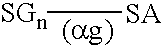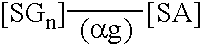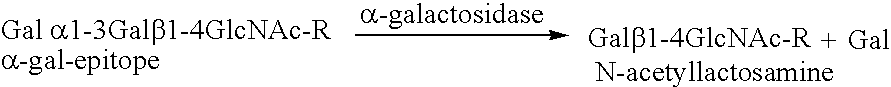Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Disaccharidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Disaccharidases are glycoside hydrolases, enzymes that break down certain types of sugars called disaccharides into simpler sugars called monosaccharides. In the human body, disaccharidases are made mostly in an area of the small intestine's wall called the brush border, making them members of the group of "brush border enzymes".
Method of screening for potential anti-metastatic and anti-inflammatory agents using mammalian heparanase as a probe
Qualitative and quantitative methods of testing an agent for its potential at inhibiting glycosidase catalytic activity, the methods including the steps of interacting a glycosidase enzyme with a glycosidase substrate in a presence of the agent and qualitatively or quantitatively evaluating an effect of the agent on the catalytic activity of the glycosidase enzyme toward the glycosidase substrate. Preferably the glycosidase enzyme is a heparanase enzyme and the glycosidase substrate is, respectively, a heparanase substrate.
Owner:INSIGHT BIOPHARMLS +1
Peritoneal dialysis fluid
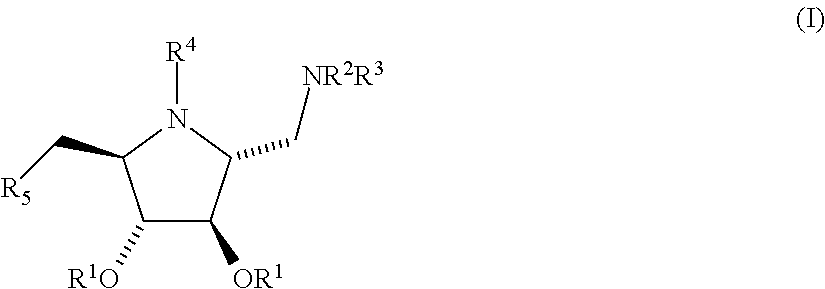
Peritoneal dialysis fluids and the use thereof for performing peritoneal dialysis are disclosed. The peritoneal dialysis fluid comprises a physiologically acceptable aqueous solution containing physiologically acceptable inorganic anions and cations and, as an osmotic agent, at least one sugar derivative, at concentrations sufficient for the removal of water and solutes from a patient by peritoneal dialysis. The sugar derivative is a compound of formulawherein each SG, which may be the same or different, represents a residue of a physiologically acceptable metabolizable sugar, SA represents a residue of a physiologically acceptable metabolizable sugar alcohol, n is from 1 to 4 andrepresents a glycoside linkage which is cleavable by an alpha-glycosidase enzyme.
Owner:ALLIED THERAPEUTICS LTD
Selective glycosidase inhibitors and uses thereof
Owner:SIMON FRASER UNIVERSITY
Bone xenografts
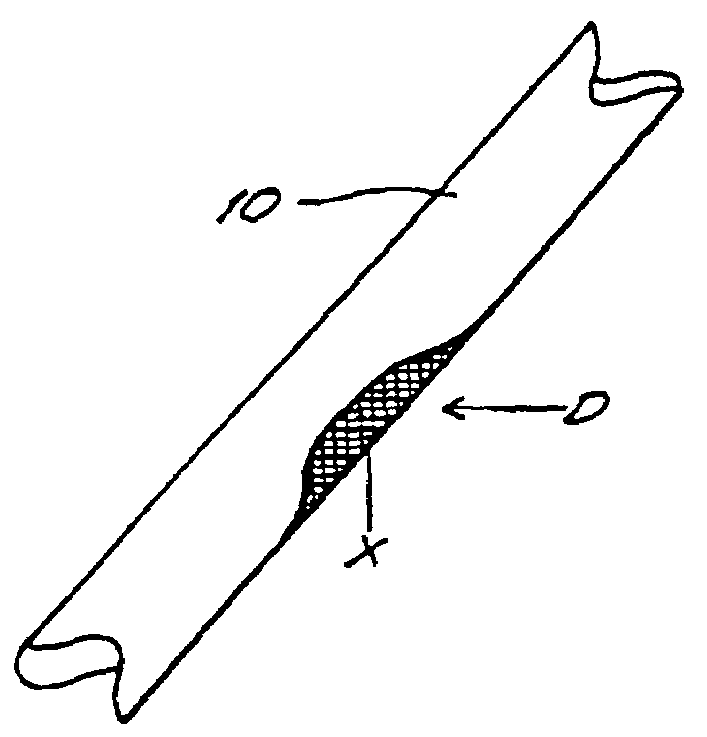
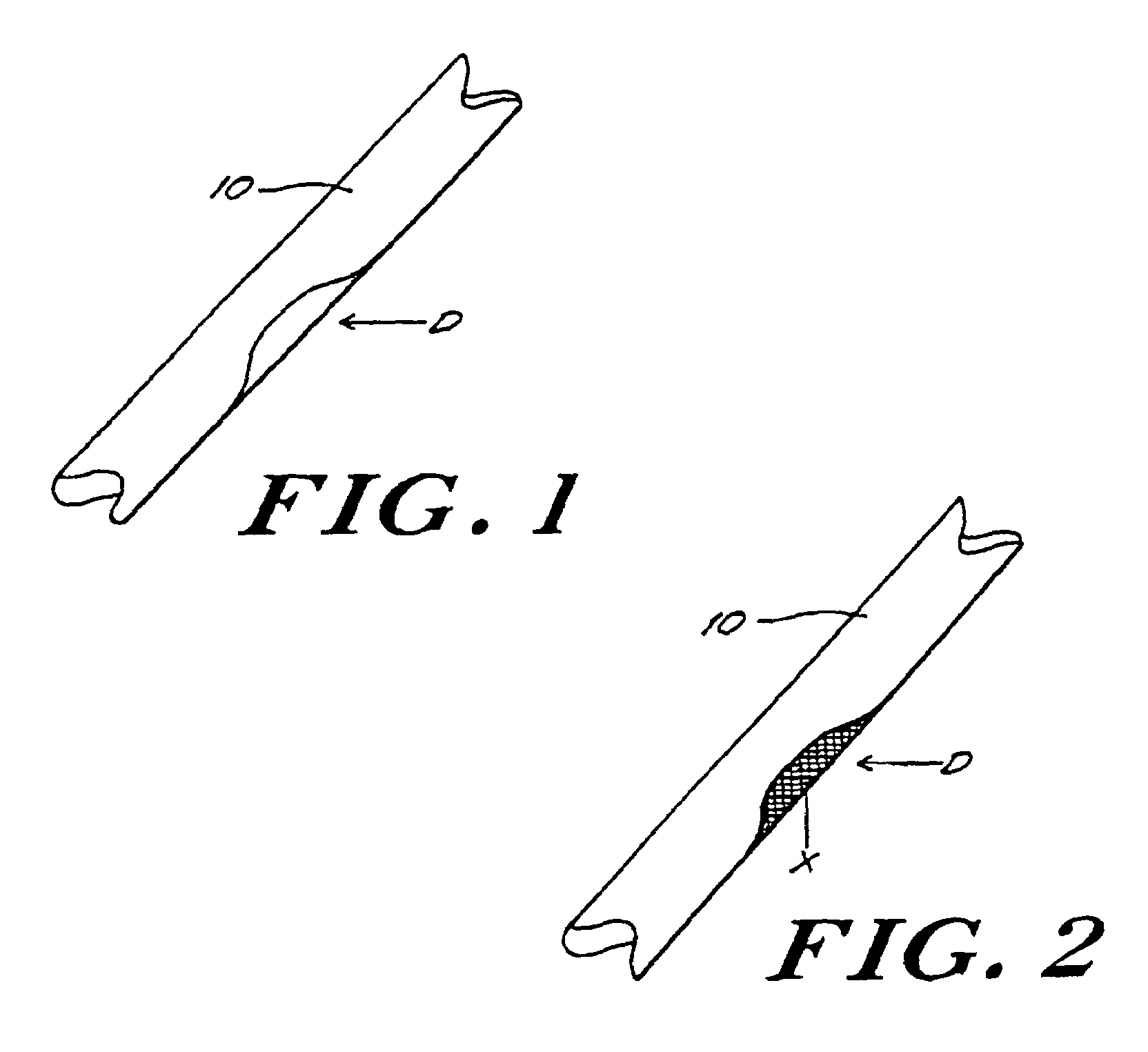
The invention provides an article of manufacture comprising a substantially non-immunogenic bone xenograft for implantation into humans. The invention further provides a method for preparing a bone xenograft by removing at least a portion of a bone from a non-human animal to provide a xenograft (X); washing the xenograft in saline and alcohol; subjecting the xenograft to a cellular disruption treatment; and treating the xenograft with a glycosidase to remove surface carbohydrate moieties. The invention also