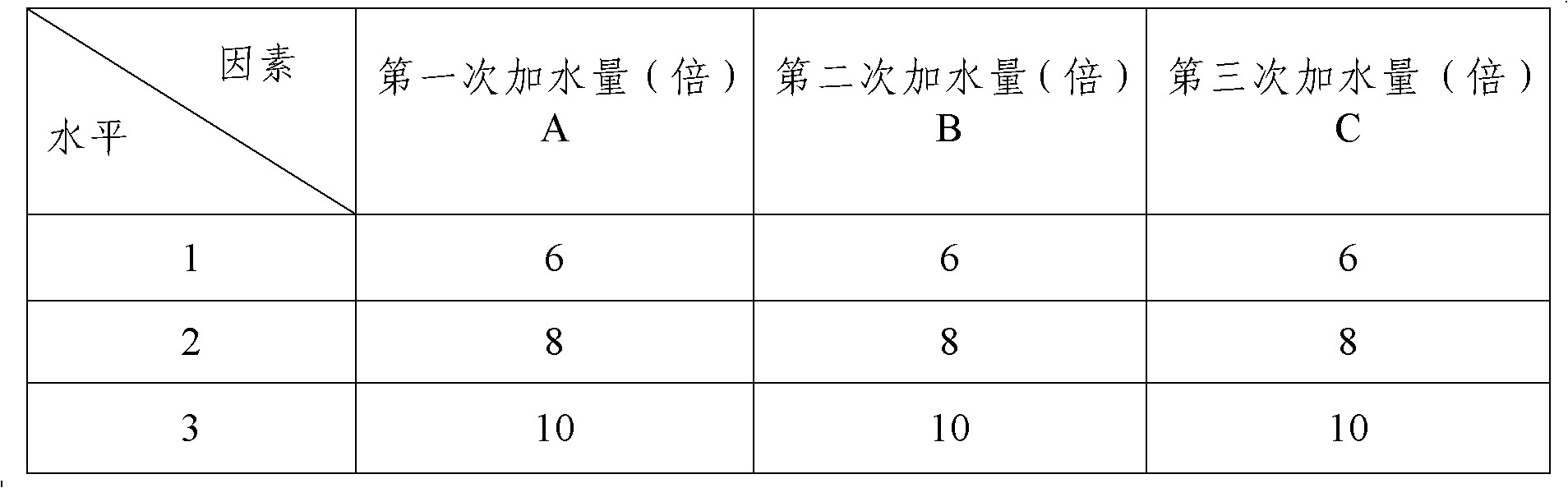
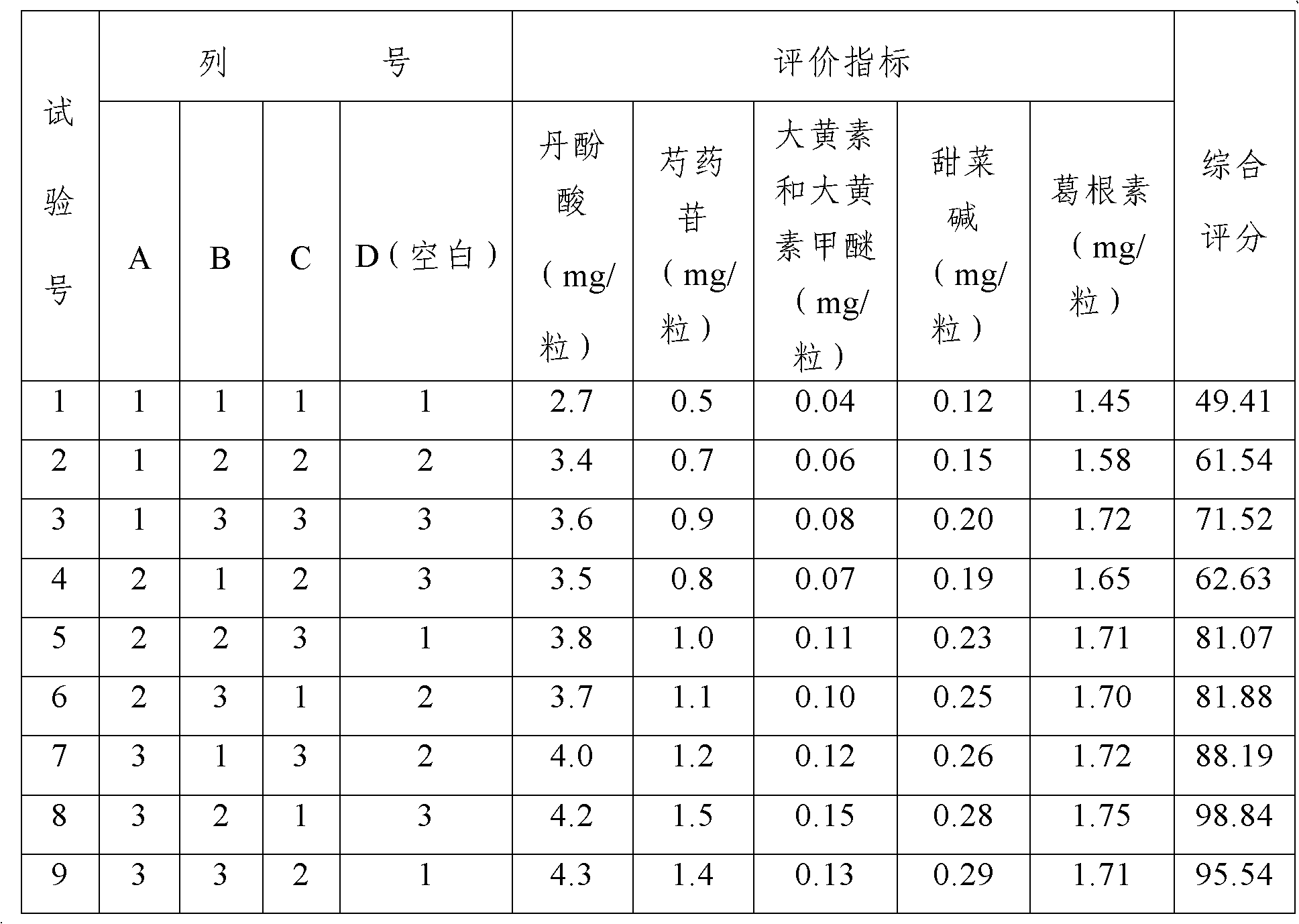
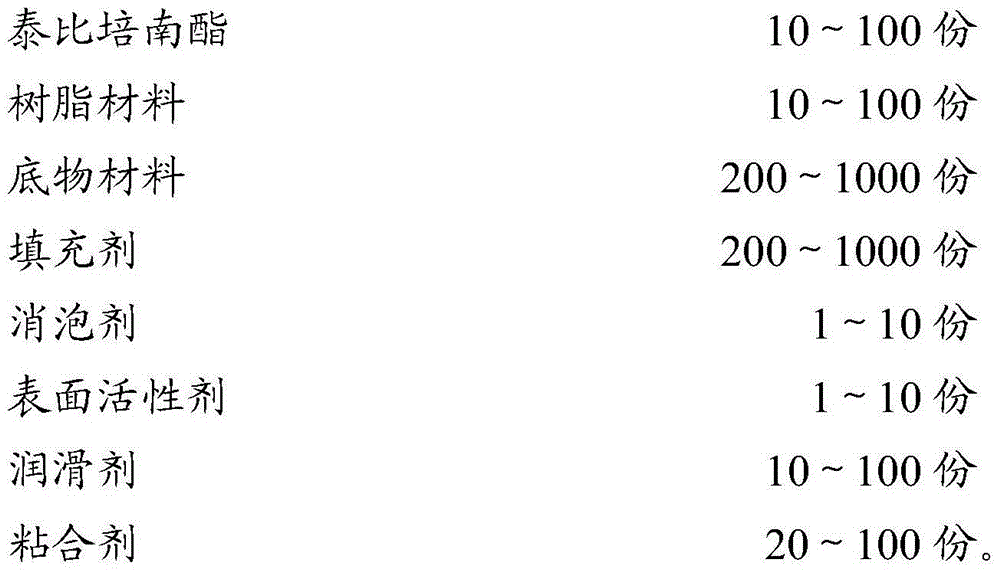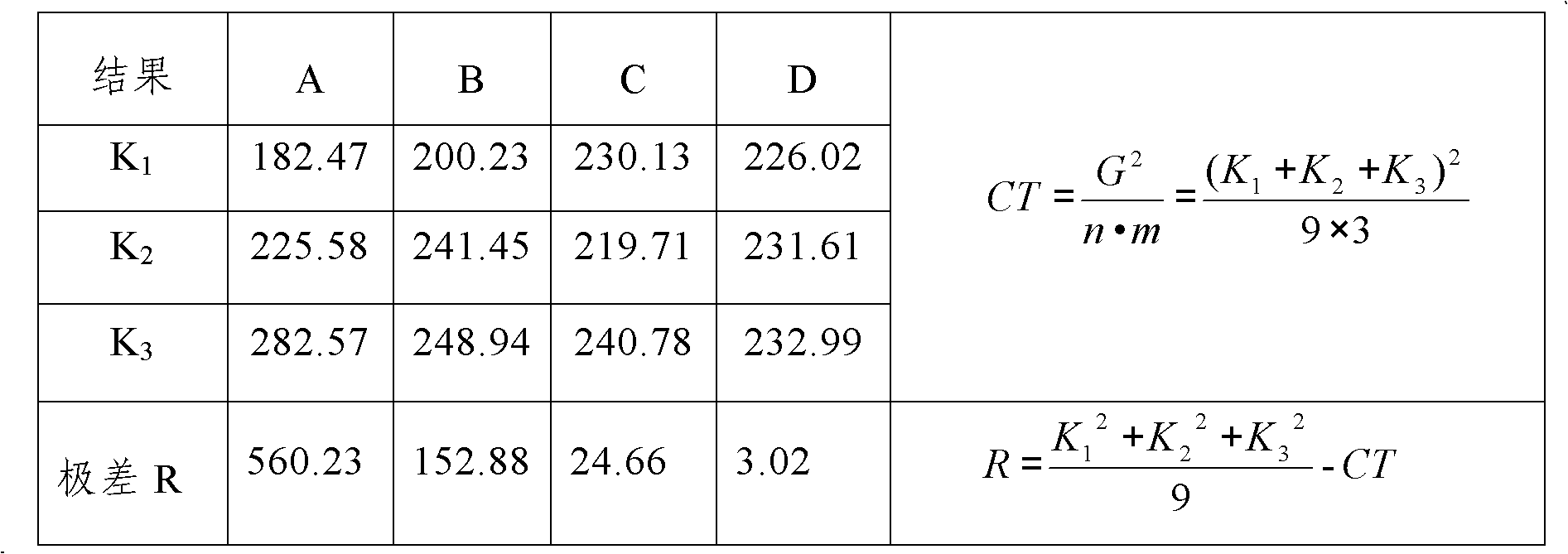Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
123results about How to "Mask bad taste" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Animal medicine inclusion compound, preparation method and application thereof
InactiveCN101954089AImprove solubilityImprove stabilityPharmaceutical non-active ingredientsSolubilityOrganic solvent
The invention discloses application of an inclusion technology in preparing animal medicines, and an animal medicine inclusion compound. The animal medicine inclusion compound can increase the dissolution velocity and the solubility of indissolvable medicines, improve the stability of the medicines which are changeable in the presence of lights or moistures and the like, and facilitate the storage of medicines. The invention also provides a method for preparing the animal medicine inclusion compound, and the method is simple in operation, can reduce the use of organic solvent, lower the cost, reduce the poisonous side effects of the medicines, and can be applied to the preparation of animal medicines.
Health-care food capsule and preparation method thereof
ActiveCN102511800AReasonable processing methodAnti-menopausal syndromeFood shapingFood preparationBiotechnologyGlycerol
The invention provides a health-care food capsule, which consists of a content and a gelatine skin, wherein the content is a mixture which is prepared from raw materials according to the following part by weight: 12-22 of maca powder, 5-15 of wolfberry extract, 5-15 of Chinese angelica extract, 2-8 of acanthopanax extract, 2-8 of epimedium extract, 1-5 of ginseng extract, 15-25 of corn oil and 35-50 of tea seed oil. The gelatine skin is prepared from edible gelatine, glycerin and purified water according to the weight ratio of 1 / 1 / 0.5. The content is obtained through crushing, burdening, mixing, grinding and degassing the raw materials. The gelatine skin takes the edible gelatine, the glycerin and the purified water as gelatine skin raw materials, the gelatine liquor is obtained after mixing, gelating and heating the gelatine skin raw materials,, then the content and the gelatine liquor in the capsule are produced through the pelleting of an automated machine, and a product has a reasonable and practicable processing method. The health-care food capsule has the effects of alleviating climacteric syndrome, increasing the sexual capacity and relieving physical fatigue, is easy to beabsorbed by the human body, has no toxic and side effects, and can be orally taken for a long time.
Owner:厦门鹰君药业有限公司
Solid preparation
The present invention provides a solid preparation comprising a crystal of [3-[(2R)-[[(2R)-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1H-indol-7-yloxy]acetic acid (Compound A), especially a crystal of Compound A having a particle size of not larger than 100 mum at the cumulative weight distribution value of 50%, and not larger than 200 mum at the cumulative weight distribution value of 95%, preferably a solid preparation having the excellent stability and the content uniformity of Compound A, which is prepared by preparing granules of the crystal of Compound A with fillers, disintegrants and binders, and then followed by mixing said granules with external excipients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
