Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Hesperetin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
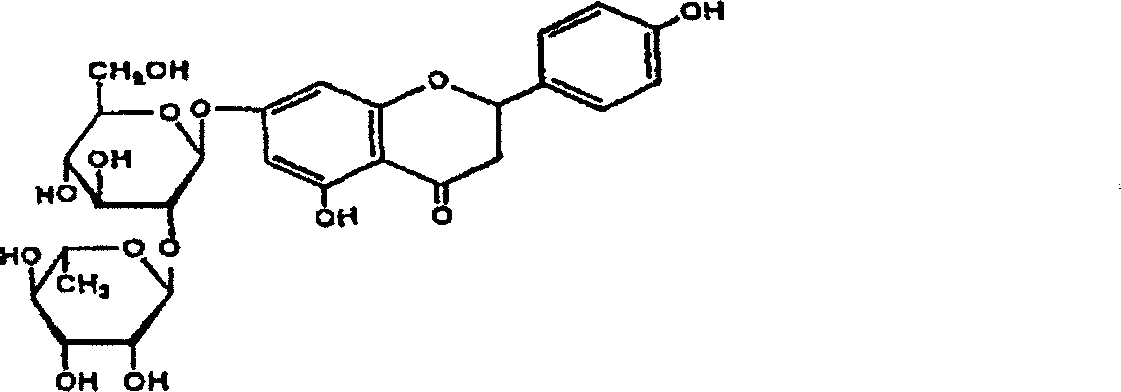
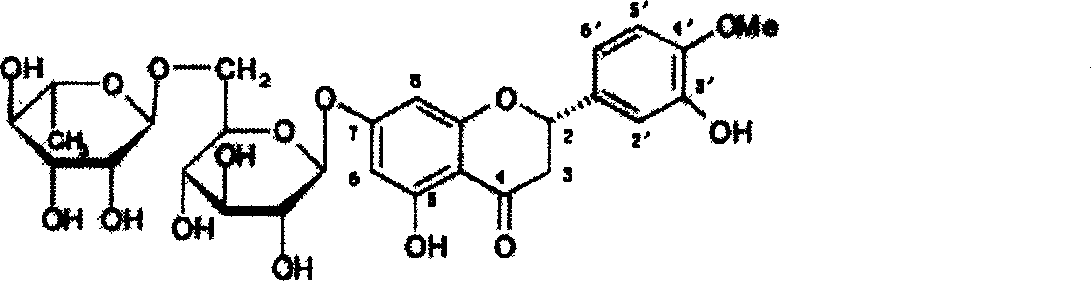
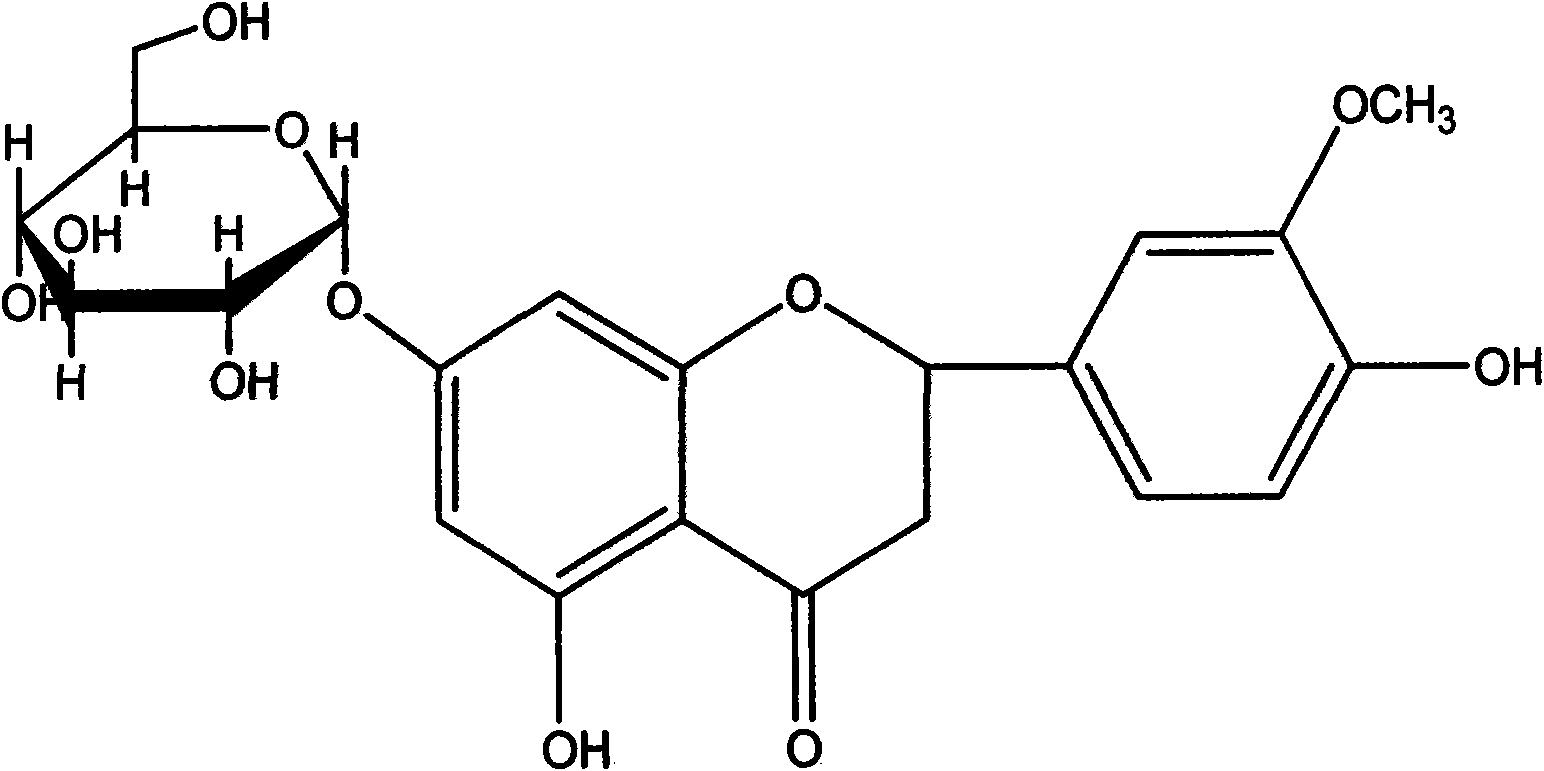
Hesperetin is the 4'-methoxy derivative of eriodictyol, a flavanone. Hesperetin's 7-O-glycoside, hesperidin, is a naturally occurring flavanon-glycoside, the main flavonoid in lemons and sweet oranges. Hesperetin (and naringenin, the parent flavanone of naringin) are not found to a significant extent in Citrus spp.
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Use of hesperetin for enhancing the sweet taste
The use of the hesperetin of the general formula (I) to enhance the sweetness of the sweet substance or to enhance the sweet olfactory impression of the flavoring agent that gives the olfactory impression of sweetness is described, wherein the hesperetin of the general formula (I) is ( 2S)-enantiomer, (2R)-enantiomer or any desired mixture of two enantiomers, the salt of hesperetin of general formula (I) contains or consists of two or more general formula ( I) a mixture of hesperetin salts, or a mixture comprising or consisting of hesperetin of general formula (I) and one or more salts of hesperetin of general formula (I).
Owner:SYMRISE GMBH & CO KG
Method for hydrolytic preparing biological tangeritin by enzyme
InactiveCN101089187AControl the hydrolysis processEasy to separate and purifyFermentationNaringinCitrus Bioflavonoids
In the invented method, citrus bioflavonoid and corresponding enzyme are used as raw materials, being dissolved in organic solvent, adding glucoside solution at concentration of 1-100g / L, under pH value of 3.0-7.0 and temperature of 20-65 deg.C for 20-60 min. After separation and purification, obtained is citrus bioflavonoid monoglycoside. With this invention, naringoside enzymolysis and cirmtim enzymolysis method for production of naringen in monoglycoside and hesperetin monoglycoside, compared with chemical method, has advantages of:easily to be controlled of enzymolysis, mild reaction, less by-products and easy to be separated of products. By using this invented method, the enzymolysis of naringoside and cirmtim for production of naringenin monocoside and hesperetin monoglycoside can be effectively controlled, and only the first glycosidic bond is broken, so obtained is highest yield of naringenin monoglycoside, hesperetin monoglycoside and rhamnose.
