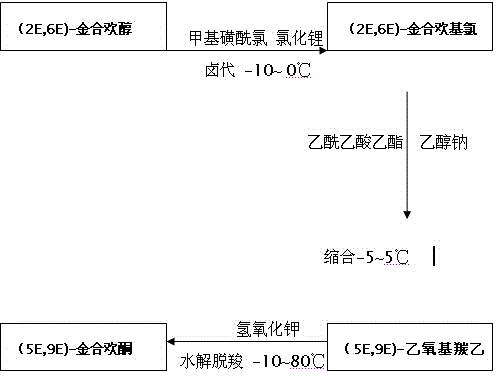
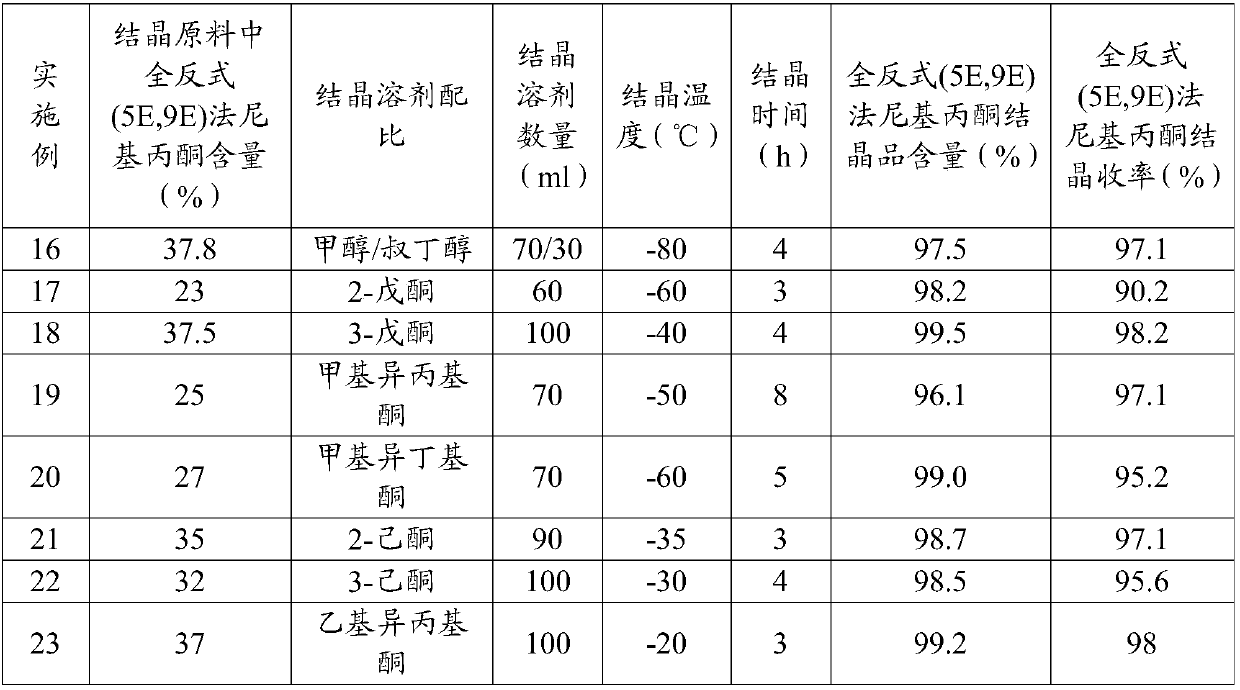
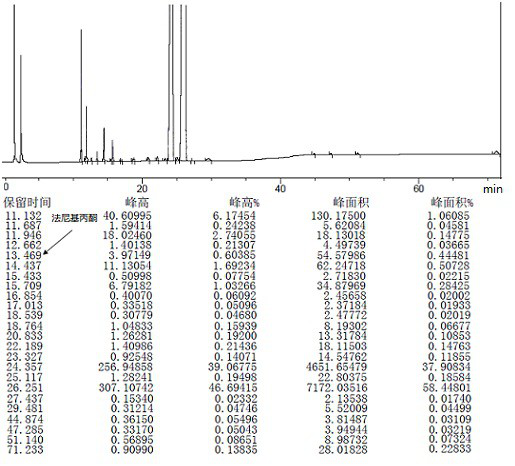
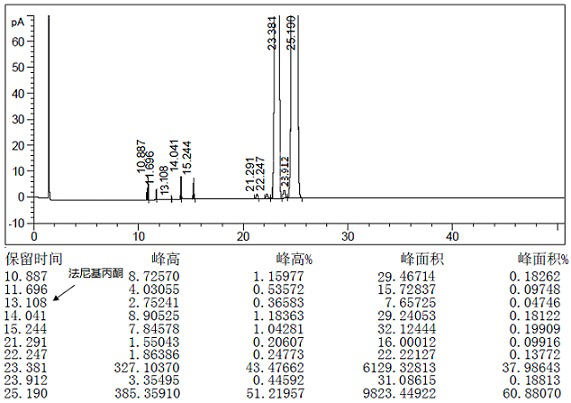
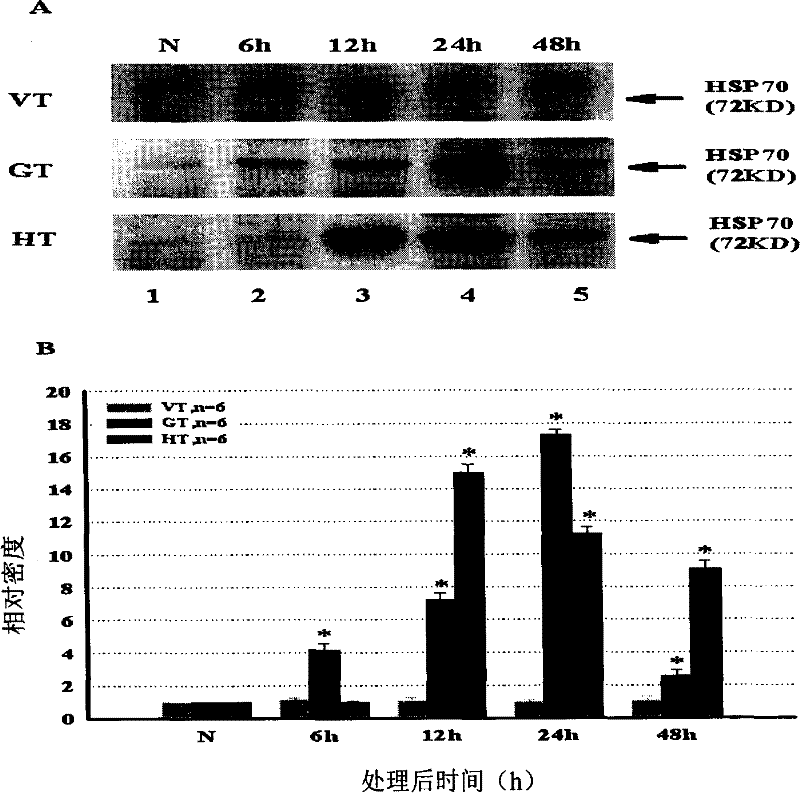
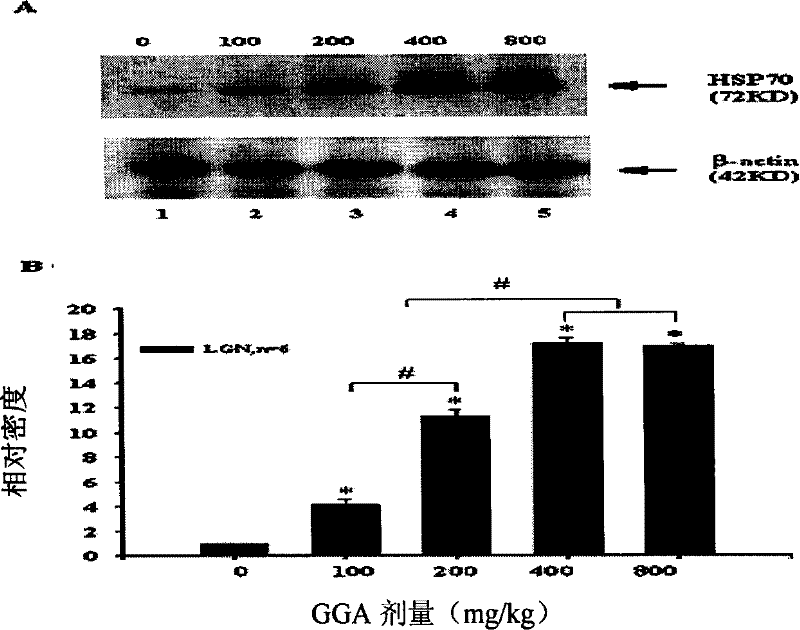
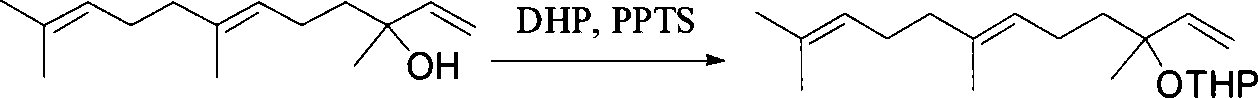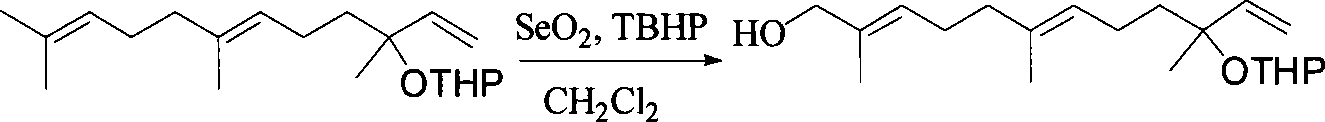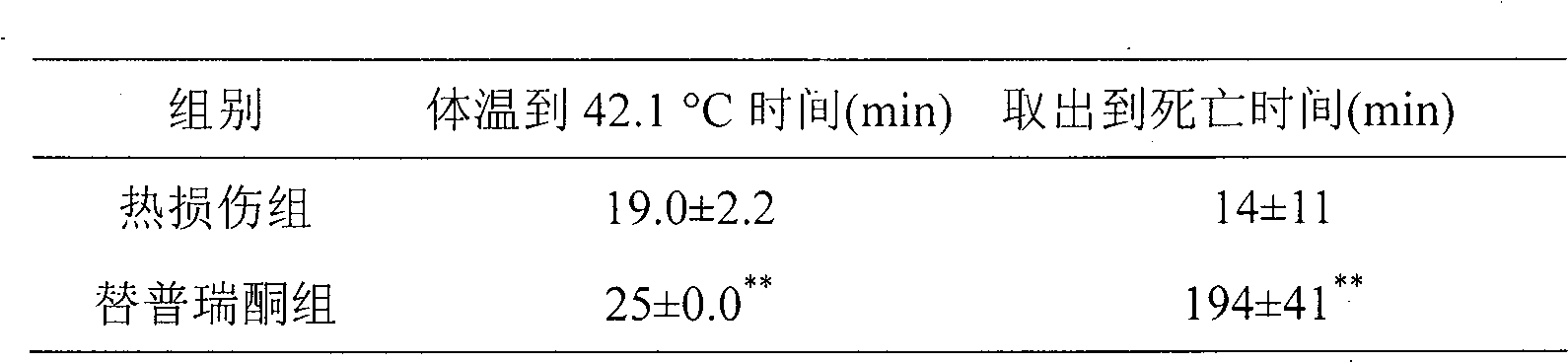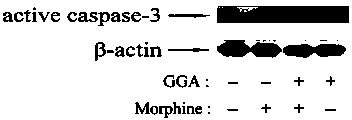Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Teprenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
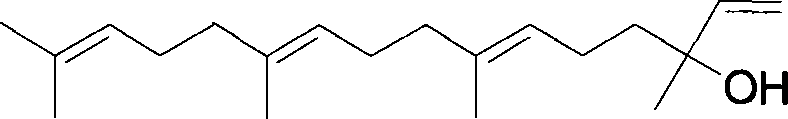
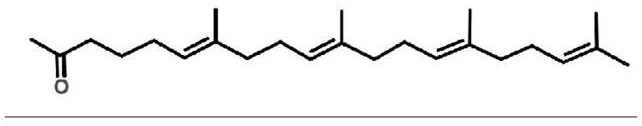
Teprenone (or geranylgeranylacetone) is a pharmaceutical drug used for the treatment of gastric ulcers. In Japan it is sold under the brand name Selbex (セルベックス).
Topical use of teprenone
InactiveUS20090214607A1Effective controlEffective preventionCosmetic preparationsBiocideSkin cellProtection Skin
Owner:SEDERMA SA
Acute hypoxia injury resistance use of teprenone
ActiveCN101450048ALow priceImprove securityOrganic active ingredientsDrug compositionsSocial benefitsOral medication
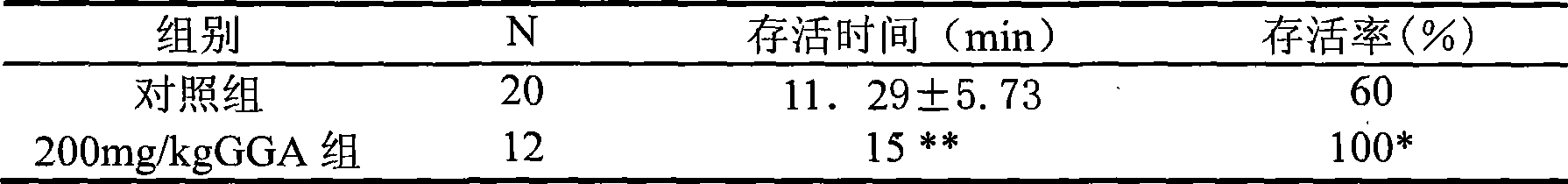
The invention relates to the action of anti-acute hypoxia injury of teprenone, under the condition of oral administration of the teprenone, the survival ratio of chmice under the acute hypoxia condition can be obviously improved, the survival time can be prolonged. The invention pours the teprenone with different concentrations into the stomach of the adult chmice, after four hours, the chmice is arranged in the acute hypoxia environment, the survival ratio and the survival time are observed, so that the survival ratio of the animal can be improved and the survival time of the animal can be prolonged under acute hypoxia condition by pouring the teprenone with certain concentration into the stomach in advance, the