'Yi Kang Bu Yuan' formulation for benefiting qi, moving blood, invigorating spleen and tonifying kidney, its preparation method and quality control method
InactiveCN1961929AImprove bioavailabilityGood disintegrationDigestive systemPharmaceutical delivery mechanismDiseaseRhizome
The invention relates to a preparation having the functions of promoting blood circulation, enlivening spleen and tonifying kidney, the process for preparation and quality control method, wherein the preparation is produced mainly from astragalus root, Chinese ephedra, Chinese angelica root, herba ecliptae, atractylodes rhizome, safflower, radix paeoniae rubrathe, peach kernels, achyranthes and cyathula root, Ligusticum wallichii, bitter orange and root of balloonflower. The preparation can be made into injections and various oral administration preparations including dripping pills, mini-pills and dispersible tablets. The invention also provides the method for determining and identifying contents of the preparation.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
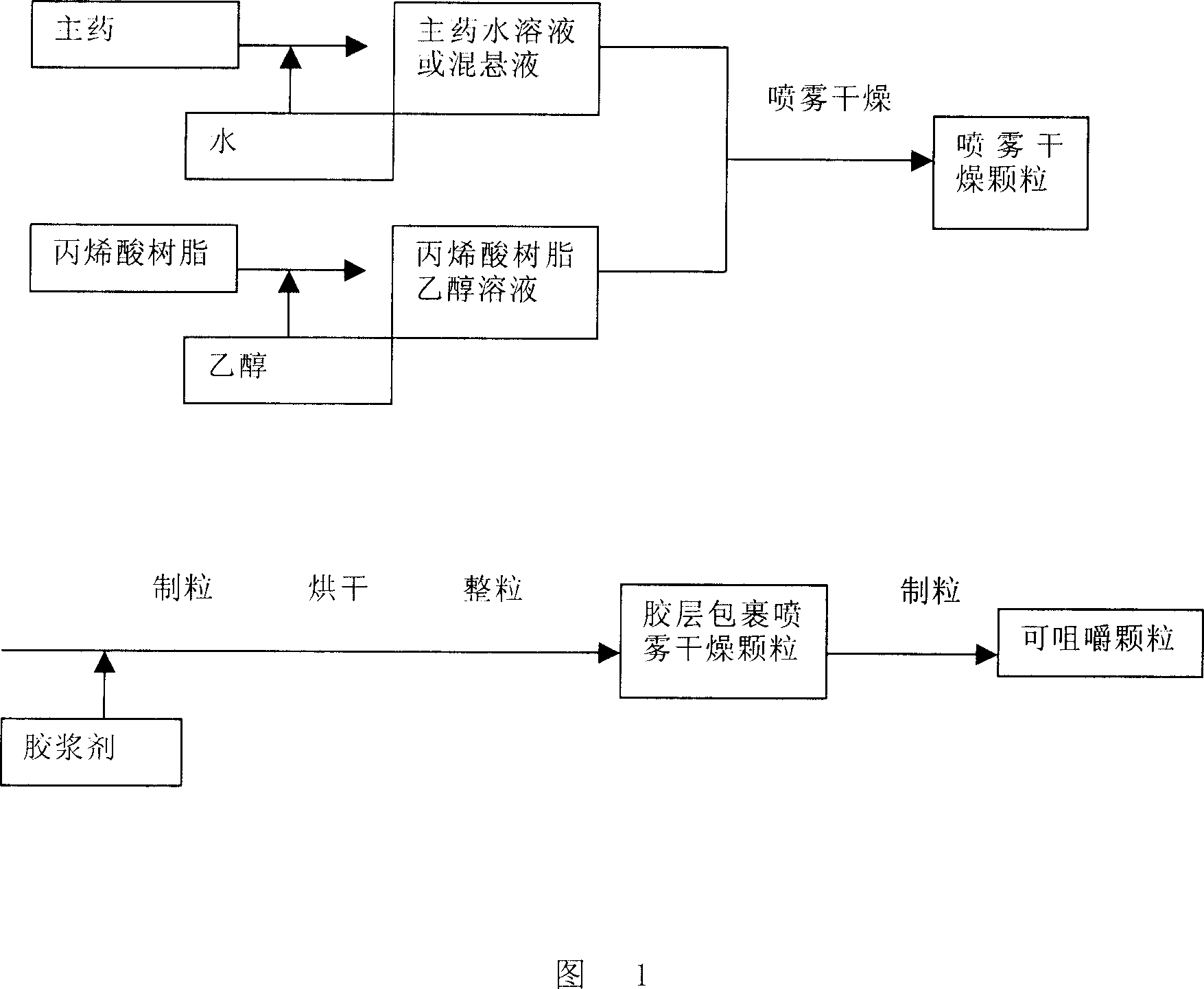
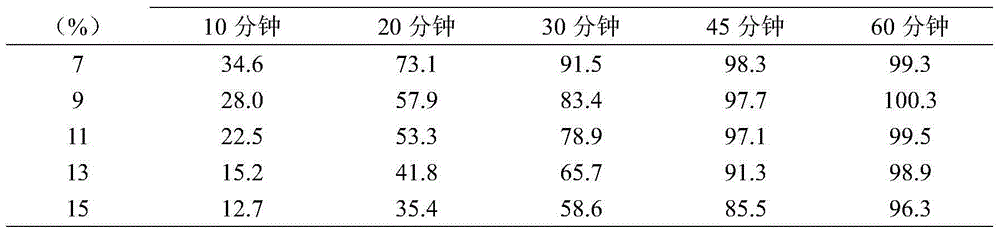
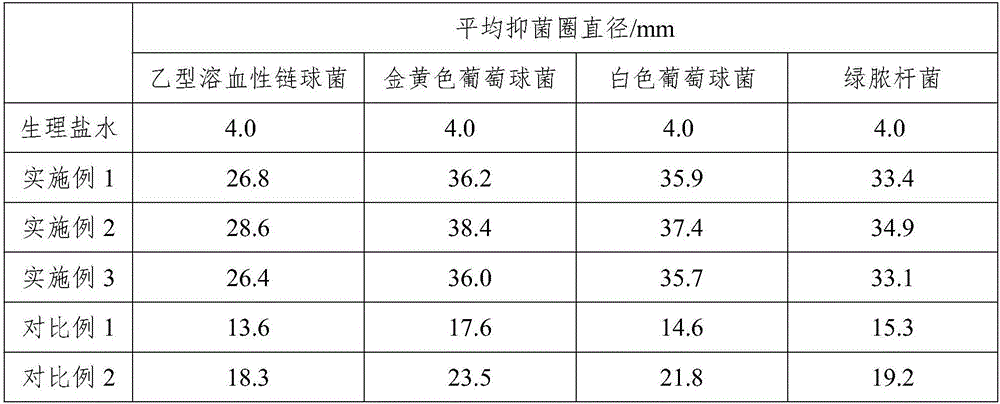
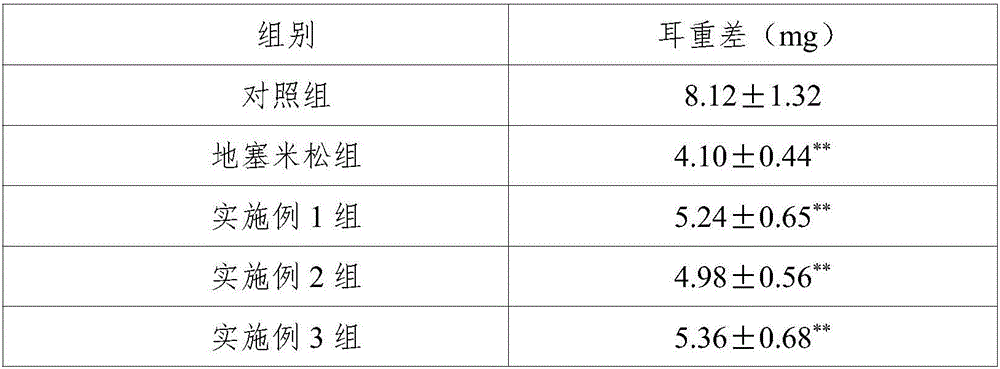
Unfavorable taste-masking drug granule, chewable formulation and preparation process thereof
InactiveCN1994468AStrong stickinessAvoid destructionPharmaceutical non-active ingredientsPill deliveryRotary evaporatorAcrylic resin
The invention relates to a particles used to shade bad taste of drug, wherein it is formed by corn element with active component and continuous polymer dress; the corn element is formed by active component and acrylic resin at 1:0.5-1:20 mass ratio; the dress is soluble gel slurry; and the drug is soluble in acid solution with bad taste. And the production comprises that: dissolving drug and soluble base material into solvent; using atomizing drier or rotation evaporator to obtain dry particles; then graining them at neutral condition via gel material; forming dress that separates drug and taste bud on the surface of particles; preparing oral agent via general method. The invention can avoid releasing bad taste in mouth but release drug in stomach.
Owner:牛祝琴 +2
Method for preparing polypeptide fruit jelly
The polypeptide fruit jelly producing process includes the following steps: mixing fruit jelly powder in 0.6-1.2 weight portions and sweetener in 0.03-15 weight portions, adding water in 59.9-67.03 weight portions via stirring for 3-10 min until complete water absorbing swelling of fruit jelly powder, heating at the boiling temperature 85-90 deg.c for 3-10 min, immediately filtering, adding preservative at 80 deg.c to the boiling point, adding essence at 75-80 deg.c, adding acidity regulating agent at 70-75 deg.c, adding polypeptide solution at 65-70, pouring, sealing, sterilizing, cooling and inspection. The present invention has good taste and health care effect, and may be used as the excellent nutrient source for athlete, valetudinarian and other special people.