Owner:ZHEJIANG UNIV OF TECH
Semisynthesis luteolin preparation new process
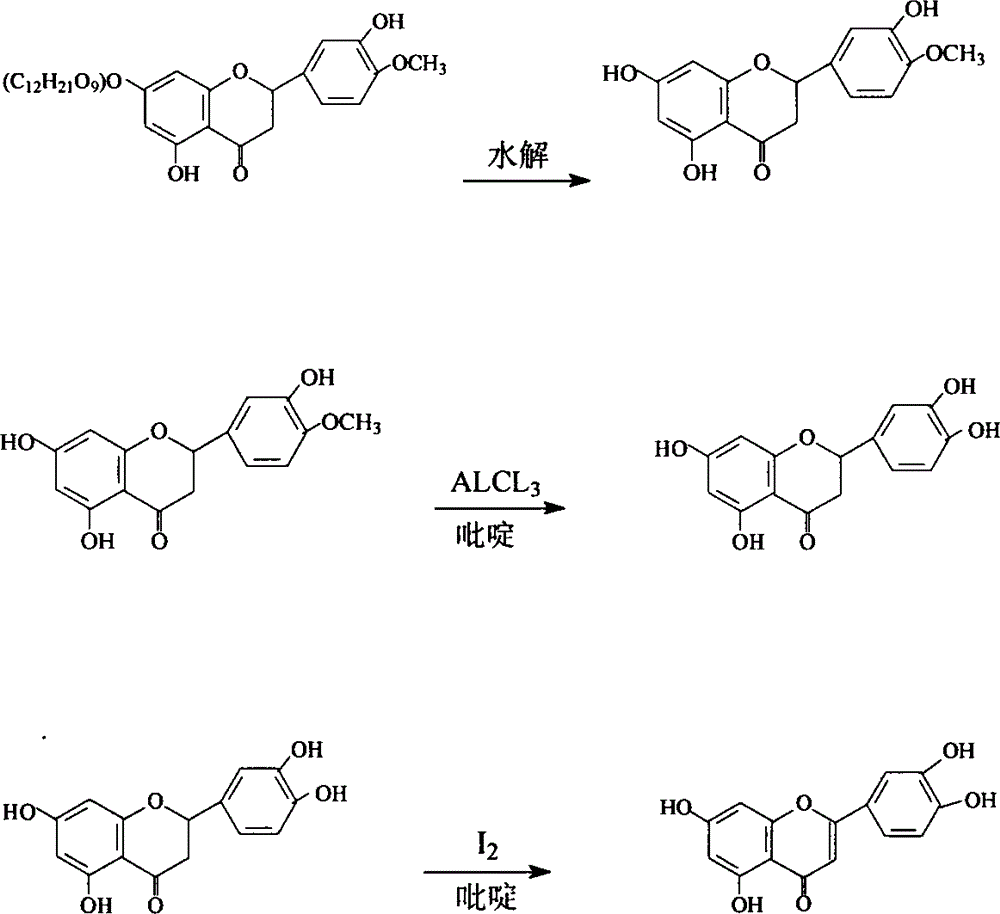
The invention provides semisynthesis luteolin preparation new process. The process includes a first step of hydrolyzing hesperidin to obtain hesperetin, a second step of demethylating the hesperetin to obtain eriodictyol, and a third step of dehydrogenizing the eriodictyol to obtain the luteolin. Compared with the prior art that the hesperidin is firstly dehydrogenized and then hydrolyzed, the process enables the iodine using amount to be greatly reduced, and production cost is low. The invention further provides technology for preparing the eriodictyol.
Owner:李玉山
Method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits
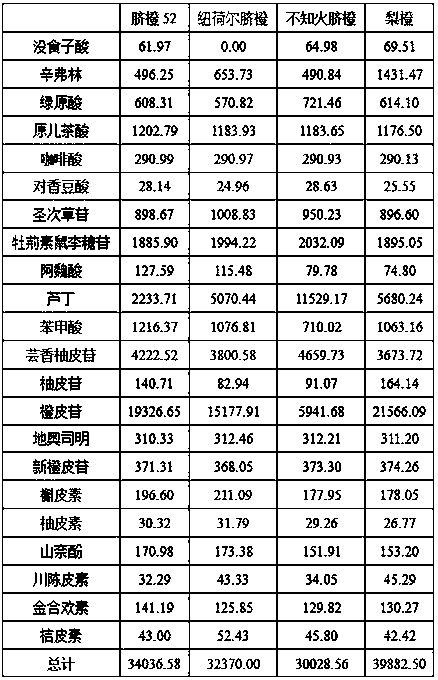
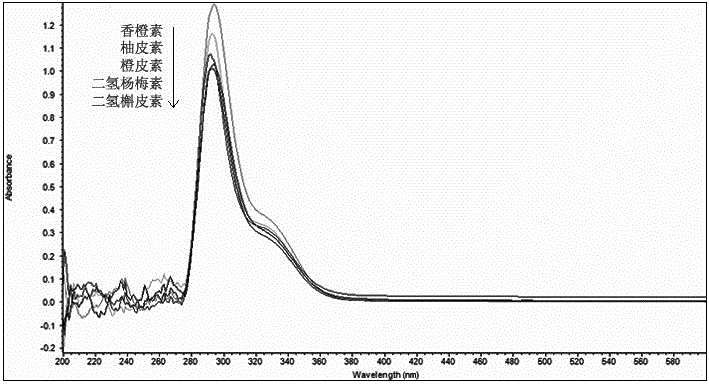
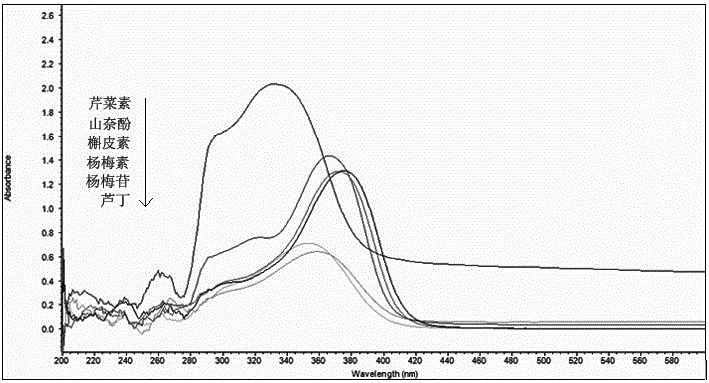
The invention discloses a method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits by adopting high performance liquid chromatography-diode array detector-fluorescence detector (HPLC-DAD-FLD). The method is capable of simultaneously determining twenty-two phenolic compounds in citrus fruits such as gallic acid, synephrine, chlorogenic acid, protocatechuic acid,caffeic acid, p-coumaric acid, rhamnosylvitexin, eriocitrin, ferulic acid, rutin, benzoic acid, narirutin, naringin, hesperidin, diosmin, neohesperidin, quercetin, naringenin, kaempferol, nobiletin,hesperetin, acacetin and the like, derivatization is not needed, and the method is high in accuracy, high in sensitivity and excellent in repeatability.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
New technology for preparing luteolin by using hesperidin
The invention provides new technology for preparing luteolin by using hesperidin. The method includes a first step of hydrolyzing hesperidin to obtain hesperetin, a second step of dehydrogenizing the hesperetin to obtain diosmetin, and a third step of demethylating the diosmetin to obtain the luteolin. Compared with the prior art that the hesperidin is firstly dehydrogenized and then hydrolyzed, the method enables iodine using amount to be greatly reduced, and production cost is low. The invention further provides new technology for preparing the diosmetin.