anti-acute hypoxia injury capability of the chmice can be improved. According to the method of the invention, the teprenone is administrated at suitable time with suitable dose, which can improve the anti-acute hypoxia injury capability of the chmice, powerful evidences can be provided for the human body experiment, if the teprenone can be popularized for human body application, good economic benefit and social benefit can be generated.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
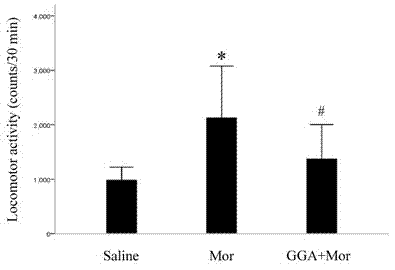
Application of geranylgeranylacetone to preparation of medicament for preventing and/or treating opiates drug addiction
InactiveCN102266312ASignificant effectImprove securityOrganic active ingredientsNervous disorderNarcotics addictionMorphine
The invention discloses application of geranylgeranylacetone to the preparation of a medicament for preventing and / or treating opiates drug addiction, relating to the technical field of medicines. Geranylgeranylacetone is taken as a medicament for preventing and treating the opiates drug addiction or is used for preparing a medicament for preventing and treating the opiates drug addiction. One or more medicinally acceptable conventional medicinal auxiliary materials can also be added into geranylgeranylacetone to prepare capsules, pills, powder, tablets, granules, oral liquids, injections and the like. Geranylgeranylacetone has the effects of antagonizing conditioned place preference behaviors caused by morphine and increase in autonomic activities induced by morphine and relieving the function of abstinence symptom action, is used for preventing and treating the opiates drug addiction, and has the advantages of remarkable curative effect, high safety, addiction resistance, low price, capability of lowering the economic burden of a patient, and the like.