Owner:CHINA RES INST OF DAILY CHEM IND
Solid preparation for treating pediatric epilepsy and preparation method thereof
InactiveCN104922075AMask bad tasteImprove stabilityNervous disorderUnknown materialsActive agentMedicine
The invention discloses a solid preparation for treating pediatric epilepsy and a preparation method thereof. The solid preparation is a dispersed preparation and comprises active agent core particles, wherein the core particles just comprise medicines for treating epilepsy or comprise the medicines for treating epilepsy and one or several excipients. Through the adoption of a taste masking mixture coating comprises the core particles of anti-epileptic medicines, coating particles are formed. The solid preparation is capable of masking the uncomfortable taste, has the function of improving the stability of the medicines, and can be used for solving the problem that children have difficulty in swallowing the bitter medicines.
Owner:黑龙江童医生儿童生物制药有限公司
Oral soft capsule compound preparations for curing cold
ActiveCN101278934AEnhance the beneficial effectMask bad tasteOrganic active ingredientsAntipyreticTraditional medicineCrystallization
The invention provides a medicine composition. The medicine composition is a soft capsule that can effectively treat cold and comprises active drug components, a solvent and a co-solvent. The soft capsule can not only solve the problems of leakage and crystallization existing in existing preparation and remarkably improve the beneficial effects of the medicine composition. Compared with products sold in the market, the medicine composition provided by the invention has more remarkable stability and better treat result.
Owner:江苏万高药业股份有限公司
Medicine for treating intestinal tract disease
InactiveCN1430956AMask bad tastePrevent and slow down decompositionOrganic active ingredientsDigestive systemDiseaseIntestinal tract diseases
A medicine in the form of capsule for treating enterogastric diseases is disclosed, whose main active component is 1,4-dimethyl-7-isopropyl-3-sodium azulenylsulfonate. Its advantages are high curative effect, and easy desintegration and absorption.
Owner:SICHUAN GUOKANG PHARMA
Oral ulcer treatment patch film and preparation method thereof
ActiveCN106063798AAddresses deficiencies that favor microbial growthImprove plasticityOrganic active ingredientsAntipyreticOral ulcersVitamin B6 synthesis
The invention belongs to the technical field of drugs and relates to an oral ulcer treatment patch film and a preparation method thereof. The oral ulcer treatment patch film mainly comprises oleanolic acid, polysaccharide sulfate, vitamin B6, chitosan, a film forming agent, glucoside, tributyl citrate and distilled water. The oral ulcer treatment patch film has a reasonable and scientific formula. Components of the oral ulcer treatment patch film interact with each other and have effects of inhibiting bacteria, diminishing inflammation and easing pain so that the oral ulcer treatment patch film can effectively relieve oral ulcer and shorten cure time. The oral ulcer treatment patch film has the advantages of good adhesion, fast dissolving speed and good uncovered film integrity, guarantees drug effects and is an ideal oral ulcer patch film.