Owner:李玉山
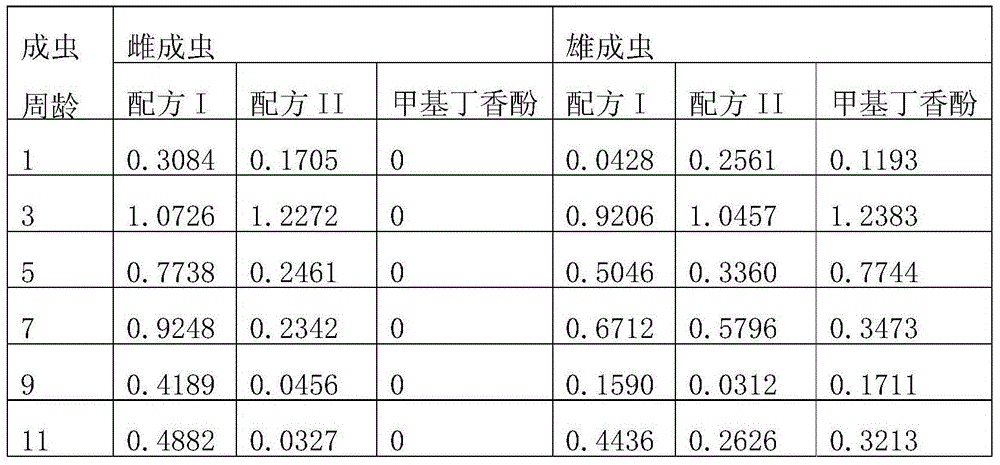
Bactrocera dorsalis amphoteric attractant as well as preparation method and application thereof
InactiveCN103548826ALow costProduct quality and safetyBiocidePest attractantsVegetable oilAntioxidant
The invention provides a bactrocera dorsalis amphoteric attractant as well as a preparation method and an application thereof. The bactrocera dorsalis amphoteric attractant comprises hesperetin, alcohols, ketones, methyleugenol, esters, vegetable oil, an antioxidant, added auxiliary components such as an antioxidant, an anti-ultraviolet agent and an anti-bactericide agent as well as a thickening agent, a slow-release agent and the like and is put in a deep color container with a cover so as to be preserved. The main components and the raw materials of the amphoteric attractant derive from natural plant extractives, so that the amphoteric attractant is environmentally-friendly and is safe for non-target organisms; the bactrocera dorsalis amphoteric attractant has a very good trapping effect on female adults and male adults of bactrocera dorsalis in combination with the integrated application of other solid sustained-release materials and is capable of effectively reducing the population cardinal number of the bactrocera dorsalis in a using region generation by generation. The bactrocera dorsalis amphoteric attractant is simple in synthesis process, low in cost and suitable for wide application and popularization in orange producing areas and other bactrocera dorsalis host crop producing areas and is also effective for injurious insects such as bactrocera zonata and melon flies.
Owner:吴小毅 +2
Method for preparing mono-glucoside through selective hydrolysis of flavone rutinoside or neohesperidoside
InactiveCN103819520AEasy to operateLow costSugar derivativesSugar derivatives preparationOrganosolvKetone
The invention relates to a method for preparing mono-glucoside through selective hydrolysis of flavone rutinoside or neohesperidoside, and belongs to the fields of chemistry and medicine. The method comprises the following steps: mixing flavone rutinoside or neohesperidoside with macroporous absorption resin in water or alkaline water solution, heating or adding acid; wherein in this process the diglucoside is dispersed, absorbed, and cured by the macroporous absorption resin; after drying, in the catalysis of acid or a dewatering agent, rhamnose in the molecule carries out condensation reactions with a ketone agent, and the rhamnose in the molecule is removed in the action of acid; eluting with an organic solvent or an alkaline water solution; and recycling the solvent or acidifying so as to obtain the flavone mono-glucoside. The method solves the problems that flavone diglucoside is hard to disperse in a ketone reagent and the selectivity of rhamnose hydrolysis is low. The method has the advantages of simple operation, easy recycling of organic solvent, low cost, high yield, and easiness in industrial production. Compounds such as hesperetin mono-glucoside, diosmetin mono-glucoside, hesperetin dihydrochlcone mono-glucoside, and the like, can be industrially produced from flavones such as flavone neohesperidoside through the method.
Owner:闻永举
Method for simultaneously measuring 11 flavonoid constituents in vine tea through HPLC
InactiveCN105717229ASharp peak shapeGood symmetryComponent separationColor/spectral properties measurementsBiotechnologyTest sample
The invention discloses a method for simultaneously measuring 11 flavonoid constituents in vine tea through HPLC and belongs to the technical field of medicinal plant constituent analysis.The method includes the steps that firstly, a mixed standard substance solution and a vine tea test sample solution are prepared respectively, then the mixed standard substance solution and the vine tea test sample solution are detected respectively through high performance liquid chromatography, and the contents of dihydromyricetin, dihydroquercetin, aromadendrin, naringenin, hesperetin, myricetin, myricetrin, kaempferol, quercetin, rutin and apigenin in a test sample are obtained through calculation.The measuring method is good in stability and reproducibility, easy to operate, accurate and reliable in result and capable of being applied to simultaneous measurement of multiple flavonoid constituents in the vine tea.
Owner:FUJIAN AGRI & FORESTRY UNIV
Method for preparing naringenin, hesperetin and mono-glucoside mixtures of naringenin and hesperetin
The invention provides a medicinal composition mainly containing naringenin, Prunin, hesperetin and hesperetin-7-0-glucoside. A medicine containing naringin, narirutin, hesperidin and neohesperidin isutilized as a raw material, an extract of the medicine is hydrolyzed, or extracting and hydrolyzing are conducted simultaneously on the extract of the medicine, and the plant extract can be obtained.The medicinal composition can be used for development and production of foods, health foods and new traditional Chinese medicines.
Owner:JILIN UNIV
Method for preparing and determining value of hesperetin reference material
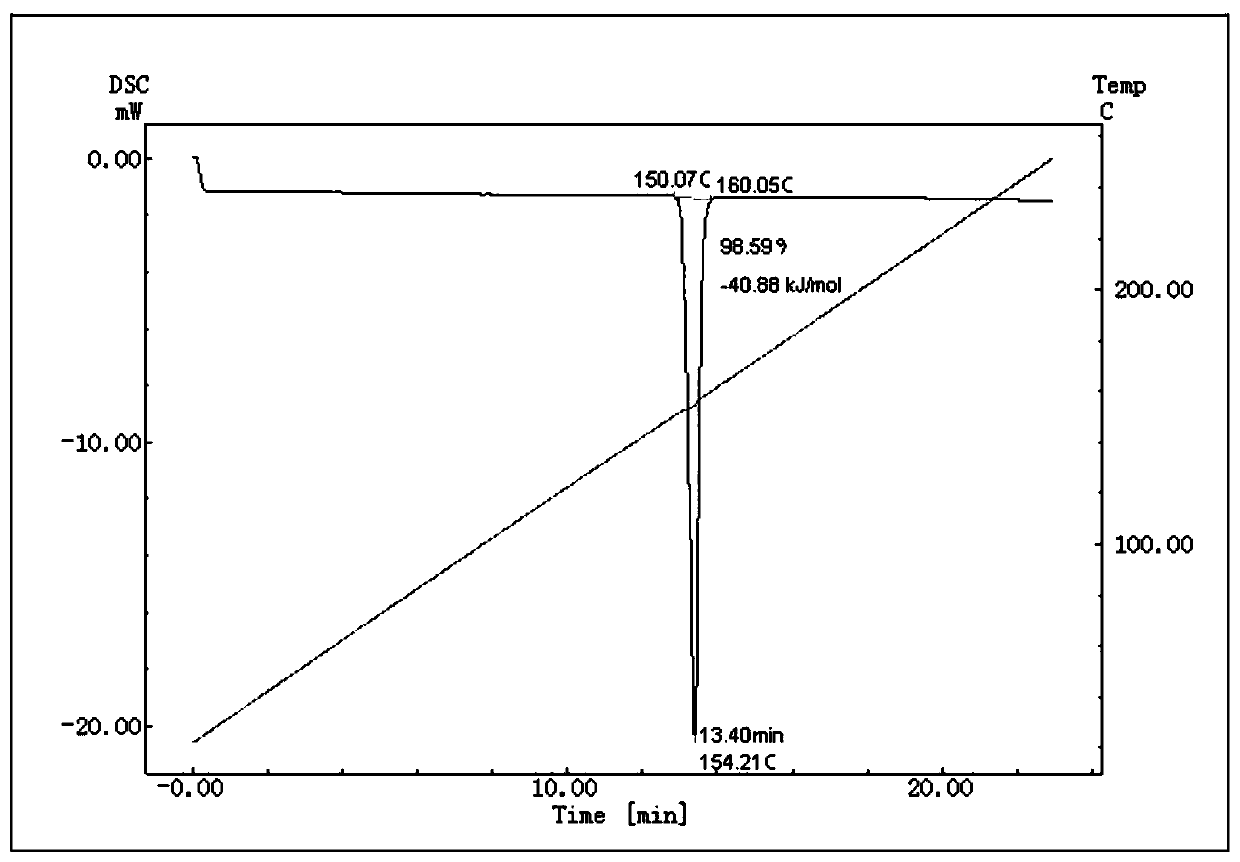
ActiveCN108918701AStable in natureAccurate and reliable characteristic valuesComponent separationSilica gelDifferential scanning calorimetry
The invention discloses a method for determining the value of a hesperetin reference material. The steps are as follows: 1) the mass percentage content of hesperetin in hesperetin raw material powderis determined by high performance liquid chromatography area normalization method, and the purity value P0 of the hesperetin raw material powder is obtained; 2) the mass percentage contents of water,organic volatile impurities and organic non-volatile impurities in the hesperetin raw material powder are determined, hesperetin purity value PHPLC-AN = P 0 * [100%-Xw-Xn-Xv] * 100% is determined by combination of the P0 determined by the step 1); 3) hesperetin purity value PDSC is determined by differential scanning calorimetry (DSC); and 4) final hesperetin purity value is calculated. The invention discloses a method for preparing the hesperidin reference material. The method for preparing the hesperidin reference material is as follows: first, preparing the reference material, to be more specific, taking a candidate reference material, purifying the candidate reference material with reverse C18 silica gel, performing rotary evaporateion, drying and storing; and determining the purity value according to the method for determining the value.