Owner:KUNMING UNIV OF SCI & TECH
Method for synthesizing (E,E) Geranyl linalool
InactiveCN101070270AOrganic compound preparationHydroxy compound preparationSulfonyl chlorideNerolidol
This invention relates to synthetic method of a ( E, E) - geranyl linalool. The invention takes (E) - nerolidol as raw material. The hydroxyl is shield by dihydropyrane, gain ( E) - nerolidol tetrahydropyrane aether; selenium dioxide and teri-butyl hydroperoxide selectively oxidize the anti-form methyl of ( E) - nerolidol tetrahydropyrane aether to gain anti-form allyl position hydroxylated oxidative product ( E, E) - 12 - hydroxy nerolidol tetrahydropyrane aether, transit halogenating reaction to gain ( E, E) - 12 - halogeno- nerolidol tetrahydropyrane aether, then take reaction with isopropyl methyl ketone that is selectively divested one proton by diisopropyl amido lithium, generate ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - ketone, use sodium borohydride to reduce to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - alcohol, takes reaction with sulfonyl chloride or sulphonic acid ester with alkali presence to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 oxygen) -16 - 6, 10, 15 - triene - 3 - alcoholic sulphonic acid ester, then divide sulphonic acid ester group under base catalysis to gain ( E, E) - geranyl linalool tetrahydropyrane aether, and by deprotection to gain ( E, E) - geranyl linalool. ËFor the configuration of ( E) - nerolidol 3 position tertiary carbon is not influenced in the course of reaction, if use ( E) - nerolidol that has optical activity as raw material, should gain optical active ( E, E) - geranyl linalool. ((E, E)-geranyl linalool can replace Teprenone and such type medicament intermediate, natural product intermediate, insect pheromone and spice etc.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Method for separating all trans-teprenone under low temperature
InactiveCN106431879ASolve the separation problemIncrease contentOrganic chemistry methodsCarbonyl compound separation/purificationAlkaneSolvent
The invention relates to a method for separating all trans-teprenone under low temperature. The method comprises the following specific steps: an ether-type organic solvent is added in low-chain liquid alkane, and full and uniform stirring is performed, such that a diluting agent is prepared; the prepared diluting agent is added into a jacketed container; a teprenone isomer mixture is added; stirring is started; circulating hot water is introduced into the jacket; stirring is carried out for uniformly mixing the materials; the circulating hot water in the container jacket is discharged, and a refrigerant is introduced; crystallization is carried out under stirring, such that teprenone crystals are obtained; the teprenone crystals are placed in a vacuum suction filtration device, and vacuum suction filtration is carried out; an upper-layer filter cake is taken out and is dissolved under normal temperature, such that all trans-teprenone is obtained. The method assists in fundamentally solving an all trans-teprenone separation problem. The separation temperature is controllable. The all trans-teprenone obtained after separation has the advantages of high content and low impurity content. During the process, solvent amount is low; the solvent can be recycled and applied mechanically; and no three-waste is generated. The method is suitable for large-scale production.
Owner:TIANJIN RUIAN MEDICAL TECH DEV
Teprenone orally disintegrating tablet prescription and its preparation method
InactiveCN1954806AMask bad tasteDisintegrates quicklyOrganic active ingredientsDigestive systemVitamin COrally disintegrating tablet
An oral disintegrating tablet of teprenone contains teprenone, filler chosen from MCC, dextrin, lactose and mannitol, disintegrant chosen from L-HPC, PPVP, CMS-Na and their mixture, flavouring chosen from nature or artificial sweetening agent, antioxidant chosen from VC, VE and BHA, lubricant chosen from magnesium stearate, magnesium lauryl sulfate and talc powder, and flowing aid chosen from superfine silicon gel powder and talc powder. Its preparing process is also disclosed.
Owner:SHANTOU UNIV MEDICAL COLLEGE
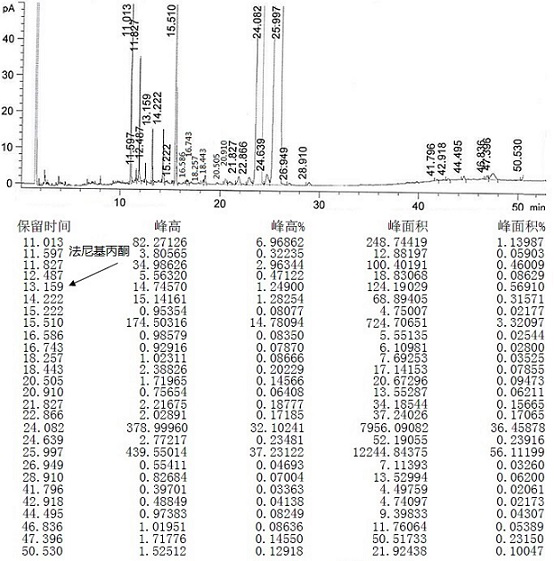
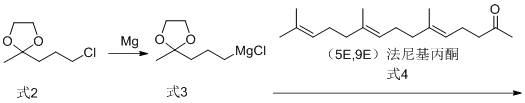
Preparation method for intermediate (5E, 9E)-farnesyl acetone of teprenone
InactiveCN104447256AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationLithium chloridePotassium hydroxide
The invention discloses a preparation method for an intermediate (5E, 9E)-farnesyl acetone of teprenone. The preparation method comprises the following steps: (1) carrying out reaction on (2E, 6E)-farnesol together with methanesulfonyl chloride and lithium chloride to obtain (2E, 6E)-farnesyl chloride; (2) carrying out reaction (2E, 6E)-farnesyl chloride together with ethyl acetoacetate and sodium ethoxide to generate (5E, 9E)-ethoxycarbonyl ethyl farnesyl acetone; and (3) decarboxylating (5E, 9E)-ethoxycarbonyl ethyl farnesyl acetone in the alkaline solution of potassium hydroxide by saponifying to obtain the (5E, 9E)-farnesyl acetone. The innovative synthesis process is used for preparing the (5E, 9E)-farnesyl acetone, the toxicity of adopted raw materials and by-products are low, and the raw materials and the by-products are easy to treat. In addition, materials react under mild reaction conditions, the preparation method is easy to operate, the yield and the purity of the (5E, 9E)-farnesyl acetone are high, and the content of isomers in (5E, 9E)-farnesyl acetone is low.
Owner:岳阳新华达制药有限公司
Synthesis methods of teprenone and intermediate thereof
ActiveCN108047011ALow purityLow yieldOrganic compound preparationOrganic chemistry methodsAcetic acidNerolidol
The invention relates to synthesis methods of teprenone and an intermediate thereof. The synthesis of the intermediate all-trans (5E,9E)-farnesyl acetone comprises: 1) in the presence of a catalyst, carrying out a carroll rearrangement reaction on the mixture of 5E-nerolidol and 5Z-nerolidol and hydrocarbonyl acetoacetate to obtain the isomer mixture of (5Z,9Z)-farnesyl acetone, (5Z,9E)-farnesyl acetone, (5E,9Z)-farnesyl acetone, and (5E,9E)-farnesyl acetone, wherein the content of (5E,9E)-farnesyl acetone in the isomer mixture is controlled at more than 18%; and 2) carrying out low-temperature crystallization separation on the isomer mixture in a moderately polar solvent to obtain the all-trans (5E,9E)-farnesyl acetone. According to the present invention, the high-purity all-trans (5E,9E)-farnesyl acetone is obtained by using the mixture of 5E-nerolidol and 5Z-nerolidol as the raw material through the low-temperature crystallization separation, such that the teprenone with the qualified quality can be conveniently obtained.