Owner:宁波市诚德医疗科技有限公司
Film agent containing alkannin and resisting oral ulcer inflammation and preparation and application thereof
InactiveCN107049993AEasy to storeEasy to carryOrganic active ingredientsAntipyreticOral ulcersDrug release
The invention discloses a film agent containing alkannin and resisting oral ulcer inflammation, and preparation and application thereof. The film agent is prepared from the following materials in parts by weight: 0.01--0.05 part of alkannin, 1-3 parts of gamma-cyclodextrin, 0.25-15 parts of chitosan quaternary ammonium salt, 0.5-5 parts of polyvinylpyrrolidone K90, 5-30 parts of bletilla hyacinthine gum, 0.5-2 parts of gelatin, 3-10 parts of ethyl cellulose, 90-97 parts of alcohol and 20-50 parts of distilled water. The film forming agent is prepared according to a certain method and comprises two layers, namely a complete drug-loaded film and a blank film, one side of the drug-loaded film is stuck to the affected part, the blank film can stop drug from dissolving out in an oral cavity, so that the effect of one-way drug release is achieved, simultaneously bad taste of main medicines is covered, the drug is gathered to the affected part, the treating effect is improved, further the pain of patients is greatly relieved and the action time of the medicines is prolonged; and the effective-component alkannin in the medicine film has the anti-inflammation and bacteriostatic effects, namely inflammation diminishing, astringency and antipyresis. Simultaneously, the film agent is convenient to store and carry and can be easily industrialized.
Owner:南京紫源康医药科技有限公司
Capecitabine granule and preparation method thereof
ActiveCN103356488AImprove stabilityMask bad tasteOrganic active ingredientsGranular deliverySide effectAdhesive
The invention relates to an oral anti-tumor drug capecitabine granule and a preparation method thereof. The capecitabine granule exists in the form of a cyclodextrin inclusion compound and is prepared from capecitabine serving as a raw material drug and auxiliary materials comprising cyclodextrin, a diluting agent, a disintegrating agent, an adhesive and a flavoring agent by wet granulation. The capecitabine granule disclosed by the invention not only covers the bitterness of a capecitabine drug and enhances the drug compliance of a cancerous person, but also enhances the stability of the capecitabine in gastrointestinal tracts and accelerates the digestion velocity, thereby preventing the peak valley phenomenon of the blood concentration of a drug, outstandingly reducing the adverse reactions of the drug, such as irritation and toxic side effect, on the gastrointestinal tracts due to the stable release of capecitabine in the gastrointestinal tracts, enhancing the drug safety and better taking the anti-tumor effect.
Owner:QILU PHARMA HAINAN
Oral disintegrated rupatadine tablet and its preparing method
InactiveCN1985816AMask bad tasteDisintegrates quicklyOrganic active ingredientsPill deliveryDiseaseOlder people
The present invention provides a kind of oral disintegrated rupatadine tablet and its preparation process. The oral disintegrated rupatadine tablet has recipe comprising rupatadine in 5-40 mg each as main medicine component as well as filler, disintegrant, corrective, antioxidant, lubricant, etc. The present invention is used in treating allergic rhinitis, hay fever and other diseases. The medicine of the present invention has simple preparation process, convenient taking, fast acting and obvious curative effect, and is especially suitable for old people, children and patient with dysphagia.
Owner:SHANTOU UNIV MEDICAL COLLEGE
Topiramate sustained release preparation and preparation method thereof
ActiveCN102824302AStable and effective plasma concentrationLess side effectsOrganic active ingredientsNervous disorderIon exchangeEpileptic seizure
The invention relates to a medicine sustained release preparation, and particularly relates to a topiramate sustained release preparation and a preparation method thereof. The topiramate sustained release preparation is prepared by sustained release particles and pharmaceutical auxiliary materials, wherein the sustained release particles comprise 15-30% of topiramate, 30-60% of an ion-exchange resin, 12-25% of an impregnant and 5-15% of a sustained release coating material. The topiramate sustained release preparation has stable and effective plasma concentration which is lower than the peak concentration of a quick release preparation, reduces incidence rate of side reactions, and can control epileptic seizures well. The topiramate sustained release preparation is taken once every day, and employs the ion ion-exchange resin, so that unpleasant taste of topiramate can be covered; and adaptability for patients is increased. The topiramate sustained release preparation can be well-received by doctors and patients.
Owner:HAINAN PULIN PHARMA +1
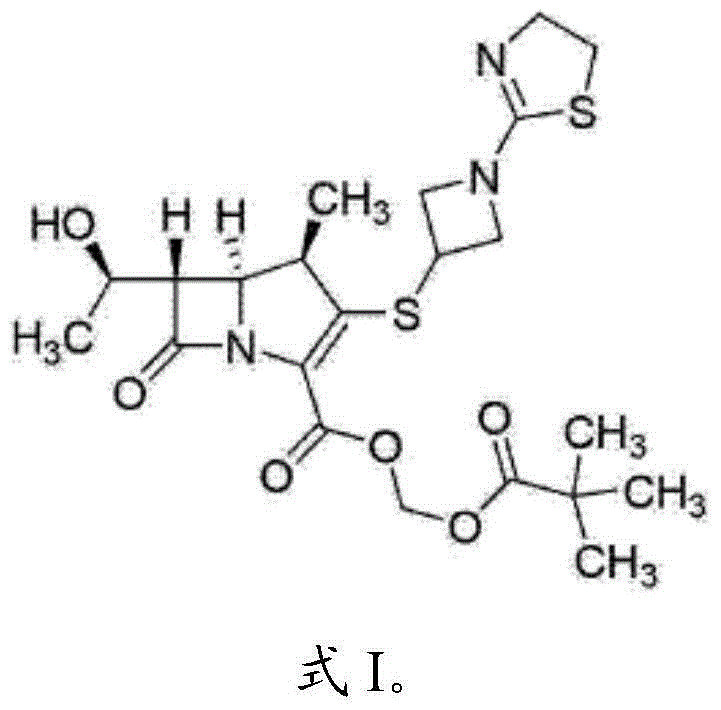
Pharmaceutical preparation of tebipenem pivoxil composition and preparation method of pharmaceutical preparation
ActiveCN104013583APoor maskingHigh dissolution rateAntibacterial agentsOrganic active ingredientsFiller ExcipientDissolution
The invention belongs to the technical field of pharmaceutical preparations and discloses a pharmaceutical preparation of a tebipenem pivoxil composition and a preparation method of the pharmaceutical preparation. The pharmaceutical preparation of the tebipenem pivoxil composition comprises the following components in parts by weight: 10-100 parts of tebipenem pivoxil, 10-100 parts of a resin material, 200-1000 parts of a substrate material, 200-1000 parts of a filler, 1-10 parts of a defoamer, 1-10 parts of a surfactant, 10-100 parts of a lubricant and 20-100 parts of a binder. The pharmaceutical preparation of the tebipenem pivoxil composition has good dissolution rate and stability and also covers the bad taste of tebipenem pivoxil, and has a good taste. The preparation method provided by the invention further improve the dissolution rate of medicines, and the bitterness caused by tebipenem pivoxil is effectively covered, the effectiveness and safety of the preparation are guaranteed and the medicament compliance of the patient is improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
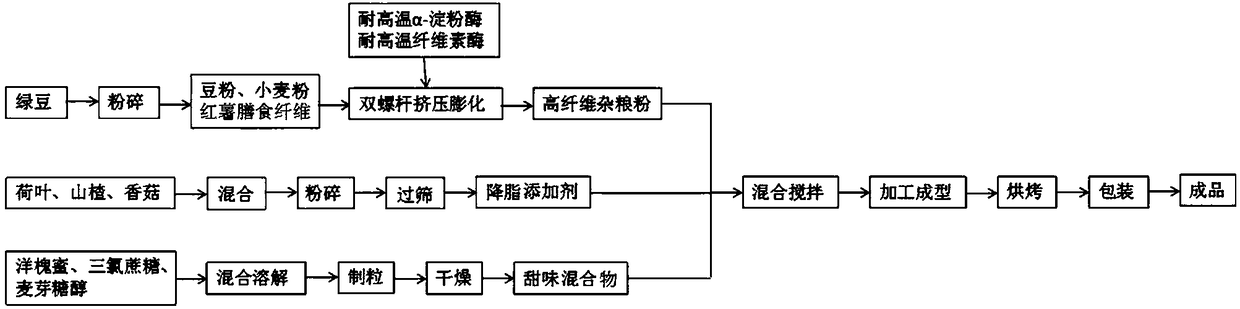
Making method of high-fiber bean crisp pastries
InactiveCN108391692AGreat tasteImprove digestibilityDough treatmentPre-baking dough treatmentMaltitolAlpha-amylase
The invention relates to a making method of high-fiber bean crisp pastries. The making method comprises the following steps of (1) preparing 60-80-mesh mung bean powder; (2) mixing the mung bean powder with wheat flour and sweet potato dietary fibers, adding high temperature resistant alpha-amylase and high temperature resistant cellulase, performing activation, adjusting the moisture content of materials to be 15-20%, pouring the treated materials into a twin-screw extrusion bulking machine to obtain bar-shaped semi-finished products, performing drying, and performing crushing to 100-120 meshto obtain puffed powder; (3) preparing an additive capable of reducing blood lipid from raw materials of dry products of lotus leaves, haws and shiitake mushrooms; (4) mixing robinia honey with sucralose and maltitol, performing dissolving, performing granulation, performing drying, and performing granule separation to obtain a sweet mixture; (5) mixing the puffed powder with the additive capableof reducing blood lipid and the sweet mixture, adding glutinous rice flour, eggs and olive oil, and performing uniform stirring to obtain dough; (6) performing baking to obtain pastries; and (7) encapsulating products. The invention provides the making method of high-fiber bean crisp pastries having the function of reducing blood lipid, the mouth feel of coarse cereals is significantly improved,and the digestibility is improved.