Owner:SOUTHWEST UNIVERSITY
Traditional Chinese medicine processing method
ActiveCN101612234AImprove pharmacological activityAntipyreticMetabolism disorderInternal capsuleChinese drug
The invention relates to a traditional Chinese medicine processing method, in particular to a traditional Chinese medicine processing method for fructus aurantii. The traditional Chinese medicine processing method comprises the following steps: the internal capsule of a traditional Chinese medicine raw material is cut out by using a knife, silt is washed, the traditional Chinese medicine raw material is soaked in water for 6 to 24 hours, an iron pressing component is used for extruding the traditional Chinese medicine raw material after being soaked, and then the traditional Chinese medicine raw material is put on a wooden stand, placed in an environment with the temperature of 30 DEG C to 35 DEG C and the relative humidity of 60% to 80% for 6 to 8 days, taken out, sliced firstly and then aired to obtain a traditional Chinese medicine decoction pieces, wherein, the traditional Chinese medicine raw material is the fructus aurantii. In the fructus aurantii after being processed by using the traditional Chinese medicine processing method, the existence of hesperetin-7-O-beta-D-glucopyranoside can be discovered by detection. hesperetin substances usually have a certain medicine effect, and the hesperetin-7-O-beta-D-glucopyranoside has stronger pharmacological activity. The traditional Chinese medicine taking the fructus aurantii as a representative is placed in an environment with high temperature and high humidity, processed and shaped by adopting the iron pressing component, which is a typical camphor type processing method.
Owner:江西樟树天齐堂中药饮片有限公司
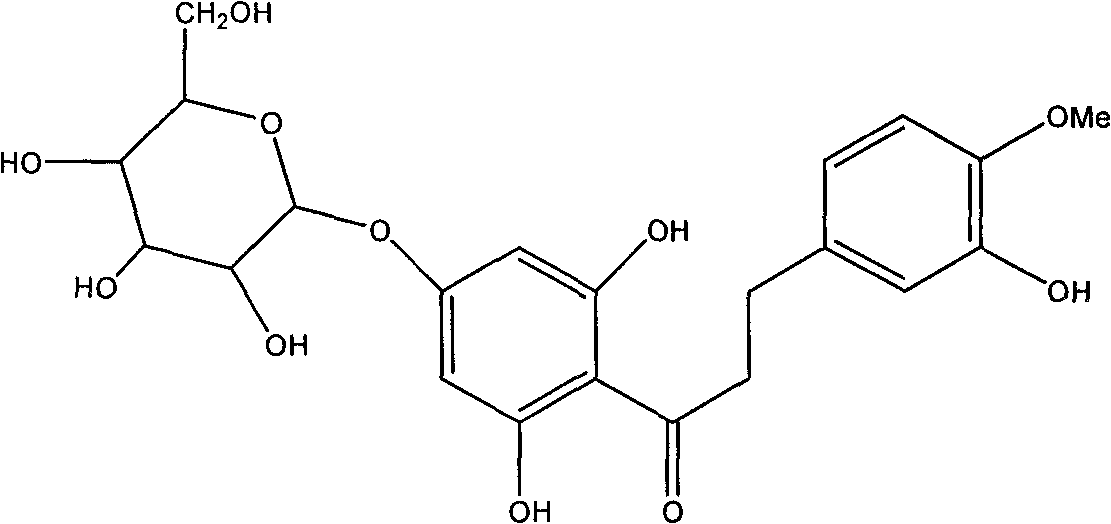
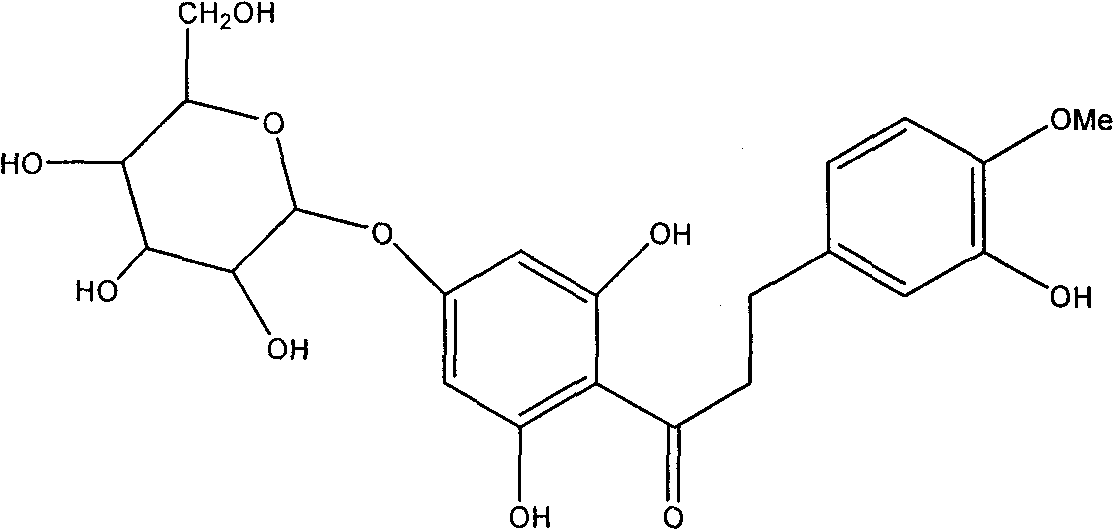
Hesperetin dihydrochalcone-7-O-glucoside and preparation method and application thereof
InactiveCN101787062AImprove sweetness qualitySugar derivativesPharmaceutical non-active ingredientsSucroseSaccharum
The invention discloses a hesperetin dihydrochalcone-7-O-glucoside, wherein the compound is obtained by generating a rhamnose deglycosylation reaction of new hesperidin dihydrochalcone in water or a water and organic solvent system under an enzyme action. The sweetness multiple of the hesperetin dihydrochalcone-7-O-glucoside prepared by the invention is about 1800 times of sucrose, and the hesperetin dihydrochalcone-7-O-glucoside can be widely used for industries of foods, medicines, feed, daily use chemicals and the like and used as a sweetening agent, a flavoring agent, a flavor enhancer and the like.
