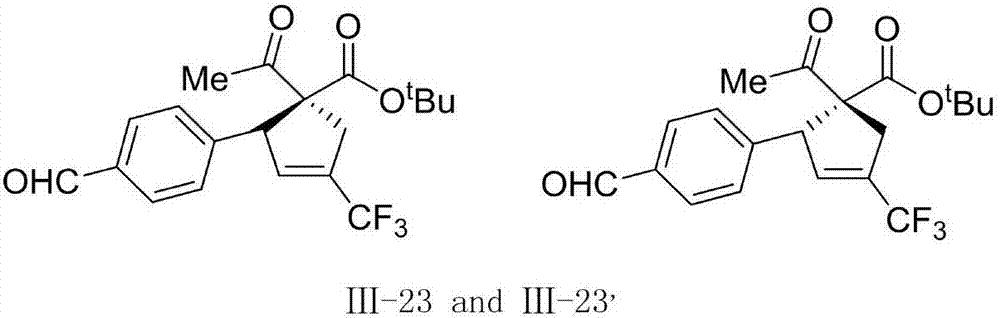
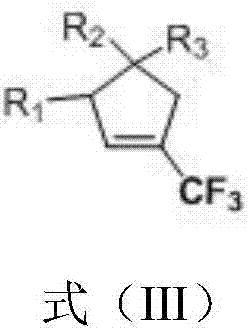
1-trifluoromethyl-tetrasubstituted cyclopentene derivatives as well as preparation method and application thereof
A kind of technology of trifluoromethyl and cyclopentene, applied in the field of chemical substances and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] First weigh (3-(trifluoromethyl)but-3-en-1-ynyl)benzene (0.5mmol), silver nitrate (50mol%) in a dry reaction tube, add toluene under nitrogen atmosphere, and then Inject triethylamine (0.5 mmol) and 1,3-dicarbonyl compound - acetylacetone (1 mmol). Then, react at room temperature, and monitor the reaction by thin-layer chromatography silica gel (TLC) and ultraviolet during the reaction until (3-(trifluoromethyl)but-3-en-1-ynyl)benzene completely disappears. After the reaction was completed, it was filtered with diatomaceous earth and the solvent was removed by rotary evaporation under low pressure, and then the crude product was separated and purified by column chromatography (petroleum ether: ethyl acetate = 50:1) to obtain 1-trifluoromethyl-3-benzene Diacetyl-4,4-diacetyl-1-cyclopentene III-1 (70 mg, 47%).
[0039]
[0040] white solid. Mp 58.9-60.1°C. 1 H NMR (400MHz, CDCl 3 )δ7.32–7.25(m,3H),7.13–7.09(m,2H),6.21-6.18(m,1H),5.08-5.03(m,1H),3.81(dd,J=17.4,1.4Hz...
Embodiment 2
[0042] With 4-formyl-(3-(trifluoromethyl)but-3-en-1-ynyl)benzene (0.5mmol), 1,3-dicarbonyl compound——acetylacetone (1mmol) as raw materials, For other operations refer to Example 1, the reaction was stirred for 24 hours, purified by silica gel column chromatography (petroleum ether: ethyl acetate = 20:1), to obtain 1-trifluoromethyl-3-(4-formylphenyl)-4,4 - Diacetyl-1-cyclopentene III-2 (126 mg, 78%).
[0043]
[0044] white solid. Mp 77.7-79.4°C. 1 H NMR (500MHz, CDCl 3 )δ9.98(s,1H),7.81(d,J=7.8,2H), 7.31(d,J=7.8Hz,2H),6.18(s,1H),5.17(s,1H),3.79(d ,J=17.4Hz,1H),2.78(d,J=17.4Hz,1H),2.22(s,3H),1.58(s,3H). 13 C NMR (126MHz, CDCl 3 )δ202.42(s), 201.81(s), 191.41(s), 144.06(s), 135.94(s), 135.59(q, J=4.8Hz), 131.13(q, J=34.2Hz), 130.06( s), 129.75(s), 121.65 (q, J=269.9Hz), 78.99(s), 53.73(s), 34.92(s), 28.14(s), 26.60(s). 19 F NMR (471MHz, CDCl 3 )δ -66.11(s).HRMS(ESI) calcd for C 17 h 15 f 3 NaO 3 :347.0865,found:347.0863.
Embodiment 3
[0046] With 4-cyano-(3-(trifluoromethyl)but-3-en-1-ynyl)benzene (0.5mmol), 1,3-dicarbonyl compounds——acetylacetone (1mmol) as raw materials, For other operations refer to Example 1, the reaction was stirred for 24 hours, purified by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1), to obtain 1-trifluoromethyl-3-(4-cyanophenyl)-4,4 - Diacetyl-1-cyclopentene III-3 (115 mg, 72%).
[0047]
[0048] white solid. Mp 92.0-94.1°C. 1 H NMR (500MHz, CDCl 3 )δ7.57(d, J=8.5Hz, 2H), 7.23(d, J=8.5Hz, 2H), 6.16-6.14(m, 1H), 5.12(d, J=1.9Hz, 1H), 3.72( dd,J=17.4,1.2Hz,1H),2.77 (dd,J=17.4,1.3Hz,1H),2.19(s,3H),1.57(s,3H). 13 C NMR (126MHz, CDCl 3 ( q, J=270.1Hz), 118.13(s), 112.04(s), 78.84(s), 53.59(s), 34.89(s), 28.11(s), 26.63(s). 19 F NMR (471MHz, CDCl 3 )δ-66.15(s).MS(70eV):M / Z(%):321,43(100).HRMS(EI)calcd for C 17 h 14 f 3 o 2 N:321.0977,found:321.0976.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com