Owner:ZHEJIANG UNIV OF TECH
Novel compound propolis preparation and preparation method thereof
InactiveCN101797282AImprove immunitySuppress organismsNervous disorderAnthropod material medical ingredientsPropolisFreeze-drying
The invention relates to a novel compound propolis preparation and a preparation method thereof. The compound propolis product is prepared form the following ingredients: supercritical propolis, supercritical perilla frutescens seed oil, supercritical glossy ganoderma spore oil and ethanol extracted supercritical propolis residue. A main technical method has the following steps: removing impurities from raw materials; pulverizing raw materials; carrying out CO2 supercritical extraction; preparing microcapsule emulsion; carrying out vacuum freeze drying; carrying out pulverization; stirring and mixing all ingredients; and carrying out sterilization, capsule filling and package. The product of the invention belongs to the health care food with higher nutritional value, and has various health care effects of blood pressure reduction, blood fat reduction, antioxidation, immunity improvement and the like.
Owner:YANGZHOU UNIV
Clarithromycin dispersible tablet preparation method
InactiveCN104337778AQuality improvementStable useAntibacterial agentsOrganic active ingredientsSolubilityPrill
The present invention provides a clarithromycin dispersible tablet preparation method, which comprises: carrying out ultrafine crushing on clarithromycin and part of lactose into micro-powder with the diameter of less than 10 [mu]m to obtain mixed powder A; taking the remaining filler and part of a disintegrant, and mixing to form mixed powder B; mixing an adhesive and purified water to prepare an aqueous solution with the mass fraction of 1-10%; mixing the mixed powder B with the mixed powder A in an equal increase manner, placing into a three-dimensional mixer to mix for 30-40 min, adding to the adhesive aqueous solution to prepare a soft material, and screening with a 16-24 mesh sieve; drying at a temperature of 50-70 DEG C, and screening the whole particle with a 16-24 mesh sieve; externally adding the remaining disintegrant, a lubricant and a sweetener to the particles to obtain a material C; and tableting the material C to obtain the clarithromycin dispersible tablets. According to the invention, the clarithromycin and part of the lactose are subjected to ultrafine crushing, such that the particle size is small, the specific surface area is increased, the adsorption property and the solubility are correspondingly increased, the bitterness of the product can be reduced, and the patient compliance can be improved; and with the process, the clarithromycin dispersible tablet with characteristics of stable quality, rapid drug dissolution and no bad odor can be prepared.
Owner:哈药集团人民同泰医药股份有限公司
Medicinal composition for treating gastrointestinal disorders as well as preparation method and application thereof
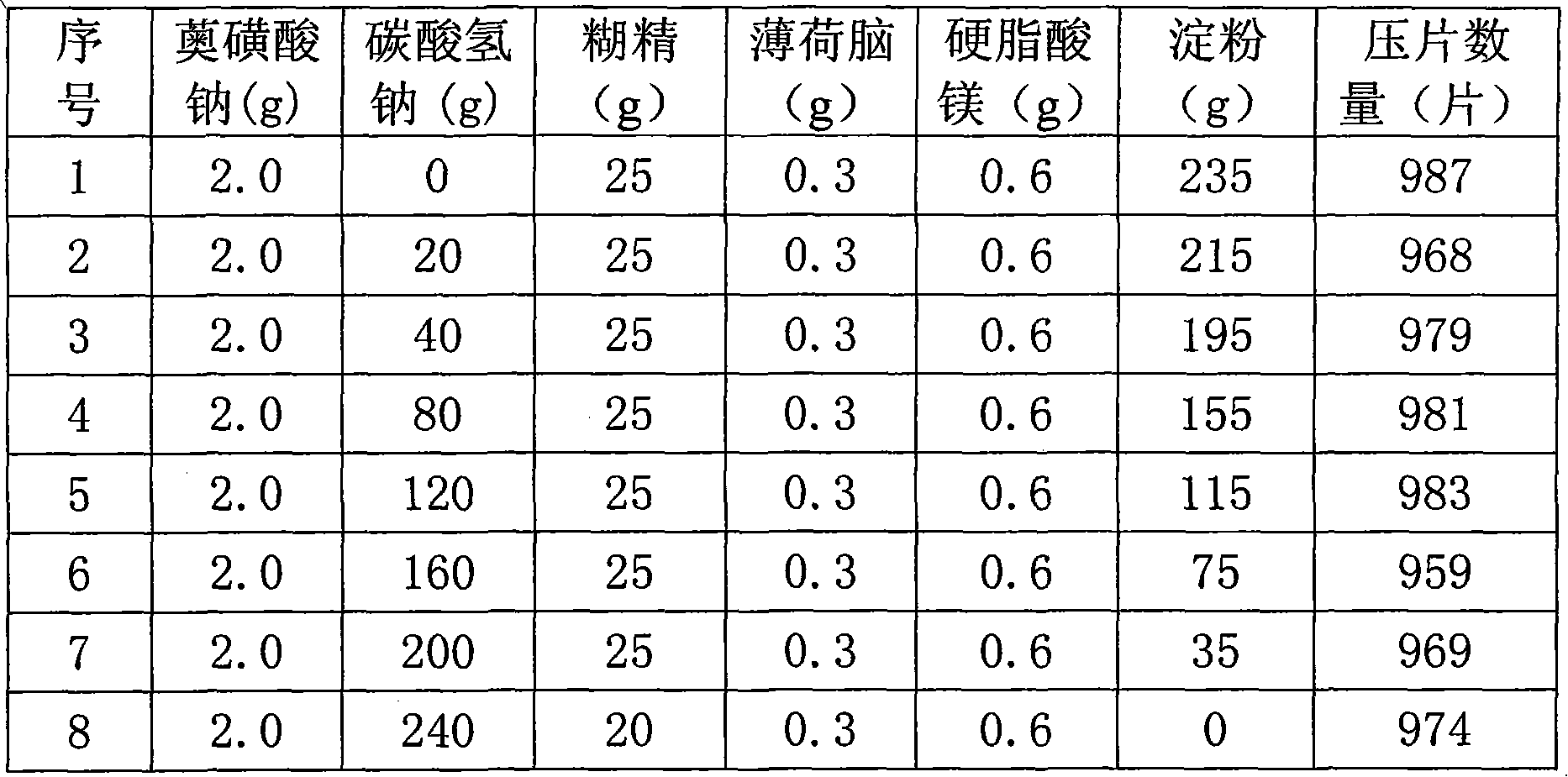
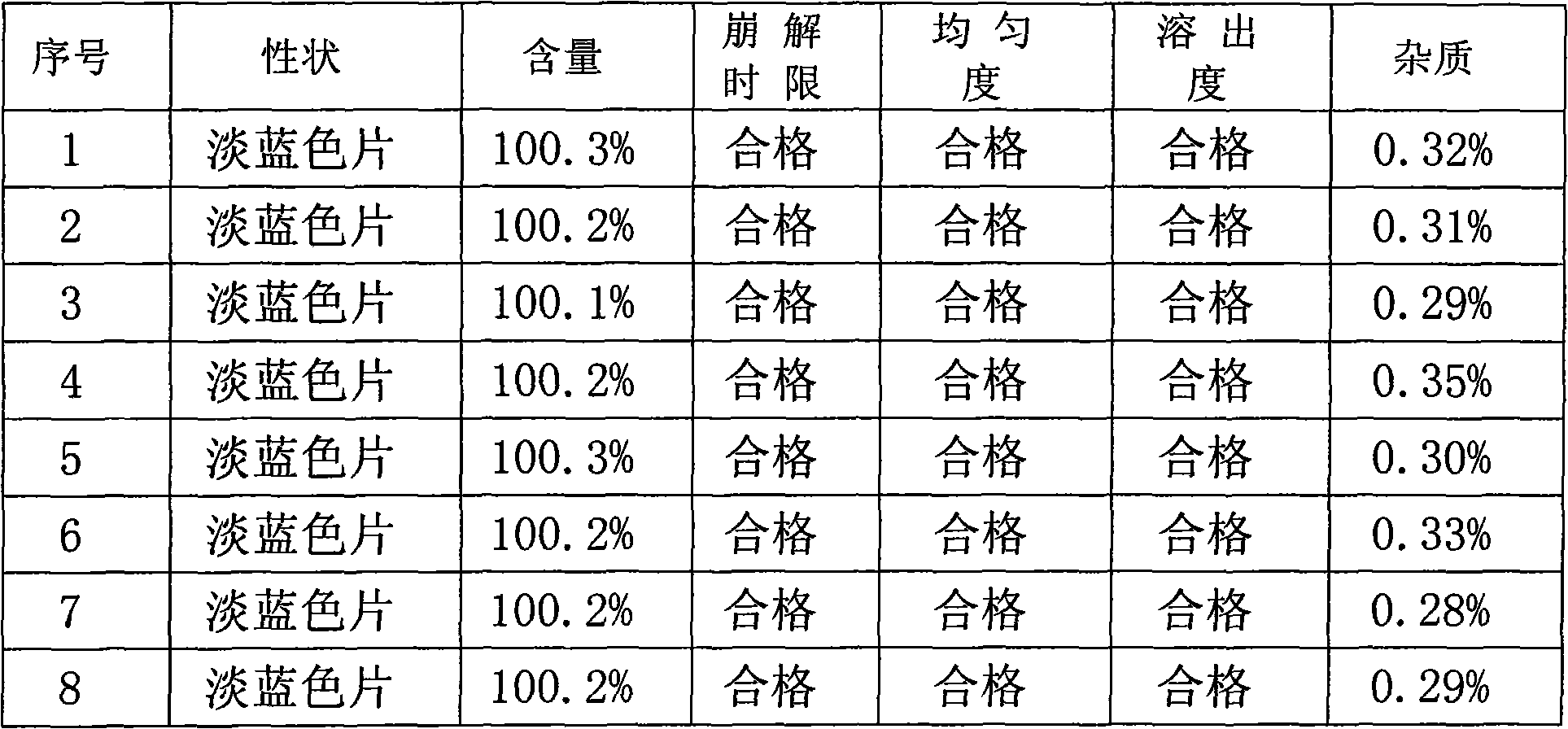
ActiveCN102114010AQuality improvementMask bad tasteAntibacterial agentsInorganic active ingredientsDecompositionSodium bicarbonate
The invention provides a medicinal composition for treating gastrointestinal disorders, and the medicinal composition is prepared from the following main components in parts by weight: 0.5 to 10 parts of sodium azulene sulfonate and 20 to 300 parts of sodium bicarbonate. The invention also provides a preparation method and application of the medicinal composition. The medicinal composition can achieve the medical effect of synergy by compatibly using the sodium azulene sulfonate and the sodium bicarbonate, has stable quality and strong controllability, has the functions of light protection, damp prevention and the like, and can cover defect taste of the medicament, so that decomposition of the medicament can be prevented or retarded, and the medicament stability is improved to ensure the curative effect of the medicament is exerted better. The medicinal composition is easily disintegrated and quickly absorbed, is convenient for clinical application, and provides a novel option for clinical treatment.
Owner:SICHUAN GUOKANG PHARMA
Beverage capable of lowering blood sugar and preparation method thereof