Owner:GUANGDONG FOOD IND INST +1
Beta-glucuroide isozyme BglA protein and application thereof
InactiveCN101864439AGuaranteed stabilityImmunogenic effectsMicroorganism based processesEnzymesBeta-glucosidaseIsozyme
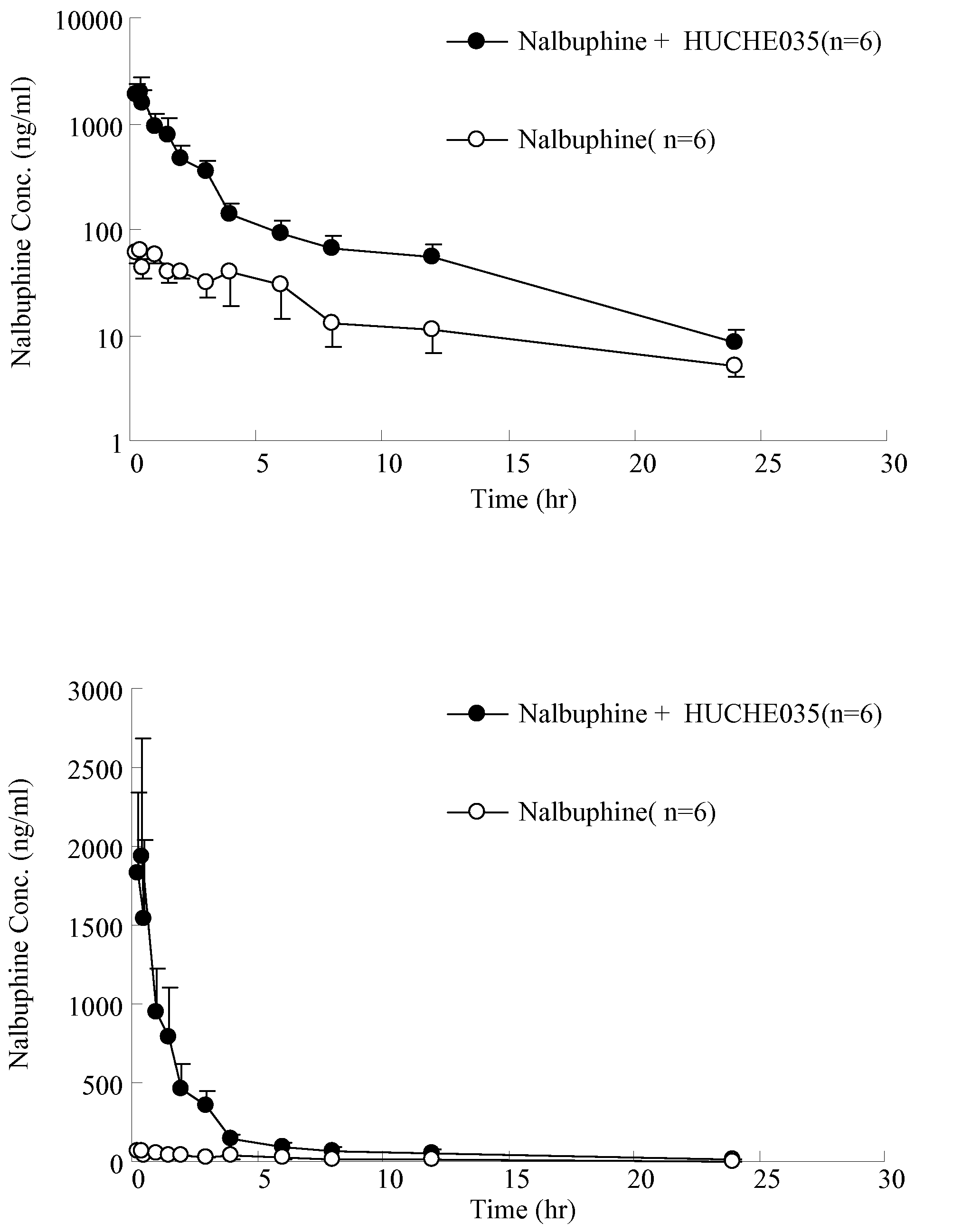
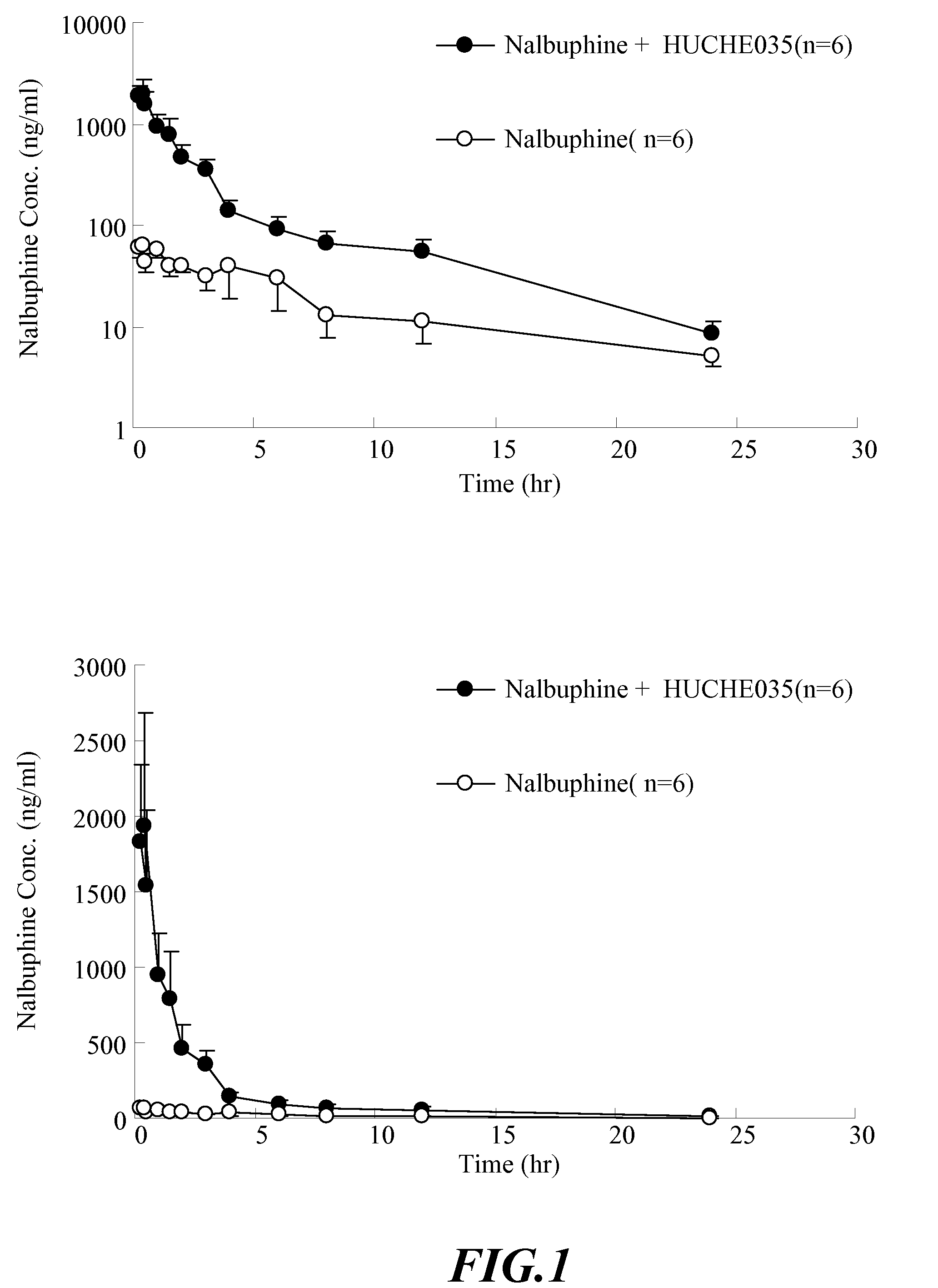
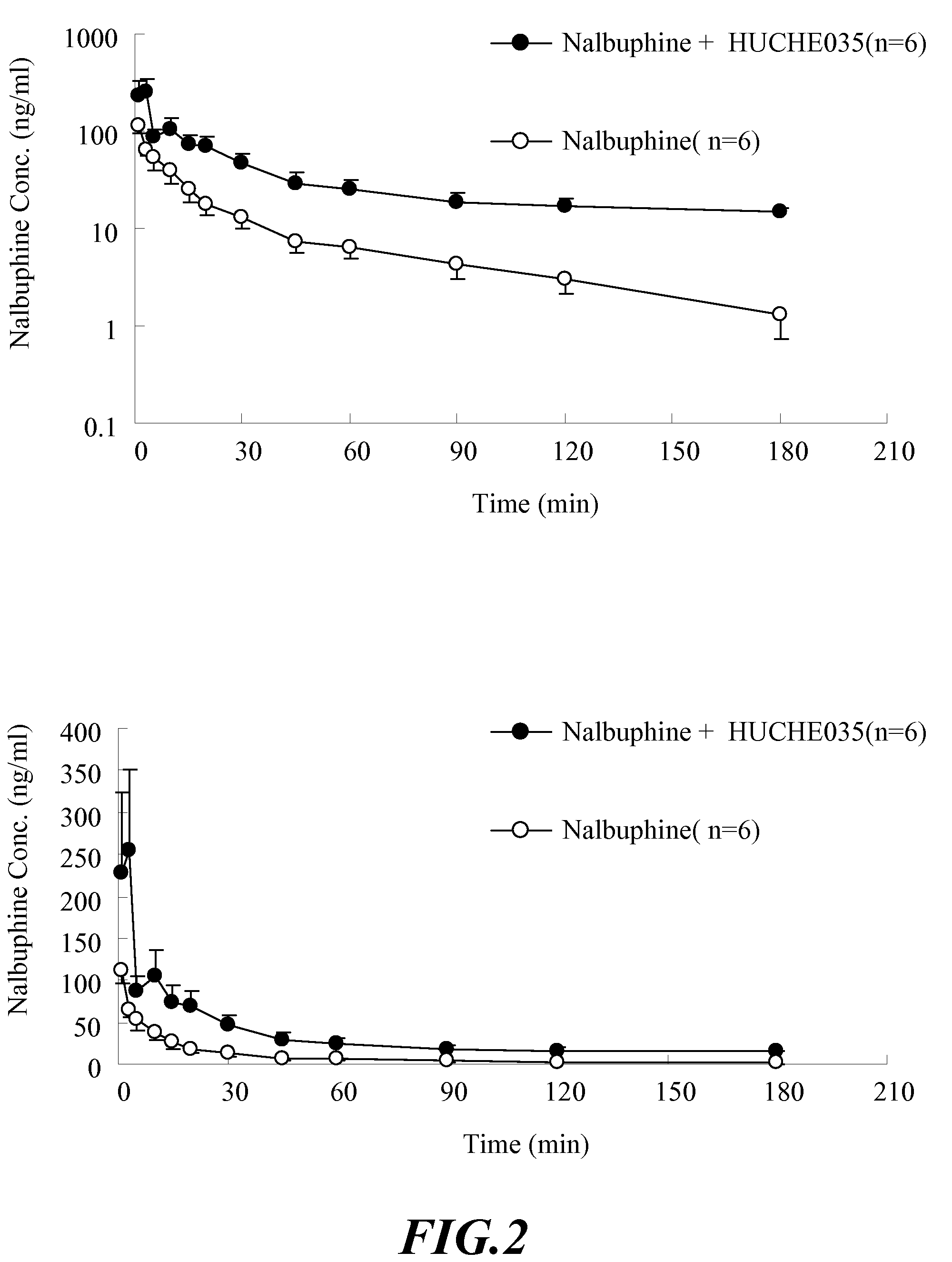
The invention discloses amino acid sequences shown as SEQ ID NO:1 and SEQ ID NO:2 of a gene for coding beta-glucuroide isozyme BglA. The BglA protein with high beta-glucuroide activity is cloned and expressed by using an ATCC (American Type Culture Collection) standard fungus strain S.singularis and adopting a modern molecular biology, aurantiamarin is biologically converted by using the BglA protein, and the glycoside is converted into aglycon, application individual difference in people of glycoside traditional Chinese medicine is eliminated, the bioavailability of the glycoside traditional Chinese medicine is improved, and the pharmacological response of active components of the glycoside traditional Chinese medicine is enhanced. In the invention, pharmacokinetics parallel comparative experiments of two dose modes of an oral dose and a vein dose of the aurantiamarin and hesperetin are simultaneously carried out in a laboratory room for the first time, which finds that the hesperetin is easy to absorb and is more slowly eliminated in comparison with the aurantiamarin, thereby complementing the reason of the bioavailability of the aurantiamarin lower than that of the aglycon.