Owner:ZHEJIANG NHU CO LTD
New application of teprenone
InactiveCN104958281ASignificant effectImprove securityOrganic active ingredientsNervous disorderAbstinencePharmaceutical drug
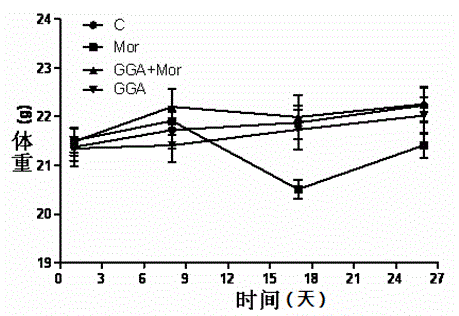
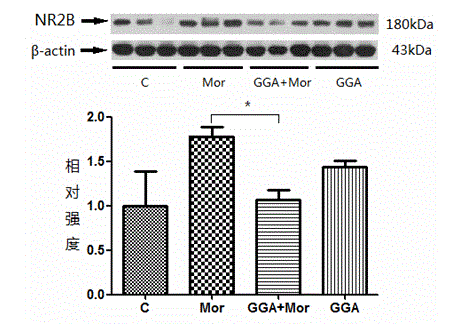
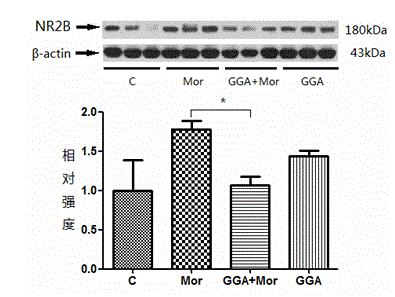
The invention discloses new application of teprenone, namely application to preparing a medicine for preventing and / or treating weight reduction caused by opioid drug habituation and abstinence relapsing, and relates to the technical field of medicines. The teprenone can resist mice weight reduction caused by morphine habituation and abstinence; in addition, in terms of the molecular level, GGA obviously restrains NAc area NR2B expression rise induced by morphine relapsing lighting; and the teprenone can be used for preventing and treating weight reduction caused by opioid drug habituation and abstinence relapsing.
Owner:KUNMING UNIV OF SCI & TECH
Application of geranylgeranylacetone to preparation of medicament for preventing and/or treating opiates drug addiction
InactiveCN102266312BSignificant effectImprove securityOrganic active ingredientsNervous disorderNarcotics addictionMorphine
The invention discloses application of geranylgeranylacetone to the preparation of a medicament for preventing and / or treating opiates drug addiction, relating to the technical field of medicines. Geranylgeranylacetone is taken as a medicament for preventing and treating the opiates drug addiction or is used for preparing a medicament for preventing and treating the opiates drug addiction. One ormore medicinally acceptable conventional medicinal auxiliary materials can also be added into geranylgeranylacetone to prepare capsules, pills, powder, tablets, granules, oral liquids, injections andthe like. Geranylgeranylacetone has the effects of antagonizing conditioned place preference behaviors caused by morphine and increase in autonomic activities induced by morphine and relieving the function of abstinence symptom action, is used for preventing and treating the opiates drug addiction, and has the advantages of remarkable curative effect, high safety, addiction resistance, low price,capability of lowering the economic burden of a patient, and the like.
Owner:KUNMING UNIV OF SCI & TECH
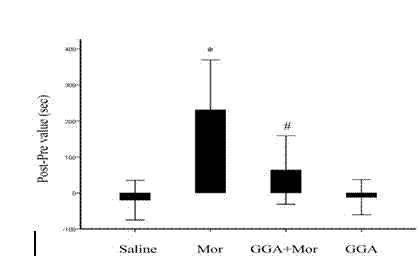
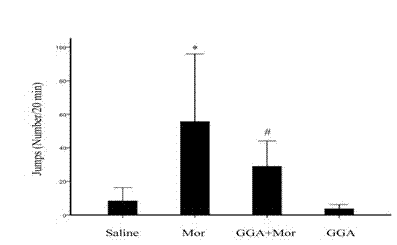
Application of Teprenone in preparing drug for preventing and/or treating readdiction in opiates narcotics
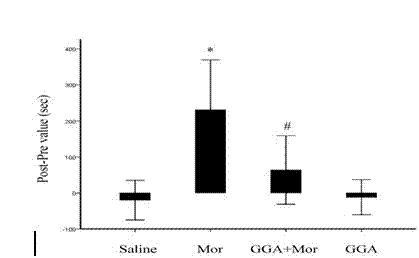
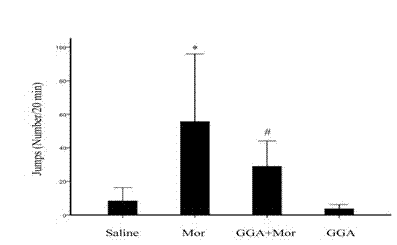
The invention discloses a novel application of teprenone, and particularly relates to an application in preparing a drug for preventing and / or treating readdiction in opiates narcotics, and relates to the technical field of medicine. The experiment result shows that after the teprenone is orally taken for 7 days, the conditioned place preference test is carried out on re-addiction mouse after the morphine addiction disappears; when GGA is previously orally taken, the mouse conditioned place preference caused by the readdiction in the morphine can be remarkably inhibited; the expression of NAc-zone NR2B induced by readdiction of morphine can be remarkably inhibited on the molecular level, and the teprenone can be used for preparing the drug for preventing and / or treating opiates narcotics.
Owner:KUNMING UNIV OF SCI & TECH
Acute hypoxia injury resistance use of teprenone
ActiveCN101450048BLow priceImprove securityOrganic active ingredientsDrug compositionsSocial benefitsAcute hypoxia
The invention relates to the action of anti-acute hypoxia injury of teprenone, under the condition of oral administration of the teprenone, the survival ratio of chmice under the acute hypoxia condition can be obviously improved, the survival time can be prolonged. The invention pours the teprenone with different concentrations into the stomach of the adult chmice, after four hours, the chmice isarranged in the acute hypoxia environment, the survival ratio and the survival time are observed, so that the survival ratio of the animal can be improved and the survival time of the animal can be prolonged under acute hypoxia condition by pouring the teprenone with certain concentration into the stomach in advance, the anti-acute hypoxia injury capability of the chmice can be improved. According to the method of the invention, the teprenone is administrated at suitable time with suitable dose, which can improve the anti-acute hypoxia injury capability of the chmice, powerful evidences can be provided for the human body experiment, if the teprenone can be popularized for human body application, good economic benefit and social benefit can be generated.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
A kind of preparation method of teprenone
ActiveCN103739470BHigh purityImprove conversion rateCarbonyl compound separation/purificationPreparation from heterocyclic compoundsDistillationOrganic layer
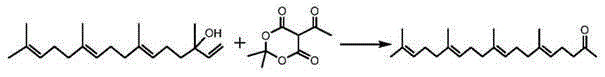
The invention discloses a method for preparing teprenone. The method comprises the following steps: (a) mixing geranyl linalool, acetyl Meldrum's acid and aluminum isopropoxide according to a molar ratio of 1: (1.2-1.5): (0.02-0.1), dissolving the mixture in a paraxylene solvent, heating up to 50-160 DEG C, refluxing for 6-10 hours, cooling and removing the solvent to obtain a teprenone mixture; (b) washing and extracting the teprenone mixture, mixing organic layers, and drying to obtain a crude product of teprenone; (c) carrying out molecular distillation, impurity removal, decompression rectifying and impurity removal on the crude product of teprenone to obtain a teprenone product. The method is simple and easy to control, the conversion rate of the product is high, and the prepared product is high in purity, stable in quality and high in safety, thereby being convenient to promote and use in clinical and industrial production.