The invention discloses a beverage capable of lowering blood sugar. Each 1kg of the beverage capable of lowering the blood sugar is composed of following raw materials according to mass: 250g-350g of bitter gourds, 6g-10g of tartary buckwheat, 0.5g-1.0g of pectin, 10g-20g of isomaltulose, 0.2g-0.4g of sodium isoascorbate, 0.2g-0.3g of sodium copper chlorophyllin, 0.05g-0.15g of stevioside, 0.8g-1.4g of edible essence and the balance of pure water. The beverage capable of lowering the blood sugar takes the bitter gourds as a main raw material and the tartary buckwheat is added; the bitter gourds and the tartary buckwheat are natural vegetables or plants so that medicine components are safely and greatly reduced; the beverage is a health-care product beverage, and the side effects and the toxicity of the medicine are reduced and the blood sugar lowering effect is very good; meanwhile, the beverage capable of lowering the blood sugar also has the effects of lowering blood pressure, lowering blood lipids, promoting a digestion function, and losing weight and beautifying.
Owner:郝振东
Health-care food for lowering blood pressure
InactiveCN102726708AAuxiliary blood pressure loweringMask bad tasteFood preparationSalvia miltiorrhizaSide effect
The invention relates to a health-care food for lowering blood pressure, which comprises the following components: 550g of Salvia miltiorrhiza, 500g of cortex eucommiae, 400g of dogbane leaf and 220g of rhizoma gastrodiae. The health-care food takes Chinese herbal medicines as components, and possesses characteristics of safety, reliability, small toxic and side effects, and is suitable for long-time administration for hypertensive patients. The components of the present invention are all in accordance with the quality standards and the hygienic requirements in The Chinese Pharmacopoeia. Human feeding tests and animal functional tests prove that the health-care food of the invention possesses auxiliary blood pressure lowering function. The used capsule form can cover the unfavorable taste of Chinese herbal medicines and is easy to be carried.
Owner:陕西功能食品工程中心有限公司
Dairy product for preventing and treating hypercholesterolemia
InactiveCN101965874AMask bad tasteHigh sensory evaluation valueMilk preparationRed yeast riceBiotechnology
The invention relates to the field of food, in particular to a dairy product for preventing and treating hypercholesterolemia. The dairy product contains 1 to 7 mass percent of red yeast rice ultra-micro powder mixture, and the red yeast rice ultra-micro powder mixture is prepared by mixing red yeast rice ultra-micro powder and beta-cyclodextrin ultra-micro powder in a mass ratio of 10: 1. The 1 to 7 percent of red yeast rice ultra-micro powder mixture contained in the dairy product ensures no side effect after long-term eating; the blood fat reducing effect of the dairy product is obvious by adding ultra-micro guar gum; the dairy product provides a means for effectively preventing the hypercholesterolemia for the sub-healthy crowd, and provides an effective and joyful fat reducing path for patients; and the added beta-cyclodextrin reduces the stimulation to the gastrointestinal tract due to direct eating of functional red yeast rice.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
New compound Chinese medicinal preparation for anti rheumatism and its preparation technology
InactiveCN1785354AMask bad tasteAdapt to development requirementsAntipyreticAnalgesicsRheumatismChinese drug
Owner:南京宇道科技开发有限公司
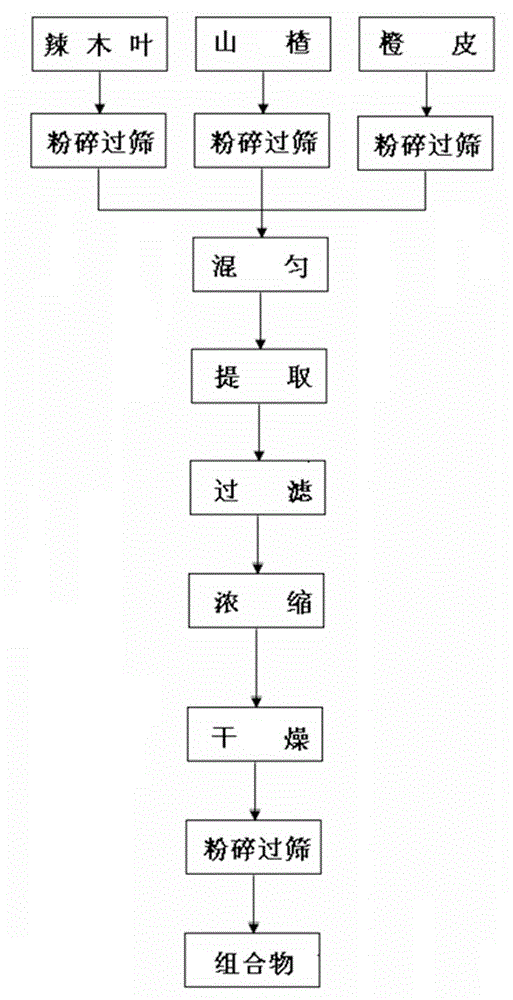
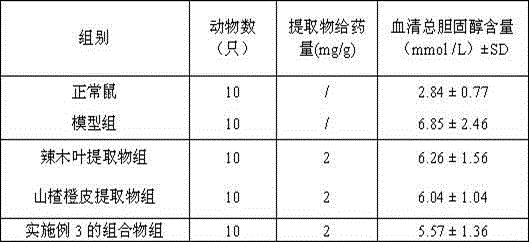
Composition conducive to lowering of cholesterol, and preparation method, preparation and application of composition
InactiveCN104435257AIncrease concentrationIncrease cholesterol-lowering functionPowder deliveryMetabolism disorderEssential nutrientVascular disease
The invention discloses a composition conducive to lowering of cholesterol, and a preparation method, a preparation and an application of the composition. The composition conducive to lowering of cholesterol is prepared from the following raw materials in parts by weight: 100-600 parts of horseradish tree leaves, 100-350 parts of hawthorns and 50-300 parts of orange peel by pretreatment, extraction and post-treatment. The horseradish tree leaves in the composition are capable of providing essential nutrient substances in human bodies and improving body health, have the efficacies of lowering cholesterol and reducing blood fat; and the hawthorn has the efficacies of promoting digestion, invigorating the stomach, promoting the circulation of qi, dissipating blood stasis and the like, is rich in abundant hesperetin and hesperidin, is capable of inhibiting the activity of reductase and cholesterol enzyme in plasma and liver, increasing emission of cholesterol, and has the functions of lowering cholesterol, reducing blood fat, resisting atherosclerosis and reducing cardiovascular diseases.