Owner:TIANJIN MEDICAL UNIV
Method for preparing hesperetin from enzymatic hydrolysis neohesperidin or hesperidin
InactiveCN106148446AHigh purityHigh efficiency and high purityFermentationChromatographic separationOrganic solvent
The invention discloses a method for preparing hesperetin from enzymatic hydrolysis neohesperidin or hesperidin. Neohesperidin or hesperidin beta-D-glucoside bond can be hydrolyzed in one step through crude enzyme liquid to prepare corresponding aglycone hesperetin. The method includes the steps of preparing the crude enzyme liquid of Penicillium decumbens through fermentation, adjusting the content and reaction time of the crude enzyme liquid, directly converting neohesperidin or hesperidin into hesperetin, dissolving or extracting conversion liquid through organic solvent, and conducting chromatographic purification to obtain hesperetin. By means of the method, the defects that a traditional chemical method is strict in reaction condition, serious in pollution, low in conversion rate and the like when used for preparing hesperetin can be overcome, and the defects that when microbial fermentation is directly adopted, operation is complicated, products are not simplex, and products are difficult to purify can be overcome.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Application of derivative of hesperetin as anti-inflammatory and immune medicine
ActiveCN1861074AEnhance pharmacological effectsQuite activeOrganic active ingredientsAntipyreticDiseaseHydrolysis
An application of the hesperetin derivative, which is prepared from hesperidin through hydrolysis to remove glycosyl, in preparing the anti-inflammatory immune medicines for treating rheumatic or rheumatoid arthritis and osteoarthritis is disclosed.
Owner:ANHUI MEDICAL UNIV
Preparation method and application of glycosylated hesperetin
PendingCN110105409AImprove solubilitySolve the problem of decomposition and absorptionOrganic active ingredientsSugar derivativesSolubilityDisaccharidase
The invention provides a preparation method of glycosylated hesperetin. The preparation method comprises the specific steps that raw materials and a glycosyl donor are put into a reaction buffer solution; after the materials and the solution are mixed evenly, glycosidase and glycosyl transferase are added in a reaction system, wherein the raw materials are hesperidin, hesperetin, an extract or a conversion product of neohesperidin and a derivative thereof. According to the prepared glycosylated hesperetin, 1-9 glycosyl groups are added on a flavonoid parent, so that the solubility of the hesperetin is greatly improved, the problem of poor solubility of the hesperetin is solved, the problems about decomposition and absorption of the hesperetin in the human body are also solved, and the bioavailability of the hesperetin in citrus is greatly improved.
Owner:FOSHAN GOLDEN HEALTH TECH CO LTD
Method for obtaining high-purity hesperetin from valeriana jatamansi residue
ActiveCN103667385AReduce the impactIncrease profitFermentationEnzymatic hydrolysisResource utilization
A disclosed method for obtaining high-purity hesperetin from valeriana jatamansi residue comprises: heating valeriana jatamansi residue from which total terpenoid is extracted, leaching for 2 times, adjusting pH of the leaching solution, utilizing macroporous adsorption resin column for gathering hesperetin, firstly eluting with deionzed water, when the eluate is cleared, then eluting with a sodium hydroxide solution with a mass concentration of about 1%, gathering the eluate, adjusting the pH value of the eluate, and performing enzymatic hydrolysis, centrifugation filtering and recrystallization to obtain high-purity hesperetin which is light yellow acicular crystal. The method is simple in operation, low in production cost and high in source utilization rate, and is easy for industrialized production.
Owner:SHAANXI DONGKE PHARMA
Application of hesperetin to preparation of medicines for preventing and treating diabetes
InactiveCN106924239AInhibits alpha-glucosidaseMetabolism disorderHeterocyclic compound active ingredientsGlycosidase inhibitorAlpha-glucosidase
The invention belongs to the field of biological medicines, relates to hesperetin or novel application of hesperetin and particularly relates to hesperetin or pharmaceutically acceptable salts thereof or application of hesperetin or pharmaceutically acceptable salts to preparation of any one of the following medicines: (1) alpha-glucosidase inhibitors; (2) medicines for treating and / or preventing diabetes; and (3) medicines for treating and / or preventing tumor. The hesperetin is capable of effectively inhibiting alpha-glucosidase and has potential for preparing medicines for preventing and treating diabetes, especially type II diabetes.
Owner:UNKNOWN +1
Amide group substituted hesperetin derivatives, preparation method thereof and application of derivatives as anti-AD (anti-Alzheimer's disease) drugs
ActiveCN106905280AStrong inhibitory activityHigh selectivityNervous disorderOrganic chemistryAcetylcholinesteraseButyrylcholinesterase
The invention belongs to the fields of pharmaceutical chemistry and pharmacotherapeutics and discloses amide group substituted hesperetin derivatives, a preparation method thereof and an application of the derivatives as AD (Alzheimer's disease) treatment drugs. Study proves that the amide group substituted hesperetin derivatives have strong inhibition property and good inhibition selectivity on acetylcholinesterase, and inhibition capacity of the derivatives on acetylcholinesterase is more than 500 times higher than inhibitory activity on butyrylcholinesterase. The study indicates that the compounds can be developed into the AD treatment drugs.
Owner:ANHUI MEDICAL UNIV
Production method of hesperidinase and application
ActiveCN105602917AIncrease enzyme activityImprove thermal stabilityMicroorganism based processesFermentationBranFermentation
The invention discloses a production method of hesperidinase and an application. The production method comprises the following steps: (1) carrying out spore-producing culture on aspergillus niger in a bran culture medium to form aspergillus niger spores; (2) carrying out fermentative enzyme production on the aspergillus niger spores in an enzyme-producing culture medium to produce the hesperidinase; and (3) carrying out separation and extraction to obtain an enzyme liquid, wherein the aspergillus niger is preserved in the China General Microbiological Culture Collection Center; the preservation name is KH005; and the preservation number is CGMCC12101. The hesperidinase produced by the aspergillus niger KH005 in an enzyme-production and fermentation culture medium has the advantages of high enzyme activity and good thermal stability. The aspergillus niger KH005 is applied to production of hesperetin by an enzymolysis approach; a new process selection is provided for production of the hesperetin; the enzymolysis approach is mild in reaction condition, good in selectivity; a parent ring of the hesperetin is not easy to denature; and the produced hesperetin is high in content and easy to separate.