Owner:乐声药业石家庄有限公司
Synthesis method of Teprenone
InactiveCN101343219BThe synthesis process is simpleReduce material consumption and energy consumptionDigestive systemCarbonyl compound preparation by condensationAcetoacetatesSulfonyl chloride
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
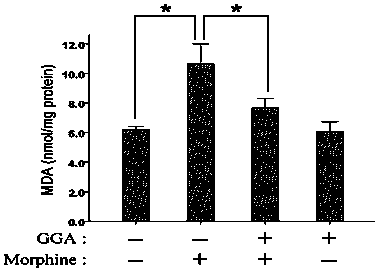
Application of teprenone in prevention and treatment of morphine-induced liver injury
InactiveCN102764248ASignificant effectImprove securityOrganic active ingredientsDigestive systemMorphinePharmaceutical drug
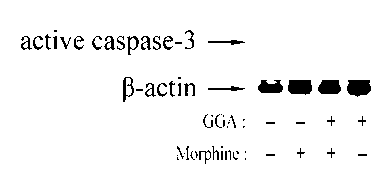
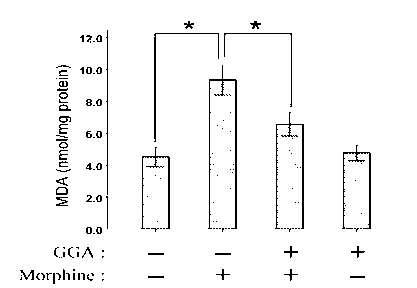
The invention relates to a new application of teprenone in preparation of a medicament for preventing and treating morphine-induced liver injury, and discloses teprenone capable of protecting liver cells from being poisoned by morphine, inhibiting morphine-induced cell apoptosis and weakening morphine-induced lipid per-oxidative injury. The teprenone can be used for preventing and treating the morphine-induced liver injury.
Owner:KUNMING UNIV OF SCI & TECH
Application of teprenone in preparing medicine used for preventing and/or treating depression
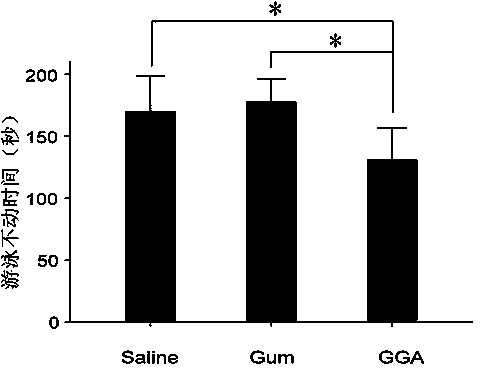
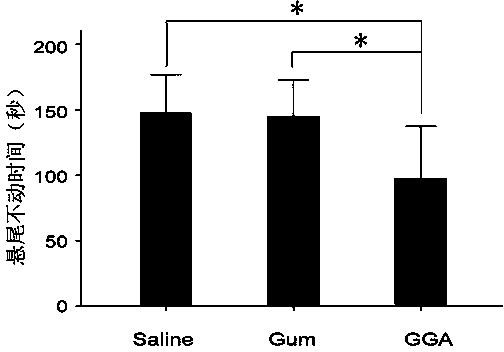
The invention discloses the application of teprenone in the preparation of drugs for preventing and / or treating depression, which belongs to the field of medical technology; the experimental results of the invention show that teprenone can protect nerve cells from the poison of dexamethasone, It can also combat the despairing behavior of mice, significantly shorten the swimming immobility time and tail-hanging immobility time of mice, and has significant curative effect, high safety and low cost.
Owner:KUNMING UNIV OF SCI & TECH
Process for synthesizing and purifying teprenone
ActiveCN103058839BImprove solubilityImprove catalytic performanceOrganic compound preparationCarbonyl compound preparationAlcoholSilica column
Owner:SICHUAN YUANJI PHARMA CO LTD
Method for synthesizing Teprenone
ActiveCN102050714BSuppress generationHigh purityOrganic compound preparationCarbonyl compound separation/purificationPurification methodsKetone solvents
The invention discloses an improved process for synthesizing Teprenone with Carroll reaction and a separation and purification method. In the method, aiming at the condition that alcohol byproducts which are difficult to separate are always produced in reaction, a treated organic aluminum catalyst is adopted, a ketone solvent is added for diluting; and low vacuum is adopted in the reaction process for rapidly transferring the low-level alcohol and the carbon dioxide generated in the reaction, and therefore, the reaction temperature is reduced, the side reaction is well controlled, the generation of the alcohol byproducts is decreased and the reaction conversion rate is higher than 95%. According to the invention, the emulsification problem in the purification process is solved so that the yield of the product before the rectification is increased to be higher than 87%, the short-distance distillation is well realized, the separated Teprenone completely meets the standards on the marketed drugs, the purity is not less than 99.0% and the yield of the high-purity product is above 66%. The improved process and the post-treatment and separation technology are simple for operation, easy for control, good in stability, achieve high reaction conversion rate and stable quality and are easy for industrialized production.
Owner:CHONGQING HEALTHY MEDICINE CO LTD
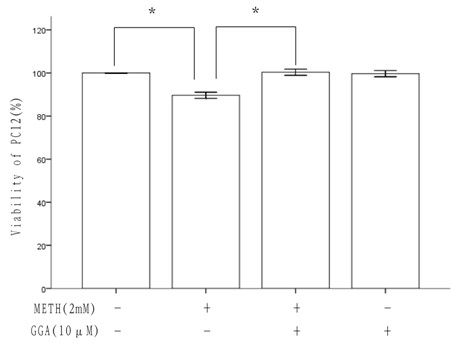
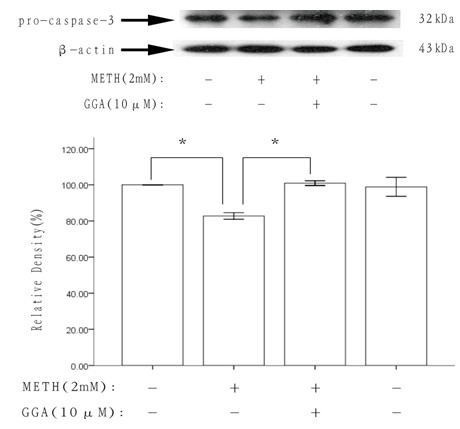
Application of teprenone in resisting neurotoxicity caused by methamphetamine
InactiveCN102600116AFree from growth inhibitionFree from apoptosisOrganic active ingredientsNervous disorderPharmaceutical drugApoptosis
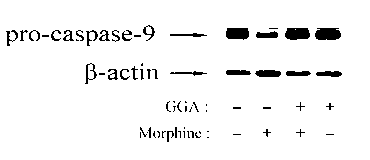
The invention discloses an application of teprenone in the preparation of drugs for preventing and / or treating neurotoxicity caused by methamphetamine, belonging to the technical field of medicines. The teprenone has the effect of resisting neurotoxicity caused by methamphetamine. A test result shows that the teprenone has the capabilities of protecting rat pheochromocytoma cells, namely PC12 cells to be free from cell apoptosis caused by methamphetamine and recovering the pro-caspase3 protein reduction caused by methamphetamine, and has the advantages of obvious treatment effect, high safety, low cost and suitability for practical popularization and application.
Owner:KUNMING UNIV OF SCI & TECH
A kind of refining method of teprenone and its intermediate
ActiveCN107501070BHigh purityEfficient removalSulfonic acid preparationCarbonyl compound separation/purificationBiochemical engineeringProcess engineering
The present invention relates to a refining method of teprenone and an intermediate used in the refining process, specifically to purify teprenone by addition reaction of bisulfite or pyrosulfite with crude teprenone. The purification method provided by the invention has a high purification yield, and the prepared teprenone refined product has high purity, has low requirements on experimental equipment, and is suitable for large-scale industrial production.