provides an article of manufacture produced by the above identified method of invention. The invention further provides a bone xenograft for implantation into a human including a portion (10) of a bone from a nonhuman animal, wherein the portion has substantially no surface carbohydrate moieties which are susceptible to glycosidase digestion. Each xenograft of the invention has substantially the same mechanical properties as a corresponding native bone.
Owner:APERION BIOLOGICS
Selective glycosidase inhibitors and uses thereof
Owner:SIMON FRASER UNIVERSITY +2
Selective Glycosidase Inhibitors and Uses Thereof
Owner:SIMON FRASER UNIVERSITY
Xenograft heart valves
The invention provides an article of manufacture comprising a substantially non-immunogenic heart valve xenograft for implantation into humans. The invention further provides methods for preparing a heart valve xenograft by removing at least a portion of a soft tissue from a non-human animal to provide a xenograft; washing the xenograft in saline and alcohol; subjecting the xenograft to cellular disruption treatment; treating the xenograft with crosslinking agents, and digesting the xenograft with a proteoglycan-depleting factor and / or glycosidase. The invention also provides an article of manufacture produced by the above-identified method of the invention. The invention further provides a heart valve xenograft for implantation into a human including a portion of a heart valve from a non-human animal, wherein the portion has extracellular components and substantially only dead cells. The extracellular components have reduced proteoglycan molecules. Each of the xenografts of the invention are substantially non-immunogenic and have substantially the same mechanical properties as a corresponding native heart valve.
Owner:APERION BIOLOGICS
Alginic Acid with Low Molecular Weight, Its Salts, Uses, Preparative Methods, Pharmaceutical Compositions and Foods
InactiveUS20100256090A1Reduce molecular weightTreat high blood pressureOrganic active ingredientsBiocideAcute hyperglycaemiaDisaccharidase
The present invention discloses an alginic acid and / or its salts with low molecular weight, wherein the weight average molecular weight of the alginic acid is from about 700 to about 4500 Daltons, and the molar ratio of guluronic acid to mannuronic acid in the alginic acid is from about 0.6 to about 19. The present invention also discloses the preparative method of making the alginic acid and / or its salts thereof, and the use of them for treating hypertension, chronic renal failure and postprandial hyperglycemia induced by glycosidase. The present invention further discloses pharmaceutical compositions and foods containing the alginic acid with low molecular weight and / or salts thereof as active component.