Owner:成都康辉生物科技有限公司
Functional blood sugar-reduction health product and preparation method thereof
InactiveCN104107269ARegulate secretionImprove effective utilizationOrganic active ingredientsMetabolism disorderSolventBlood sugar
The invention relates to a functional blood sugar-reduction health product and a preparation method thereof. The functional blood sugar-reduction health product has blood sugar-reduction assistant functions and comprises hesperetin, balsam pear, American ginseng extract and wolfberry extract. The functional blood sugar-reduction health product comprises the edible and medical materials having blood sugar-reduction effects, is safe and effective and can be used for a long time. The functional blood sugar-reduction health product can be processed to form enteric coated tablets or enteric coated capsules. The preparation method comprises that the above raw materials and water and ethanol as solvents can be processed to form extract, the extract is subjected to spray drying so that extract powder is obtained, the extract powder is processed to form tablets or capsules, and the tablets or capsules are coated. An animal functional test proves that the functional blood sugar-reduction health product has blood sugar-reduction assistant health functions and is suitable for being eaten by diabetes for a long time.
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing hesperetin, preparation method of hesperetin intermediate and biological enzyme for preparing hesperetin
The present invention provides a method for preparing hesperetin, comprising: suspending hesperidin or neohesperidin in water, and adding a sodium hydroxide solution until the hesperidin or the new hesperidin is completely dissolved, and btaining a substrate preparation solution; adding the substrate preparation solution to a buffer containing alpha-L-rhamnosidase and beta-glucosidase at a rate of0.1 to 1 mL / min for stirring at a flow rate of 0.1 to 1 mL / min to obtain a reaction solution, and after the completion of the addition, continuing the reaction for 0.5 to 1 h, the pH of the reactionsolution is 6.0 to 7.0, and the reaction temperature is maintained at 45 to 65 DEG C. The alpha-L-rhamnosidase is derived from Streptomyces, and the beta-glucosidase is derived from the genus Thermotoxin; adjusting the pH of the reaction solution to 3.0 to 5.0 to completely precipitate the solid product, and collecting the solid product to obtain the hesperetin. The method is efficient, simple, green and environmentally friendly, and can be applied to industrial mass production.
Owner:BONTAC BIO ENG SHENZHEN
Hesperidin and hesperetin as inhibitor of acyl coa-cholesterol-o-acyltransferase, inhibitor of macrophage-lipid complex accumulation on the arterial wall and preventive agent
The present invention relates to uses of hesperidin or hesperetin for inhibiting the activity of acyl CoA-cholesterol-o-acyltransferase, inhibiting the accumulation of macrophage-lipid complex on the arterial endothelium, and preventing or treating hepatic diseases in a mammal.
Owner:KOREA INST OF SCI & TECH
Alpha-L-rhamnosidase mutant enzyme, gene and expression preparation method
ActiveCN113088528ASimple stepsLow costMicroorganism based processesFermentationPichia pastorisEnzyme Gene
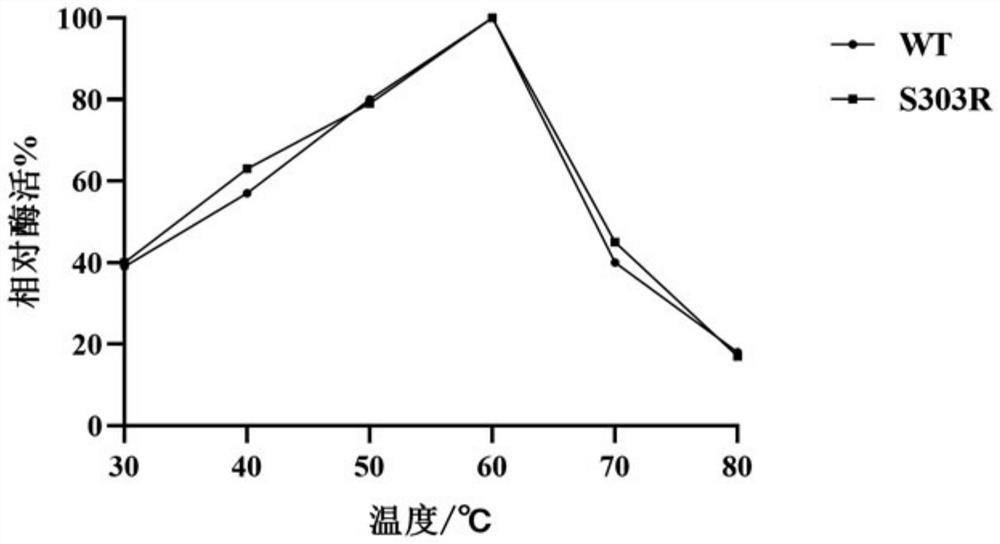
The invention discloses alpha-L-rhamnosidase capable of specifically converting hesperidin to generate hesperetin-7-O-glucoside, a gene and an expression preparation method of the alpha-L-rhamnosidase, a coding nucleotide sequence of the alpha-L-rhamnosidase is shown as SEQ ID NO: 1, and an amino acid sequence of the alpha-L-rhamnosidase is shown as SEQ ID NO: 2. The enzyme is prepared by performing site-directed mutagenesis on an alpha-L-rhamnosidase r-Rha1 gene from aspergillus niger to obtain a mutant S303R gene, and expressing the mutant S303R gene in pichia pastoris SMD1168. The enzyme only hydrolyzes hesperidin instead of other citrus flavonoids, wherein the hesperidin in crude citrus flavonoids can be specifically hydrolyzed to generate hesperetin-7-O-glucoside, so that the preparation efficiency of the hesperetin-7-O-glucoside is improved, and an important tool enzyme is provided for industrial preparation of the hesperetin-7-O-glucoside.
Owner:JIMEI UNIV
Method for preparing high-bioavailability derivatives of hesperidin
InactiveCN101445530ARapid hydrolysisImprove bioavailabilitySugar derivativesOrganic solventAcid hydrolysis
The invention discloses a method for preparing high-bioavailability derivatives of hesperidin, which is characterized in that the method obtains hesperetin-7-glucoside and hesperetin with higher purity by appropriate hydrolysis of the hesperidin and a certain separation and purification method. The preparation method is as follows: the hesperidin extracted from citrus reticulata is taken as raw material and dissolved in a certain amount of alkaline solution, a certain amount of organic solvent is added, the mixture is pre-heated after the full dissolution, a certain amount of hydrochloric acid which is also pre-heated is finally added, and hydrolysis mixed products are obtained by refluxing in a boiling water bath for a certain time, cooling, regulating pH, standing and filtering. The hesperetin-7-glucoside and the hesperetin are obtained by separating and purifying the mixture. The hesperidin which is not decomposed after separating and purifying the products can be used for recycling in the next acid hydrolysis. The method can obtain the hesperetin-7-glucoside and the hesperetin which are the high-bioavailability derivatives of the hesperidin by the simple acid hydrolysis method, thereby having the advantages of low cost and simple process; furthermore, the method can realize the recycled production and is easy to realize the industrial production.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Semi-synthesis method of luteolin and galuteolin as well as luteolin rutinoside
InactiveCN103833714AAvoid absorptionQuick joinSugar derivativesSugar derivatives preparationDehydrogenationHydrolysis
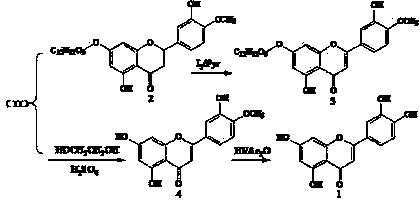
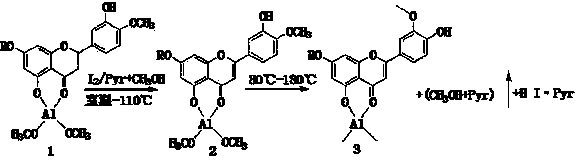
The invention relates to a semi-synthesis method of luteolin and galuteolin as well as luteolin rutinoside by using hesperidin, a semi-synthesis method of the galuteolin by using hesperetin glucoside, and a semi-synthesis method of the luteolin by using hesperetin, and belongs to the field of chemistry and medicines. The semi-synthesis method comprises the following steps: enabling the hesperidin, the hesperetin glucoside and the hesperetin to be subjected to complexation in pyridine alcohol fluid, dehydrogenizing by using iodine, directly distilling alcohol and pyridine, maintaining for a period of time in an airtight distilled state, and carrying out demethylation reaction, so that the hesperidin is generated into the luteolin rutinoside; generating the galuteolin by demethylation of diosmetin glucoside, and generating the luteolin by diosmetin; and hydrolyzing the luteolin rutinoside, so that the luteolin rutinoside is transformed into luteolin and galuteolin. The semi-synthesis method has the advantages that two steps of dehydrogenation and demethylation are combined into one step, and reaction conditions of dehydrogenation and demethylation are mild and easy to control; few reagents are used and green and environmentally friendly; and the demethylation yield is high, and the industrial production is easy. Compared with disclosed documents and patients, the semi-synthesis method has great advantages in the production of luteolin and glucosides of the luteolin.