Owner:CHENGDU GUOHONG PHARMA
Application of teprenone for preparing medicine for treating and/or preventing glaucoma
InactiveCN1903188BLow priceImprove securityOrganic active ingredientsSenses disorderDiseaseCurative effect
An application of teprenone in preparing the medicine GGA for preventing and treating glaucomas is disclosed. Its advantages are high curative effect, high safety and low cost.
Owner:BEIJING TONGREN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
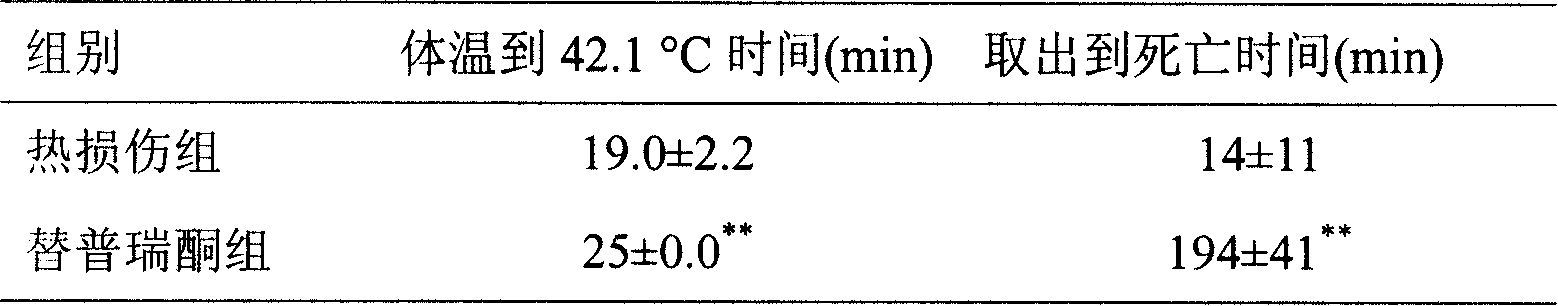
Anti-heatstroke use of teprenone
ActiveCN101164535AImprove heat damage resistanceOrganic active ingredientsDrug compositionsMedicineHeat apoplexy
The present invention relates to an application of teprenone (geranylgeranylacetone, GGA) for resisting heat apoplexy. Under the condition of peroral administration the teprenone can obviously delay heat apoplexy incidence of rat in heat environment and can prolong surviving time of the rate with heat apoplexy.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Process for synthesizing teprenone
InactiveCN105732340AOrganic compound preparationHydroxy compound preparationNerolidolGrignard reagent
The invention discloses a process for synthesizing teprenone.According to the process for synthesizing teprenone, nerolidol is adopted as an initial raw material and subjected to selenium dioxide and tert-butyl hydroperoxide oxidizing in sequence, nerolidol and dihydropyran are subjected to acid catalysis to form acetal, acetal reacts with a Grignard reagent on the presence of copper salt and reacts with methyl acetoacetate on the presence of aluminum salt, and the teprenone product consistent with the configuration of products sold on the market is obtained through four steps of reactions.According to a preparation method of teprenone, the raw material is cheap and easy to obtain, operation is easy and convenient, the reaction conditions are mild, and industrial production is expected to be achieved.
Owner:四川墨凯科技有限公司
Anti-heatstroke use of teprenone
ActiveCN101164535BImprove heat damage resistanceOrganic active ingredientsDrug compositionsSocial benefitsMedicine
The present invention relates to an application of teprenone (geranylgeranylacetone, GGA) for resisting heatstroke. Under the condition of peroral administration, the teprenone can obviously delay rat heatstroke in a heat environment and prolong surviving time of the rate with heatstroke. Different concentration of teprenone is given to adult rats by gavage, subsequently the rat heatstroke and the surviving time of the rate are observed. The result shows that a certain concentration of teprenone given by gavage in advance can obviously delay the rat heatstroke in the heat environment, and prolong the surviving time of the rat with heatstroke, and improve the anti-heatstroke ability. Through the method of the invention, the common medicine teprenone with a proper dosage is taken at a proper time, which can improve the rat anti-heatstroke ability, and provide powerful evidence for human experiment. If the method can be popularized to human application, better economic benefit and social benefit can be achieved.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Preparation method of all-trans-teprenone
PendingCN114031491AHigh process yieldReduce energy consumptionOrganic compound preparationCarbonyl compound preparationIsopropylIodide
The invention provides a synthetic method of all-trans-teprenone. According to the method, specific raw materials are selected and combined with a proper catalyst, firstly geranyl geranyl bromide is prepared, and then the geranyl geranyl bromide reacts with acetone and lithium diisopropylamide in the presence of cuprous iodide to synthesize all-trans-teprenone. The method is high in process yield and high in purity, and a new thought is provided for synthesis of all-trans-teprenone.
Owner:安徽先和医药研究有限公司
A kind of intermediate synthesized by teprenone and its application
ActiveCN109574821BSimple processReduce the generation of "three wastes"Organic compound preparationMagnesium organic compoundsCombinatorial chemistryKetone synthesis
Owner:HUANGGANG LUBAN PHARM
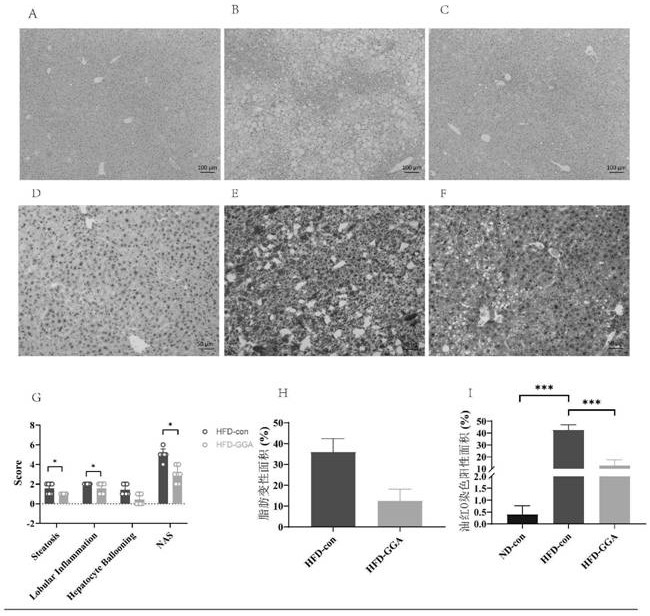
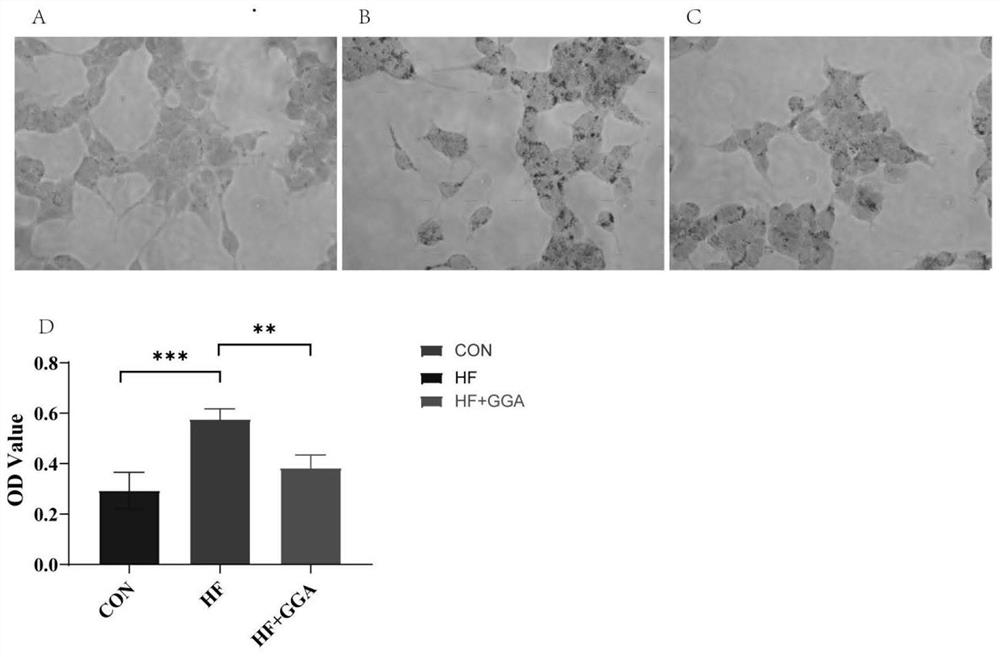
Application of teprenone in preparation of medicine for preventing and treating fatty liver/steatohepatitis
PendingCN113181146ASignificant effectImprove securityOrganic active ingredientsDigestive systemLow insulinAlanine aminotransferase
The invention discloses teprenone (the other name is pentatetraterpenone, and the chemical name is 6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraterpene-2-one). The invention also relates to application of the compound in preparation of medicines for preventing and treating fatty liver / steatohepatitis. In addition to being prepared into capsules, the compound can also be prepared into tablets, powder, granules, oral liquid, injection and other medicine forms. In-vitro test results show that after FL83b cells of an in-vitro fatty degeneration model are stimulated by GGA for 24 hours, lipid droplets in the cells are obviously reduced; and animal experiment results are as follows: 12 weeks after continuous administration of 200mg / kg of GGA to a mouse with fatty liver induced by high fat-cholesterol-fructose diet, the weight gain of the mouse with high fat diet is reduced; meanwhile, the level of serum alanine aminotransferase is remarkably reduced, and the level of cholesterol is remarkably reduced; in addition, inflammation in liver tissues is remarkably relieved, and the fatty degeneration area is remarkably reduced; the serum insulin level is obviously reduced, and the insulin resistance is obviously improved. The medicine is remarkable in curative effect, high in safety, low in price and capable of relieving the economic burden of patients.