Owner:云南云药医药研究有限公司 +1
Orally taken medicament formulation of clindamycin phosphate and preparing method thereof
InactiveCN101401814AIncrease concentrationQuick effectAntibacterial agentsOrganic active ingredientsMedicineClindamycin Phosphate
The invention discloses a clindamycin phosphate oral pharmaceutical preparation, which is mainly prepared from raw materials in the following weight ratio: (1) a buccal tablet which comprises 1 to 100 portions of clindamycin phosphate counted on clindamycin, 0 to 100 portions of disintegrant, and 5 to 500 portions of filler; (2) a filmogen which comprises 1 to 100 portions of the clindamycin phosphate counted on the clindamycin, and 5 to 500 portions of film-forming material; and (3) an oral patch which comprises 1 to 100 portions of the clindamycin phosphate counted on the clindamycin, 5 to 500 portions of the filler, and 1 to 100 portions of adhesive material. The clindamycin phosphate oral pharmaceutical preparation is directly applied to a local infection and has high local drug concentration and fast effect, and the dose is low and adverse reactions are few.
Owner:张宏宇
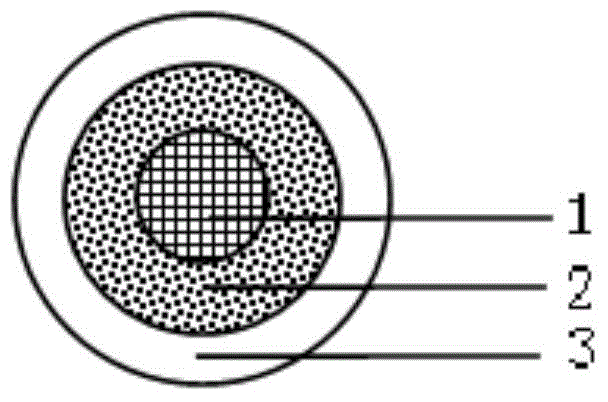
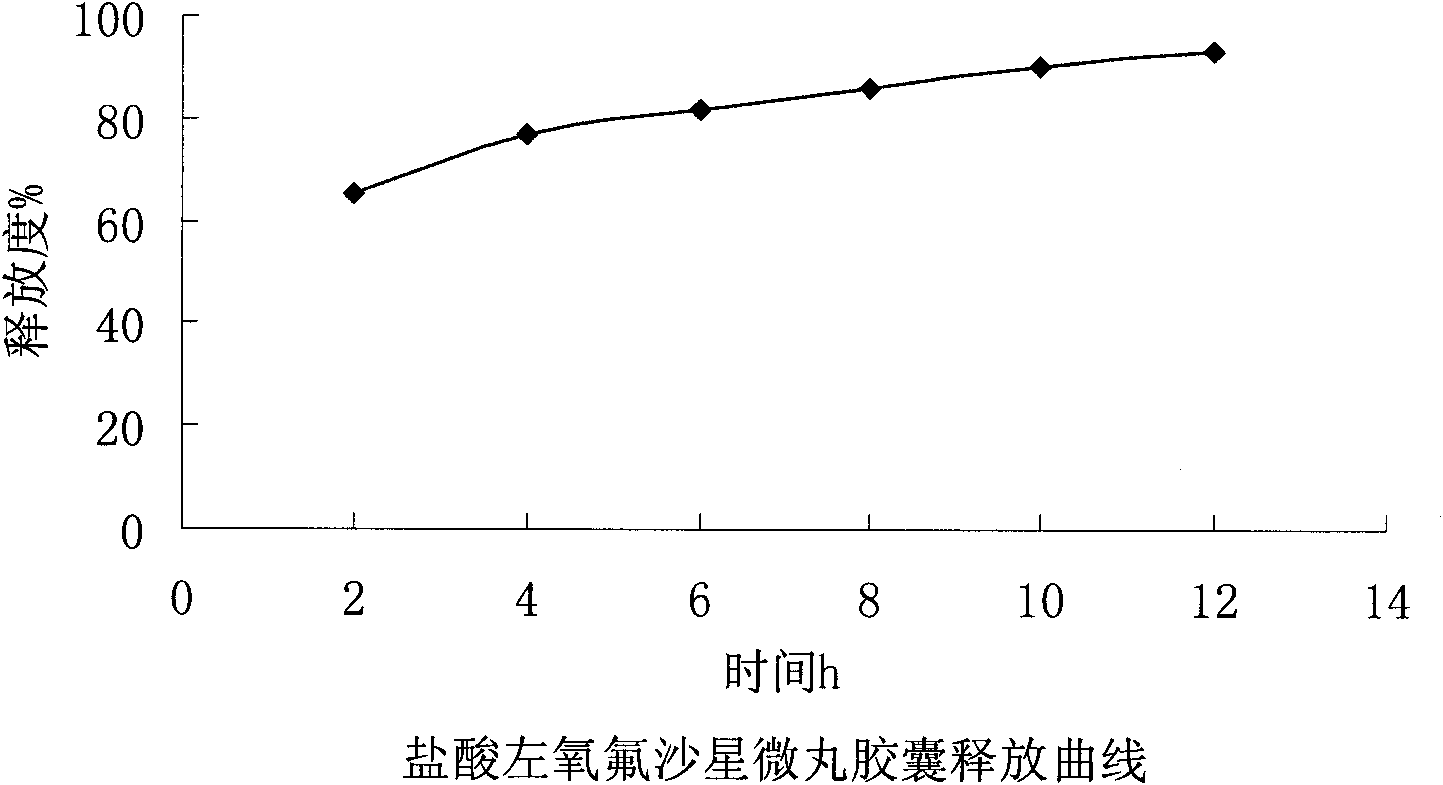
Levofloxacin hydrochloride micropill capsule and preparation method thereof
ActiveCN102106842AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
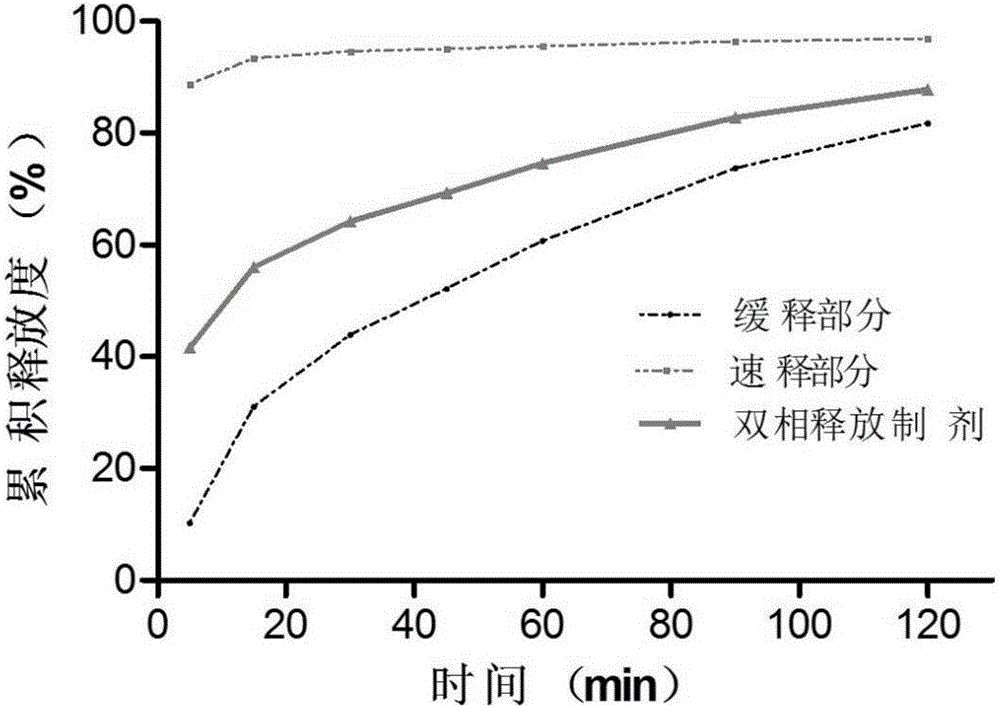
Racecadotril double-phase releasing preparation and preparation method thereof
ActiveCN106822907AIncrease contactHigh dissolution rateOrganic active ingredientsDigestive systemRacecadotrilDouble phase
The invention relates to the field of medicines, in particular to a racecadotril double-phase releasing preparation and a preparation method thereof. The racecadotril double-phase releasing preparation is prepared from medicine-containing particles with different slow-release properties and the like in percentage by mass: 10 to 90% of slow-release medicine-containing particles, 10 to 90% of quick-release medicine-containing particles, 0.1 to 0.5% of lubricant, and 0.1 to 1.0% of flavoring agent. The racecadotril double-phase releasing preparation has the advantages that the bitter taste of the racecadotril is effectively covered, the effective plasma concentration is guaranteed, the preparation technology is simple, the reproducibility is good, and the racecadotril double-phase releasing preparation is suitable for industrialized production.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Method for preparing Xinnaokang capsules
InactiveCN102000304AWith promoting blood circulation and removing blood stasisPain reliefAnthropod material medical ingredientsCapsule deliverySalvia miltiorrhizaRhizome