Owner:迁西县板栗产业研究发展中心
Composition with blood sugar reduction function and application
ActiveCN111388462AGood synergySmall doseOrganic active ingredientsMetabolism disorderPharmaceutical drugDiosmetin
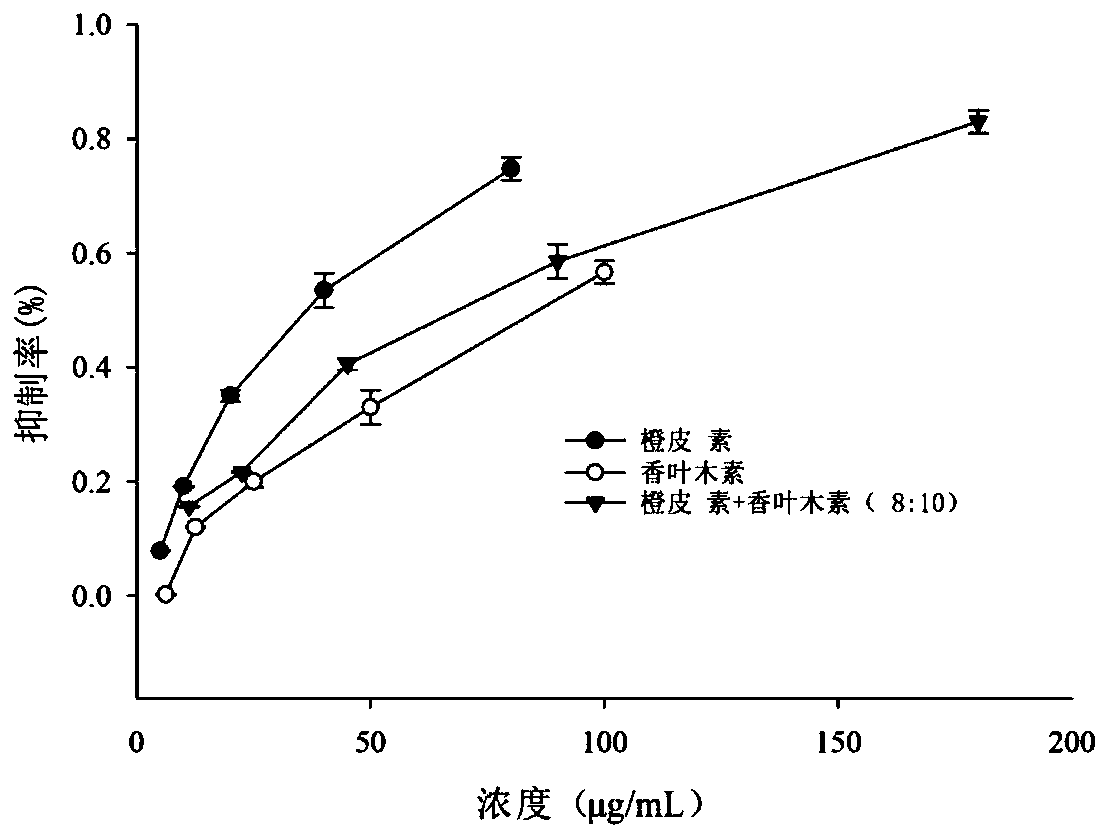
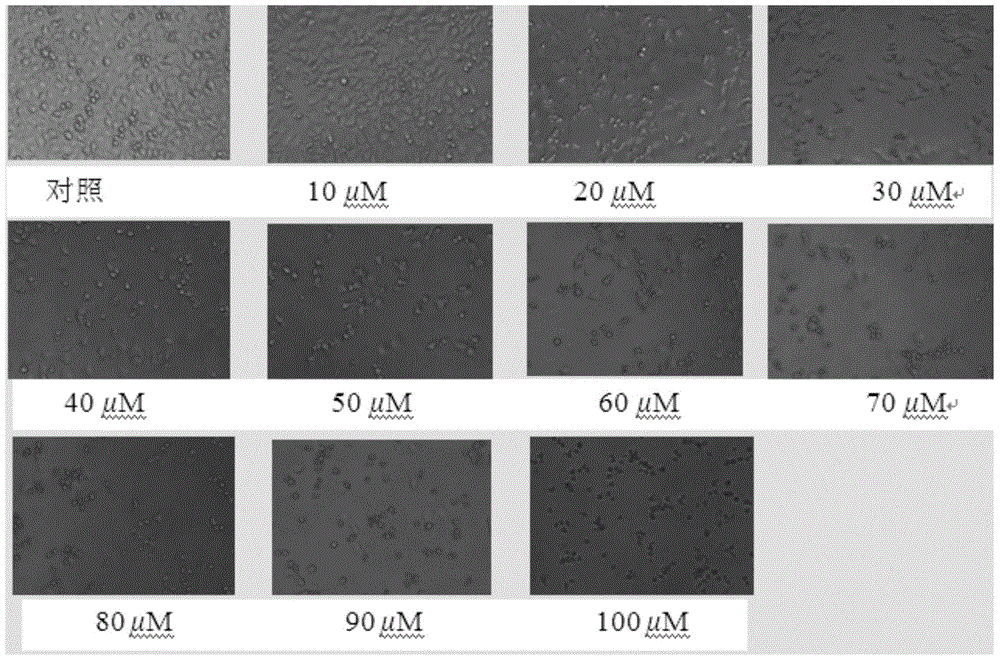
The invention discloses a composition with a blood sugar reduction function and application and belongs to the technical field of medicines. The composition with the blood sugar reduction function consists of hesperetin and diosmetin in a mass concentration ratio of (2:10)-(8-10), and preferably the mass concentration ratio of the hesperetin to the diosmetin is (3:10)-(8:10). The composition of the hesperetin and the diosmetin, which is disclosed by the invention, has a remarkable synergism in inhibiting alpha-glucosidase, has an effect prior to those of separately used flavonoids, in addition, is capable of reducing dosages of medicines and reducing drug resistance, and has wide application prospects in preparing medicines, healthcare products or foods for treating diabetes.
Owner:ZHENGZHOU FRUIT RES INST CHINESE ACADEMY OF AGRI SCI
Application of nobiletin
ActiveCN105030559ALow effective doseLittle potential side effectsOrganic active ingredientsCosmetic preparationsMedicineEpidermoid carcinoma
The invention discloses application of nobiletin, and particularly discloses application of nobiletin in preparation of health products or medicines for preventing and / or treating oral cancer. Through the anti-proliferation effects of monomeric compounds, namely hesperetin, naringenin and nobiletin, in rutaceae citrus fruits and peels on human oral epidermoid carcinoma cells, the experiments show that the hesperetin, naringenin and nobiletin have an obvious effect on inhibiting proliferation of human oral epidermoid carcinoma cells, and the effect of the nobiletin on inhibiting oral cancer cells is most obvious, so that the nobiletin can be used for preparing health products and medicines with effects on preventing and treating oral cancer and is suitable for large-scale popularization and application.
Owner:SOUTH CHINA UNIV OF TECH
Method for extracting high-purity hesperetin from immature bitter orange
ActiveCN102432575AEasy to realize industrial productionSimple processOrganic chemistrySolventHydrolysis
The invention discloses a method for extracting high-purity hesperetin from immature bitter orange, which comprises the following steps: 1. pulverizing immature bitter orange, extracting with alcohol solvent under reflux three times, filtering, merging the three filtrates, and concentrating; 2. adding inorganic acid into the concentrated solution for hydrolysis, naturally cooling to room temperature, filtering, washing the filter residues, and centrifugalizing to obtain a crude hesperetin product; and 3. dissolving the crude hesperetin product in ethanol, adding activated carbon to decolorize under reflux, filtering, concentrating, crystallizing, filtering, and drying the filtered solid matter to obtain the hesperetin crystals of which the mass purity is not lower than 95%.By using the immature bitter orange as the raw material for extraction, hydrolysis and refinement, compared with the traditional technique of extracting hesperetin from hesperidin, the invention reduces the intermediate links, lowers the production loss, enhances the production yield of hesperetin, lowers the production cost, and eliminates the hesperidin refinement, drying and other links in the original production process, thereby greatly shortening the production cycle.
Owner:XIAN XIAOCAO BOTANICAL DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com