Owner:YU CHUANXING
Separation purification process for main catechin component in tea polyphenol and glycosidase activity
InactiveCN101492440AEasy separationImprove separation efficiencyOrganic active ingredientsOrganic chemistryPurification methodsPhenolic content in tea
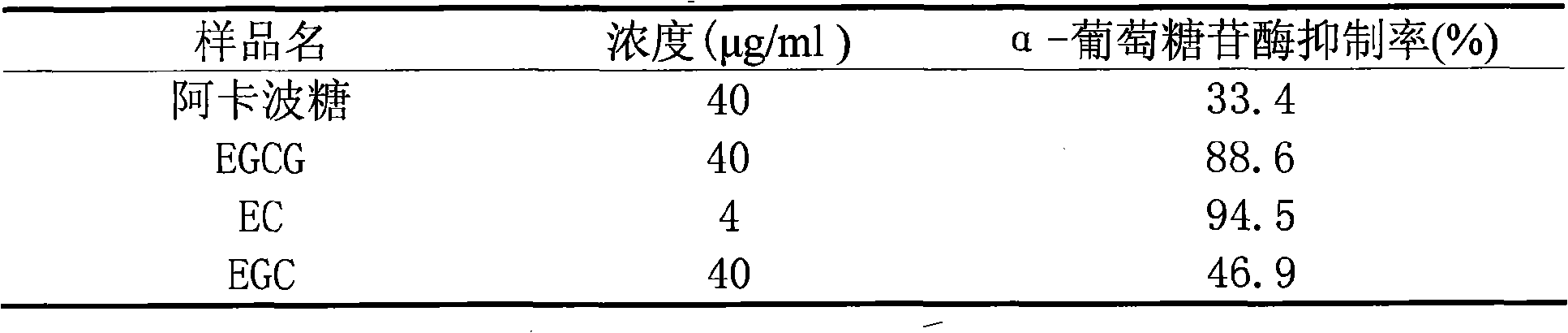
The invention discloses a separation and purification method of main catechin components in tea polyphenol and glycosidase activity thereof. In the separation and purification method, tea polyphenol extracts are absorbed by macroporous adsorption resin of nonpolar or weak polar polystyrene; water and ethanol are used for the elution; alcohol eluates, through polyamide columns for the chromatography and are eluted respectively by water and ethanol. The water recrystallization is carried out on the alcohol eluates of the polyamide columns to obtain EGCG pure products with the purity larger than 95 percent and the yield larger than 50 percent; reversed-phase C18 filled columns are used for the chromatography of water eluates of the polyamide columns to obtain EC and EGC; the ethanol of 50-80 percent is used for the recrystallization to obtain pure products of the EC and EGC with the purity all larger than 96 percent. Tests in vitro show that EGCG, EGC and EC all have inhibitory activity on alpha-glucosidase and alpha-amylase and can be used for preparing weight reduction products reducing the absorption of carbohydrates or drugs or health products lowering the postprandial blood glucose.