The invention discloses a method for preparing Xinnaokang capsules, which comprises the following steps of: crushing nine medicinal materials, namely Sichuan lovage rhizome, red flower, rhizoma alismatis, root of bidentate achyranthes, curcumae, polygala root, irkutsk anemone rhizome, spina date seed and liquorice, screening by using a 100-mesh screen and uniformly mixing; combining six medicinalmaterials of salvia miltiorrhiza, root of common peony, prepared tuber of multiflower knotweed, fruit of Chinese wolfberry, root of kudzu vine and earthworm and adding water and decocting for three times; combining the decoction, filtering, concentrating the filtrate into thick paste with the relative density of 1.35 at the temperature of 50 DEG C, adding the screened medicinal materials into thethick paste, uniformly mixing, drying, crushing and screening by using the 100-mesh screen; and adding deer heart powder, uniformly mixing and filling into capsules and obtaining the Xinnaokang capsules. The technology is simple, and the prepared Xinnaokang capsules mask the bitter taste of the medicaments, improve the medicinal stability and bioavailability, and are beautiful in appearance, convenient to take, carry and store, and accurate in dosage.
Owner:杨凌无为制药集团有限公司
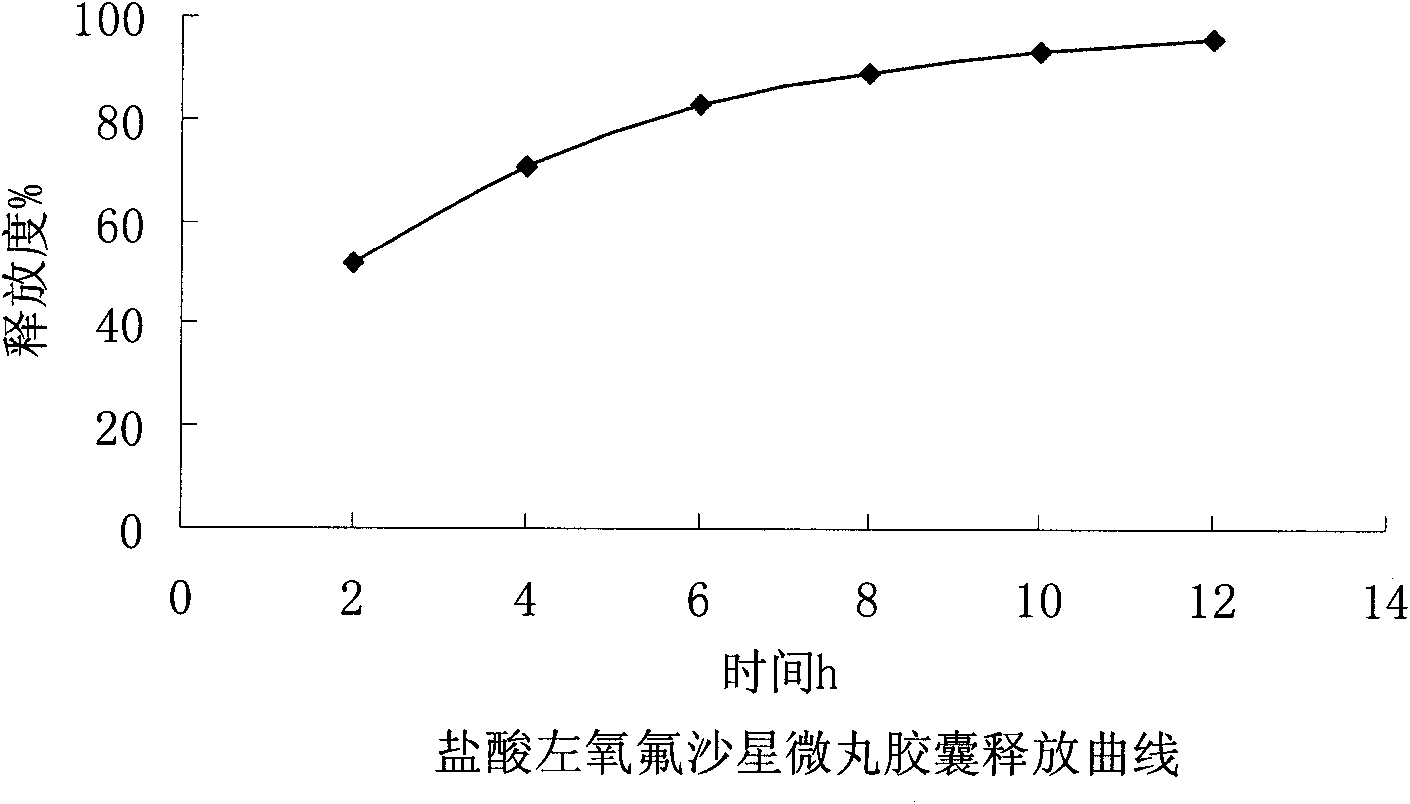
Levofloxacin hydrochloride micro-pill capsule and its preparing method
InactiveCN1813758AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsLevofloxacinGastrointestinal irritation
The present invention relates to a levofloxacin hydrochloride micropill capsule and its preparation method. It is composed of capsule external shell and micropill, the micropill is formed from levofloxacin hydrochloride, blank pill core and adhesive, every pill contains (by wt%) 10%-80% of levofloxacin hydrochloride, 15%-60% of medicine micropill pill core and 5%-30% of adhesive. Besides, said invention also provides the concrete steps of its preparation method.
Owner:范敏华
Dipyridamole oral emulsion administration system and preparation method thereof
ActiveCN106389329AImprove stabilityImprove bioavailabilityOrganic active ingredientsAntiviralsDipyridamolePediatric drug
The invention provides a dipyridamole emulsion which comprises the following components in parts by weight: 1-2.5 parts of dipyridamole, 5-75 parts of emulsifier, 0.1-15 parts of a co-emulsifier, 25-150 parts of oil, 250-500 parts of water, 0.5-1 part of a sweetening agent, 1-2 parts of an aromatic and 0.25-0.5 part of a preservative. The dipyridamole emulsion provided by the invention has the advantages of uniform texture, good stability, improved drug dissolution, easiness in absorption by the human body and high bioavailability. After high-pressure homogenization, the particle size of the emulsion is small in uniform distribution, the emulsion has good stability, the dissolution of dipyridamole is remarkably improved, and the oral bioavailability is enhanced; and flavoring agents such as the sweetening agent and the aromatic also can be added into the emulsion, so that disagreeable tastes of the drug can be effectively covered, and multiple tastes provide multiple options for children. Through a sterilization process, a little or no preservative can be added, so that the quality of the finished product of the emulsion is improved, and the safety of pediatric drugs is further enhanced.
Owner:黑龙江童医生儿童生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com