Owner:SIR RUN RUN HOSPITAL NANJING MEDICAL UNIV
Use of geranylgeranylacetone for preventing and treating renal injuries caused by morphine
InactiveCN102764249BSignificant effectImprove securityOrganic active ingredientsDigestive systemOpioidergicMorphine
Owner:KUNMING UNIV OF SCI & TECH
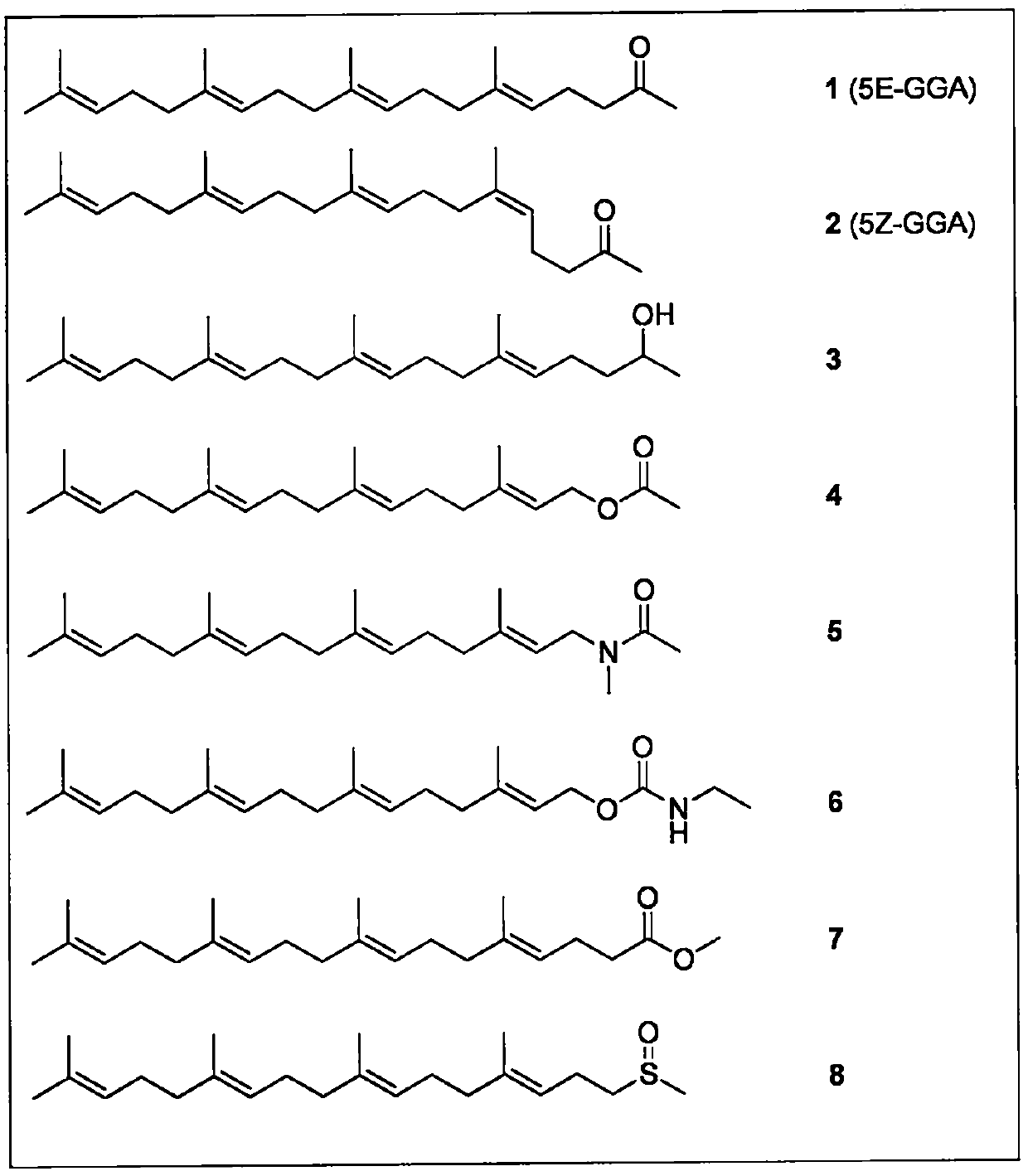
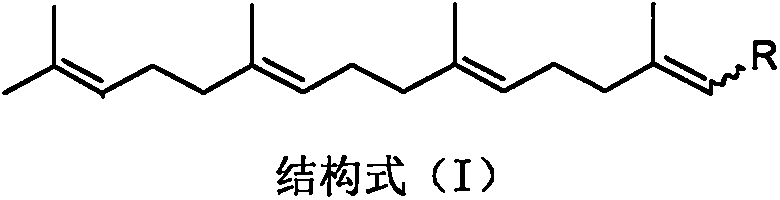
Application of teprenone and derivatives thereof in the prevention and treatment of viral hepatitis
InactiveCN108853070AGood curative effectImprove securityOrganic active ingredientsAntiviralsTeprenoneHepatitis C
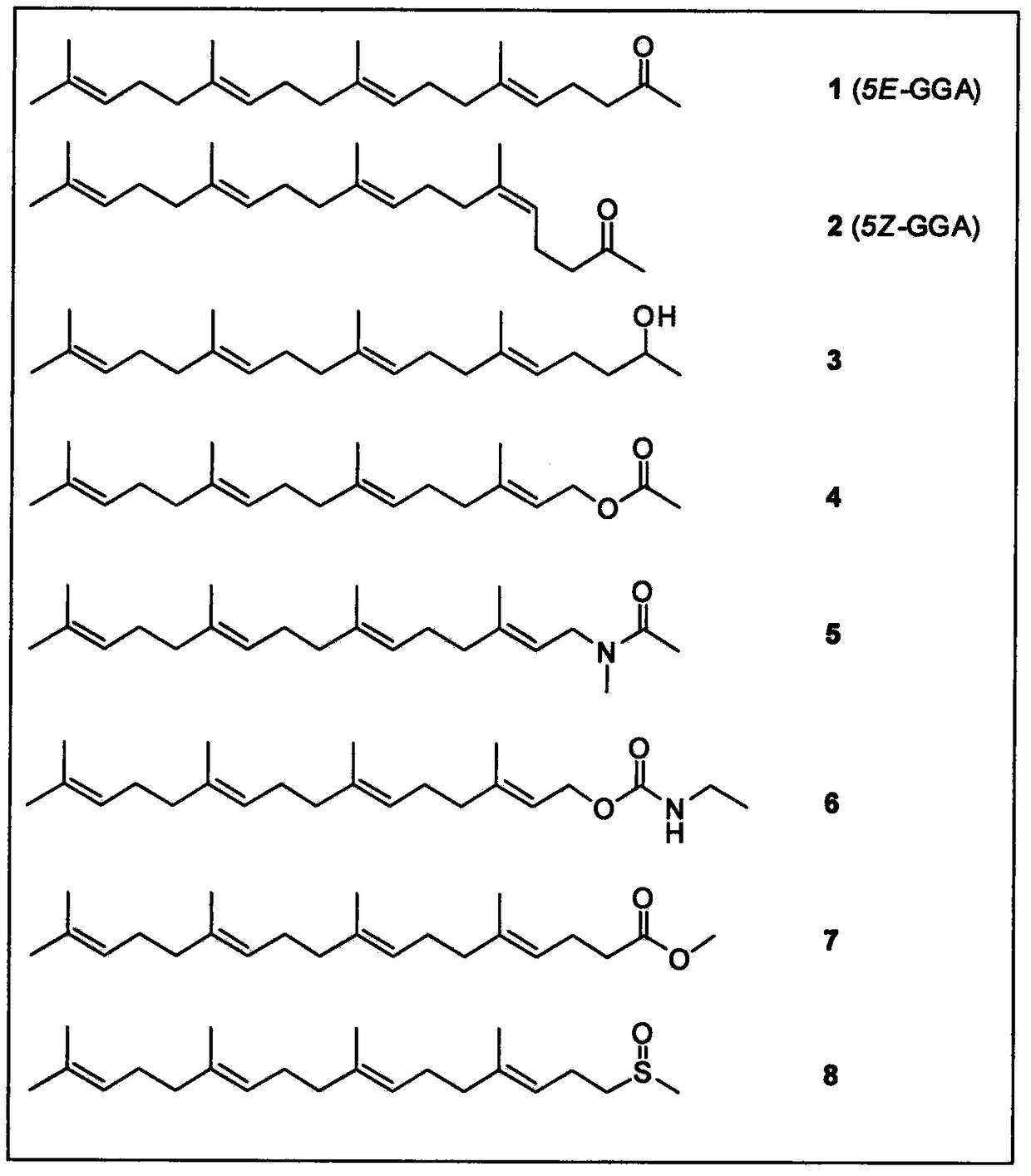
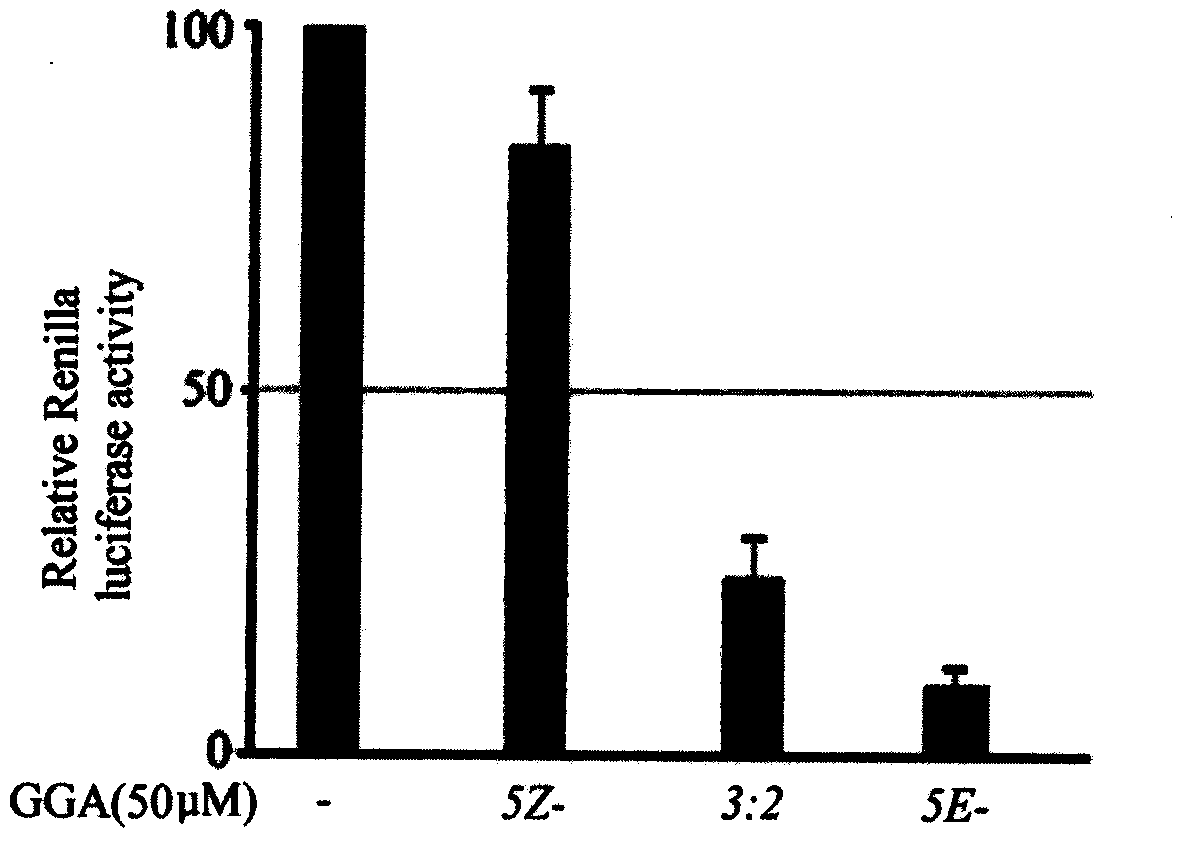
The invention relates to an application of teprenone and derivatives (structure I) thereof in the prevention and treatment of viral hepatitis, in particular for the prevention and treatment of hepatitis B and hepatitis C. The experimental results show that the antiviral activity of all-trans teprenone (5E-GGA) is significantly higher than that of monocis-preprepone (5Z-GGA), and is also higher than that of the mixture of the two preprepone isomers.
Owner:厦门信力康生物技术有限公司
Teprenone and application of derivative of teprenone in preparing drug for treating drug addiction and preventing drug addiction recurrence
PendingCN110893180APrevent relapseNervous disorderHydroxy compound active ingredientsNarcotics addictionPerylene derivatives
The invention relates to all-trans-teprenone and mono-cis-teprenone and application of a derivative (described as a formula I) of the all-trans-teprenone and mono-cis-teprenone in preparing a drug fortreating drug addiction and preventing drug addiction recurrence. The effects of the all-trans-teprenone (5E-GGA) is superior to the mono-cis-teprenone (5Z-GGA) and also a mixture of an all-trans-teprenone isomer and a mono-cis-teprenone isomer. In a mixture of cis-teprenone and trans-teprenone, the greater the weight ratio of the all-trans-teprenone is, the better the curative effect is.
Owner:厦门信力康生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com