Owner:SHANGHAI NORMAL UNIVERSITY
Selective Glycosidase Inhibitors and Uses Thereof
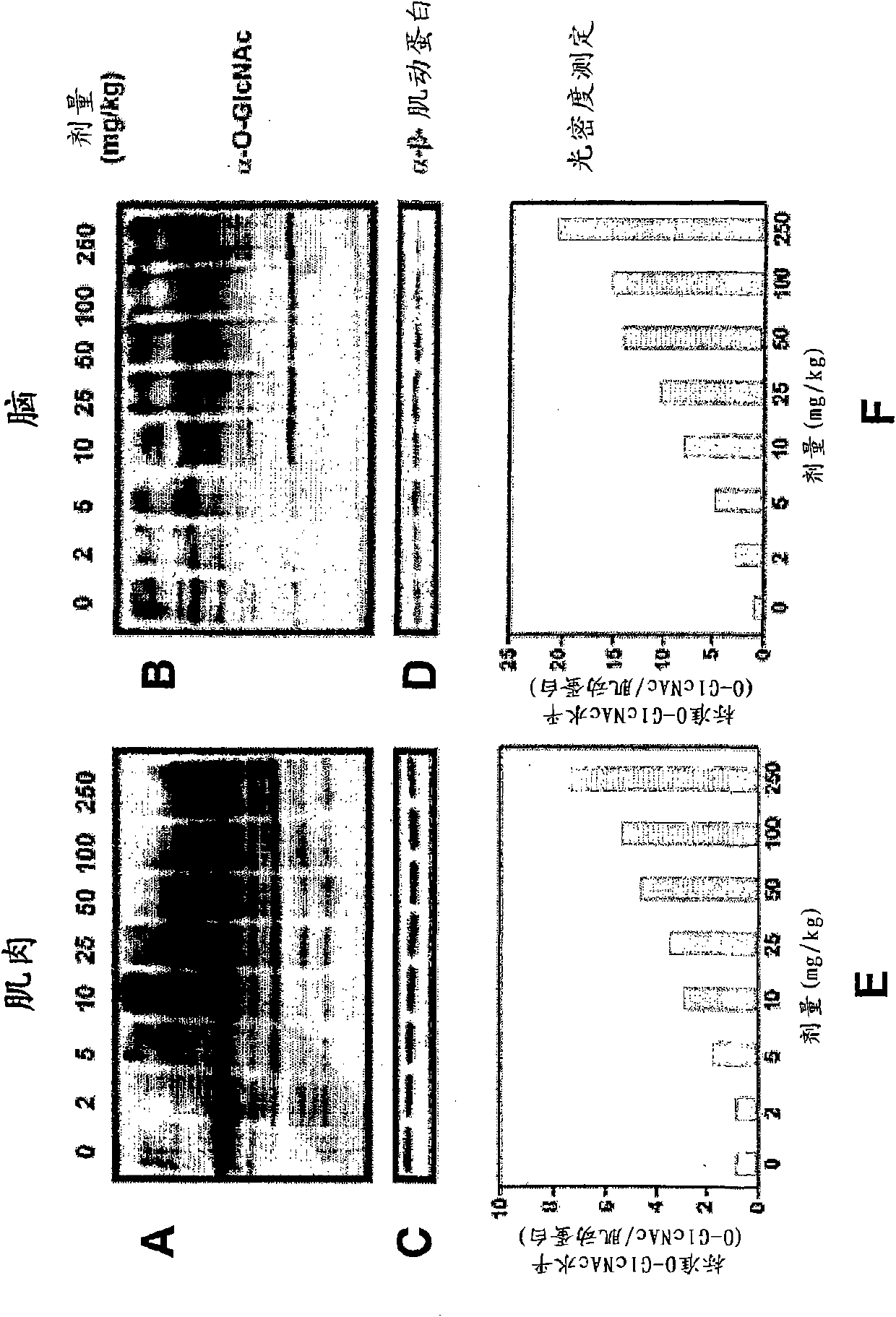
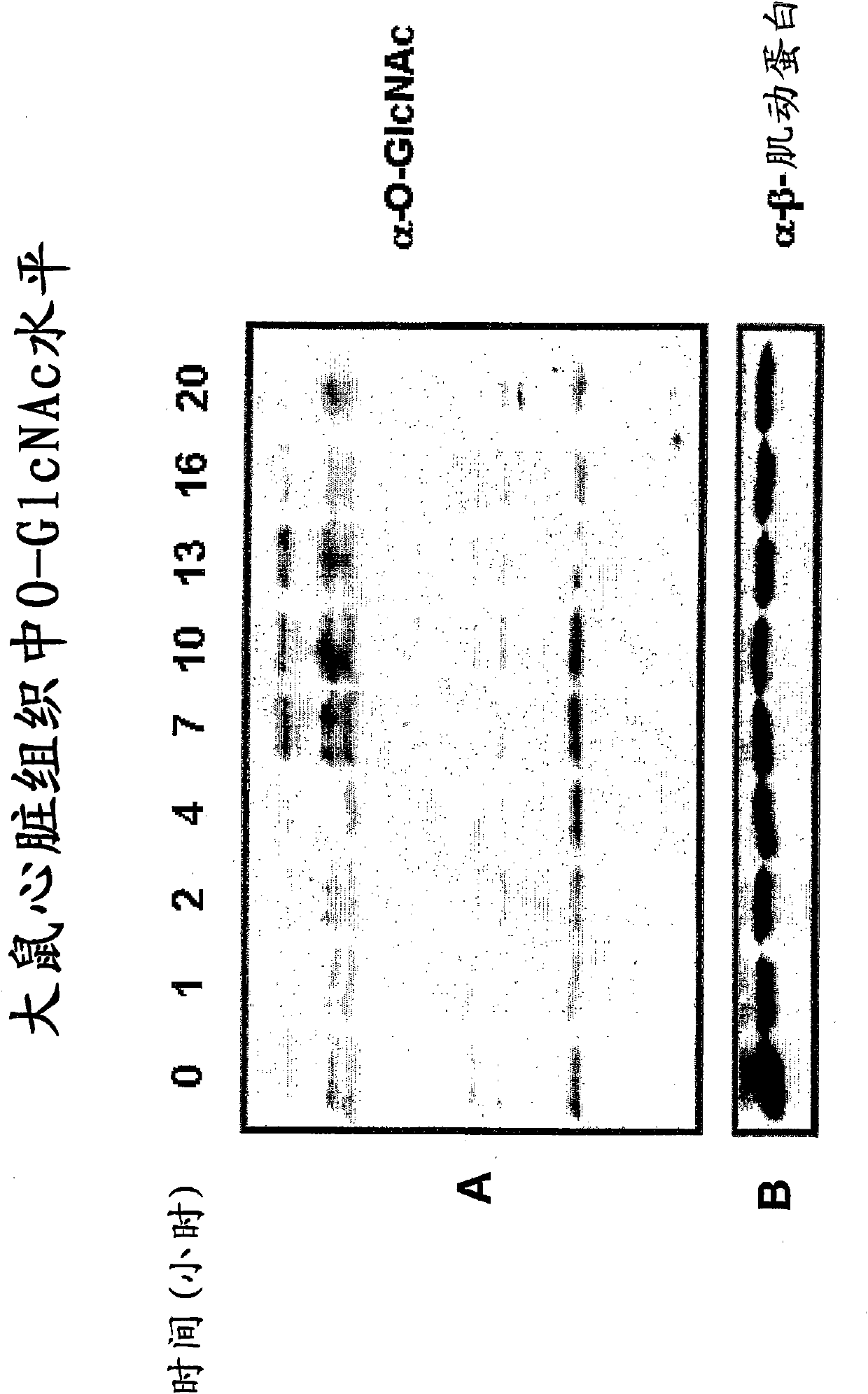
The application relates to an immoalditol compound for selectively inhibiting glycosidases, a prodrug thereof and a pharmaceutical composition comprising the compound or the prodrug The application also relates to the use of the immoalditol compound for treating diseases and disorders related to deficiency or overexpression of O-GlcNAcase, accumulation or deficiency of O-GlcNAc Such diseases and disorders include neurodegenerative diseases, tauopathy, cancers, and cardiac disorders
Owner:SIMON FRASER UNIVERSITY
Mulberry bark extract with glycosidase inhibiting function and preparation thereof
The invention discloses a white mulberry root-bark exact with the glucosidase inhibition function, a preparation method thereof and a quality control method. During the preparation of the white mulberry root-bark extract, extraction, concentration and centrifugalization are adopted, and the methods of three different types of anion-cation exchange resin columns, drying and so on are carried out to fully extract and highly concentrate the effective drugs; at the same time, the invention further provides the quality control method for carrying out the content measurement of the extract.
Owner:BEIJING WBL PEKING UNIV BIOTECH
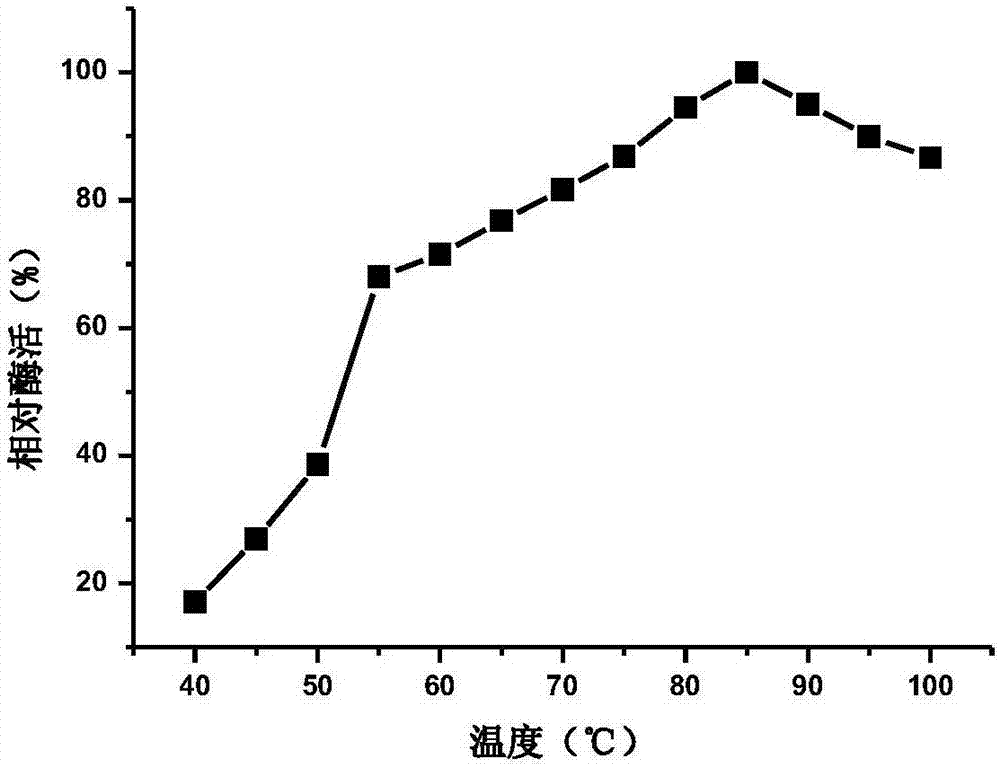
Beta-glucosidase, preparation method and application thereof
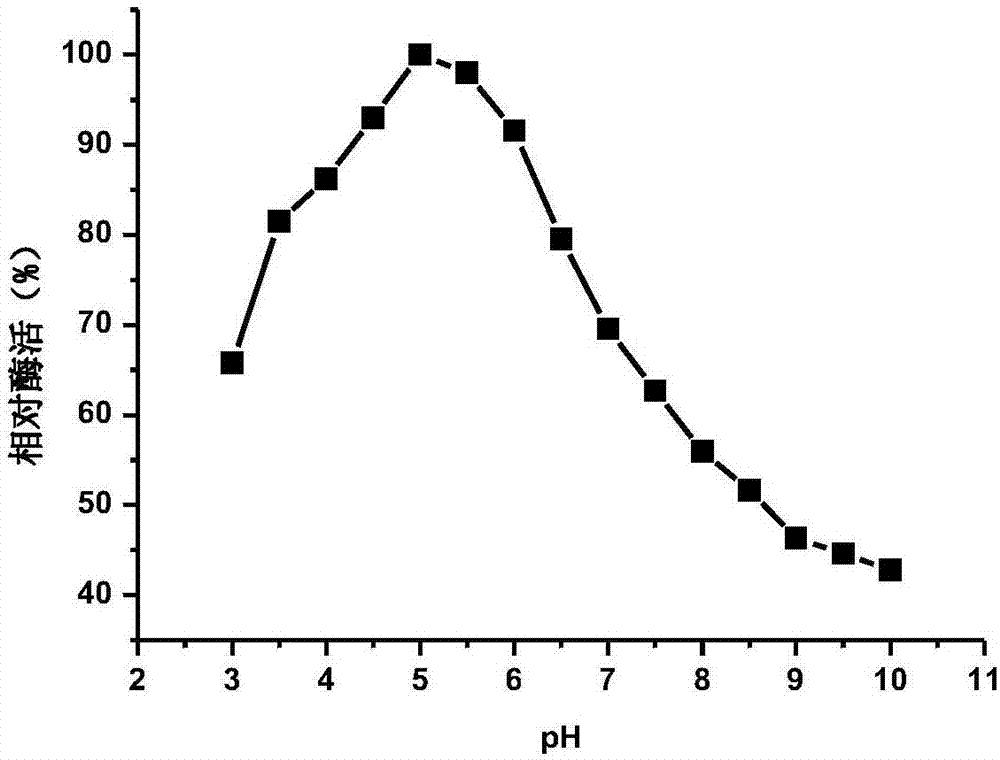
ActiveCN107384895AImprove thermal stabilityWide pH enzymatic rangeBacteriaFermentationHydrolysisAmino acid composition
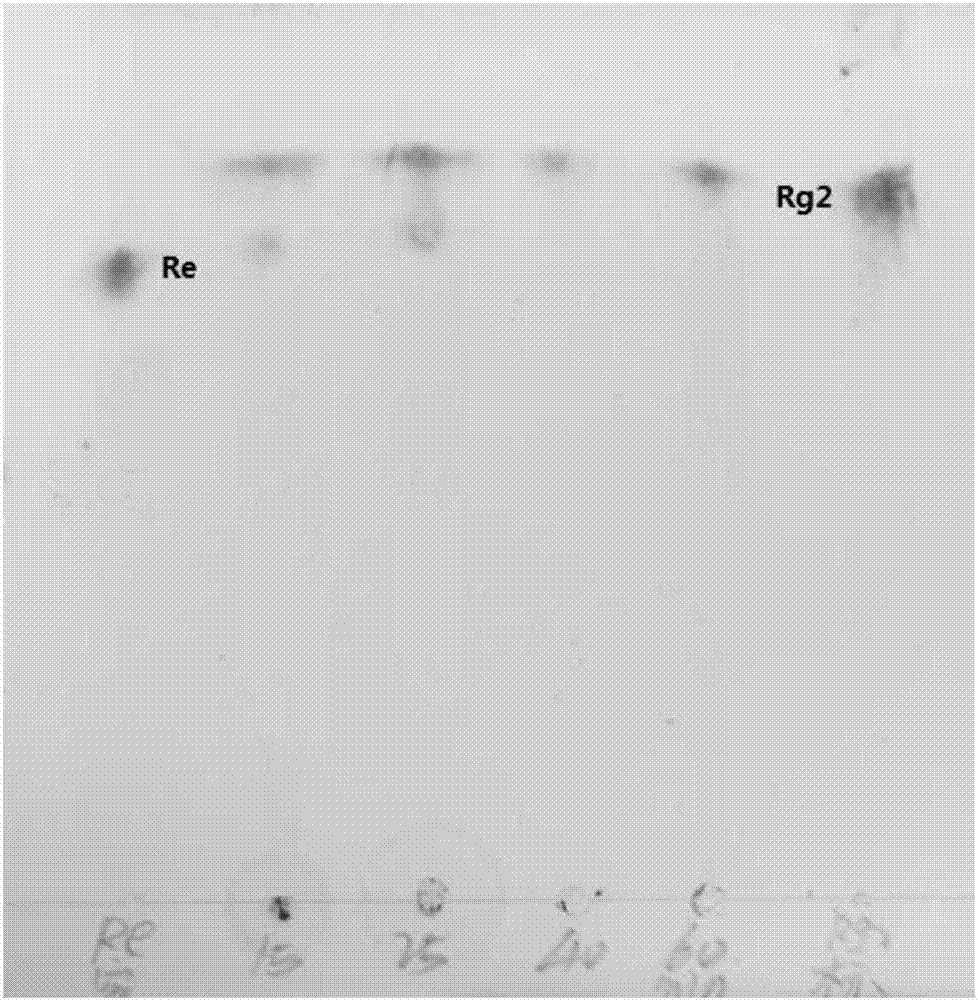
The invention provides a beta-glucosidase, a preparation method and application of beta-glucosidase, belonging to the field of genetic engineering technology and biological medicine. The beta-glucosidase is selected from the protein shown as (1) or (2) as follows: (1) the protein with amino acid sequence shown as SEQ ID NO.1 and (2) the protein with glycosidase activity and with one / more amino acid residue substituted and / or deleted and / or added in the amino acid residue sequence. The beta-glucosidase provided by the invention has high heat stability and wide pH enzymolysis scope, can be used for producing rare ginsenoside and mixture thereof, has relatively high hydrolysis capacity, is capable of converting the ginsenoside into the rare ginsenoside which has relatively high biological activity, is capable of being easily efficiently absorbed by a human body and has trace volume in the plants such as ginseng, and can be applied to various fields of food, medicine health, and the like.
Owner:JILIN AGRICULTURAL UNIV
Pharmaceutical composition having alpha-glucosidase inhibition activity, and applications thereof
ActiveCN104984346AGood hypoglycemic effectHypoglycemic effect achievedOrganic active ingredientsMetabolism disorderSide effectHypoglycemia
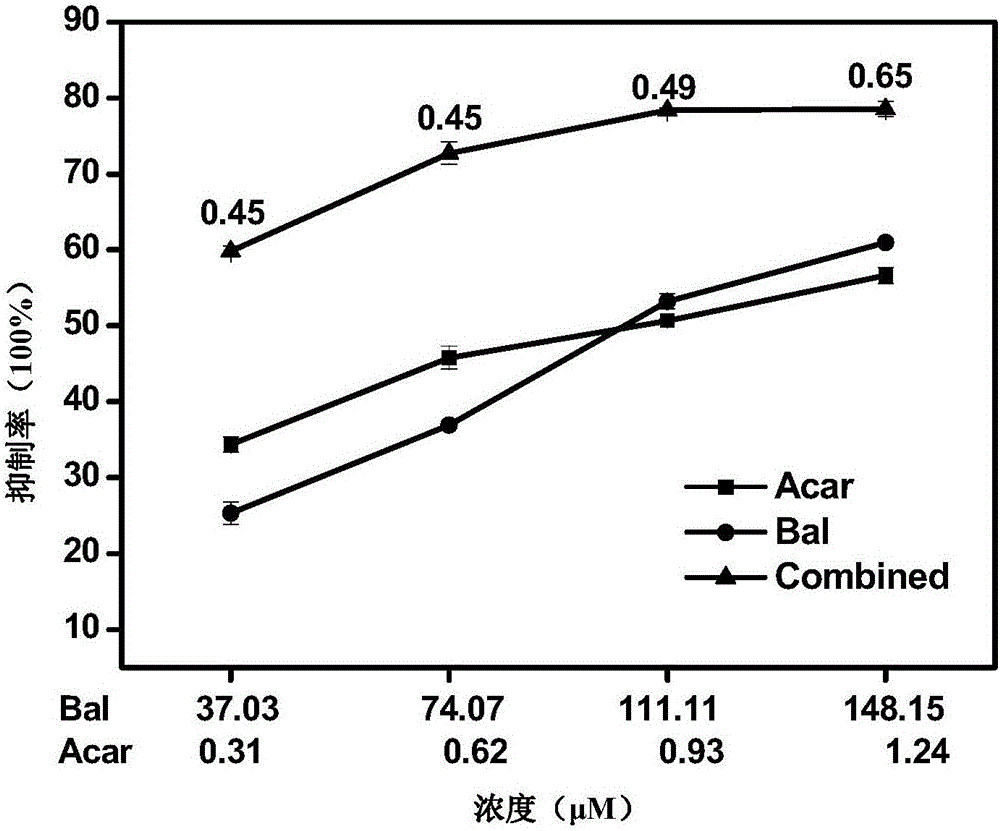
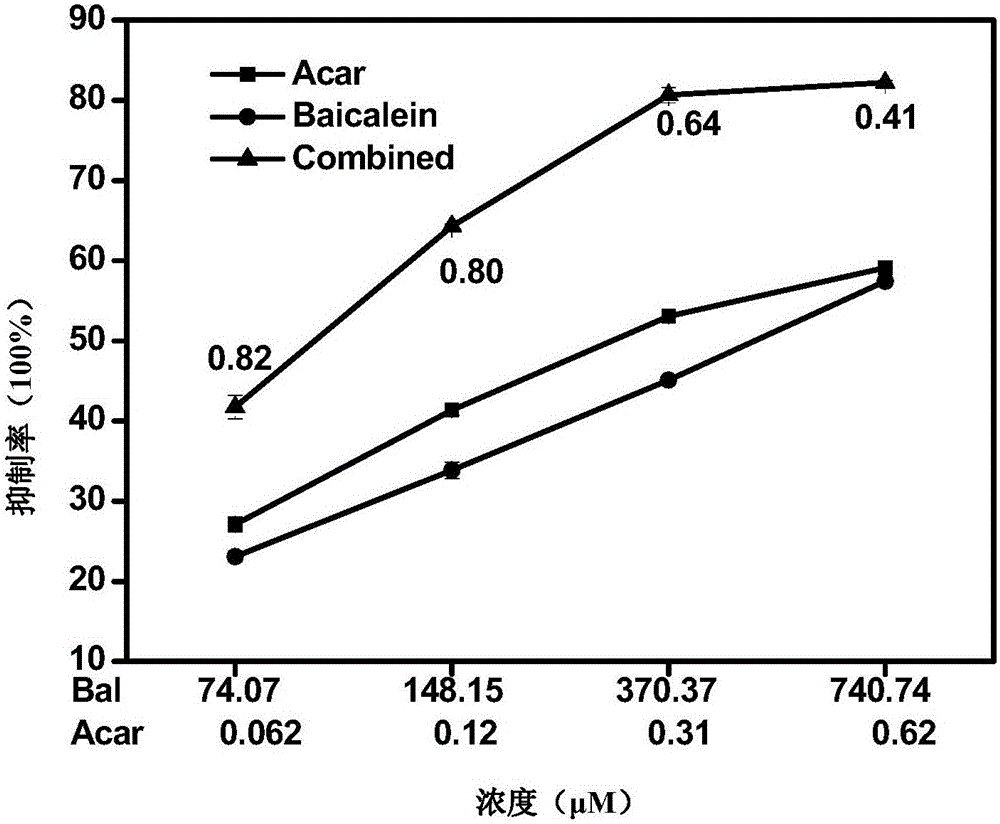
The invention relates to a pharmaceutical composition having alpha-glucosidase inhibition activity, wherein the pharmaceutical composition comprises a flavone compound and an alpha-glucosidase inhibitor, the flavone compound is at least one selected from a monomer such as baicalein, quercetin, luteolin, baicalein-7-O-glucoside and catechin, an organic salt of the monomer, and an inorganic salt of the monomer, and the alpha-glucosidase inhibitor is at least one selected from a monomer such as acarbose, voglibose and miglitol, an organic salt of the monomer, and an inorganic salt of the monomer. According to the present invention, the pharmaceutical composition can effectively reduce postprandial blood glucose, can inhibit the activity of alpha-glucosidase adopting starch, maltose and sucrose as substrates, and less uses the alpha-glucosidase inhibitor, such that the efficacy can be improved, the side effect of the alpha-glucosidase inhibitor can be effectively reduced, and hypoglycemia and other problems easily caused by drug combination are effectively solved.
Owner:上海皋鱼医药科技有限公司
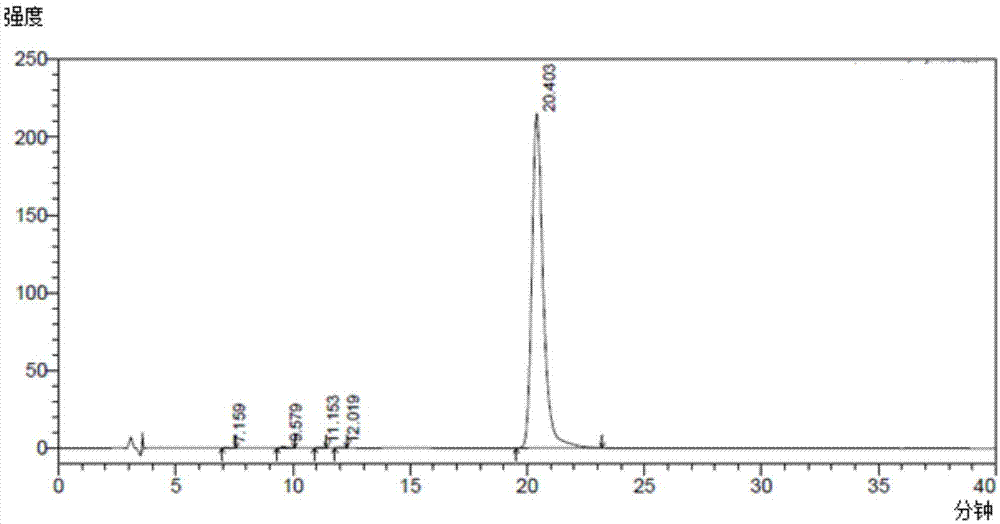
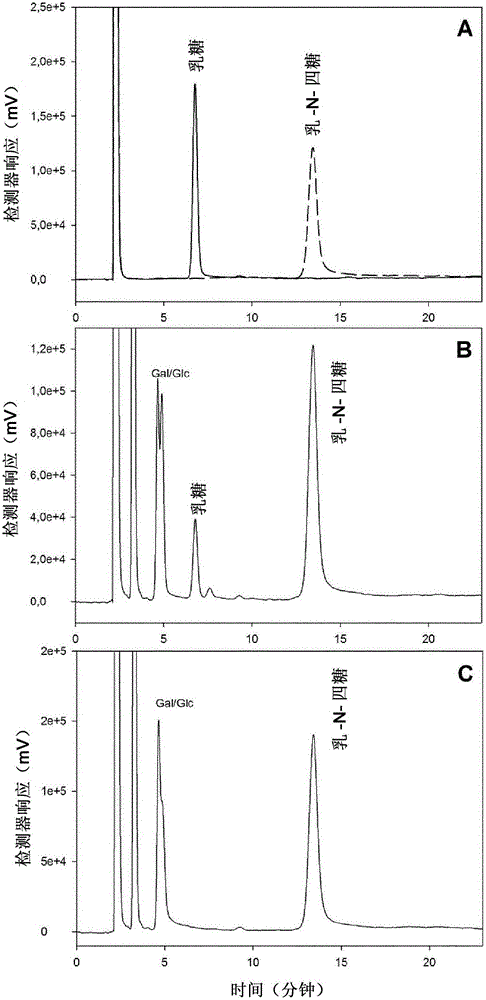
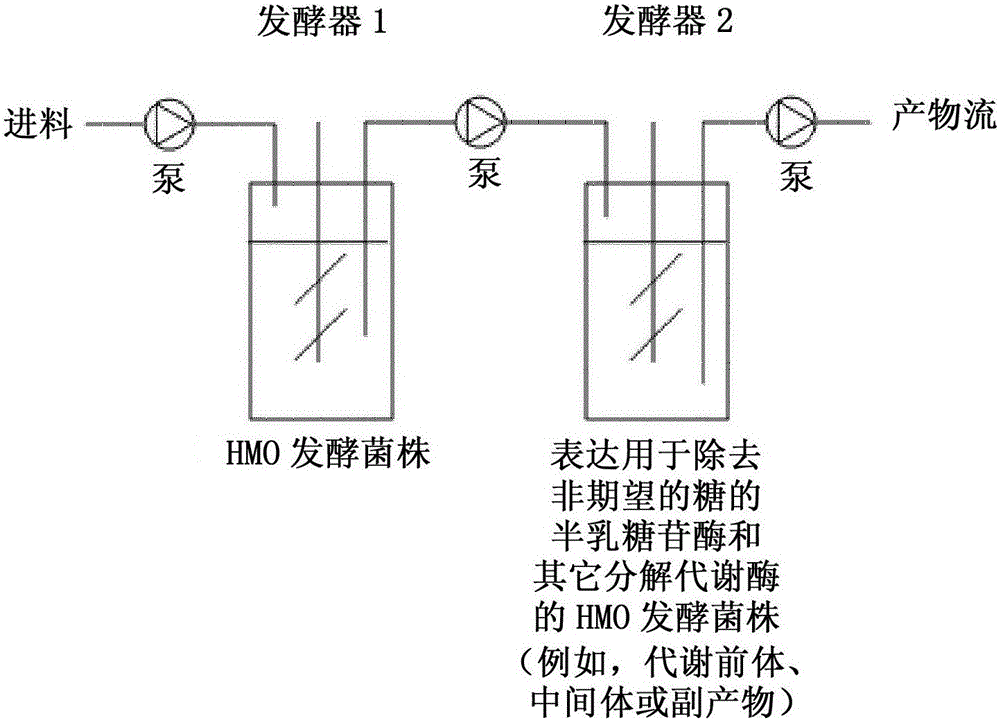
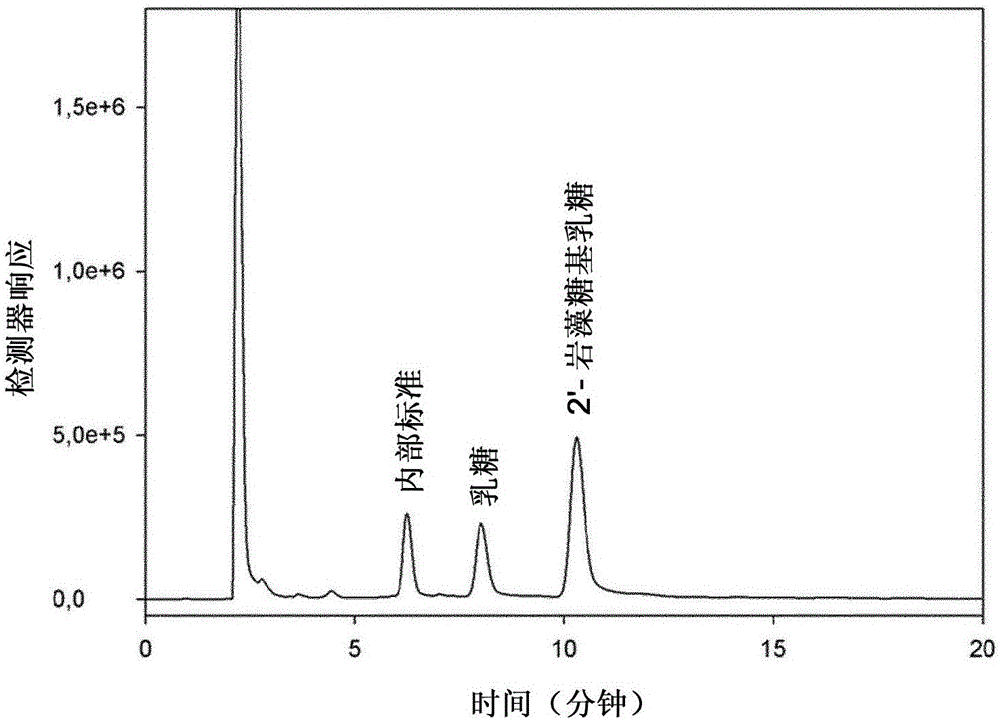
Production of oligosaccharides
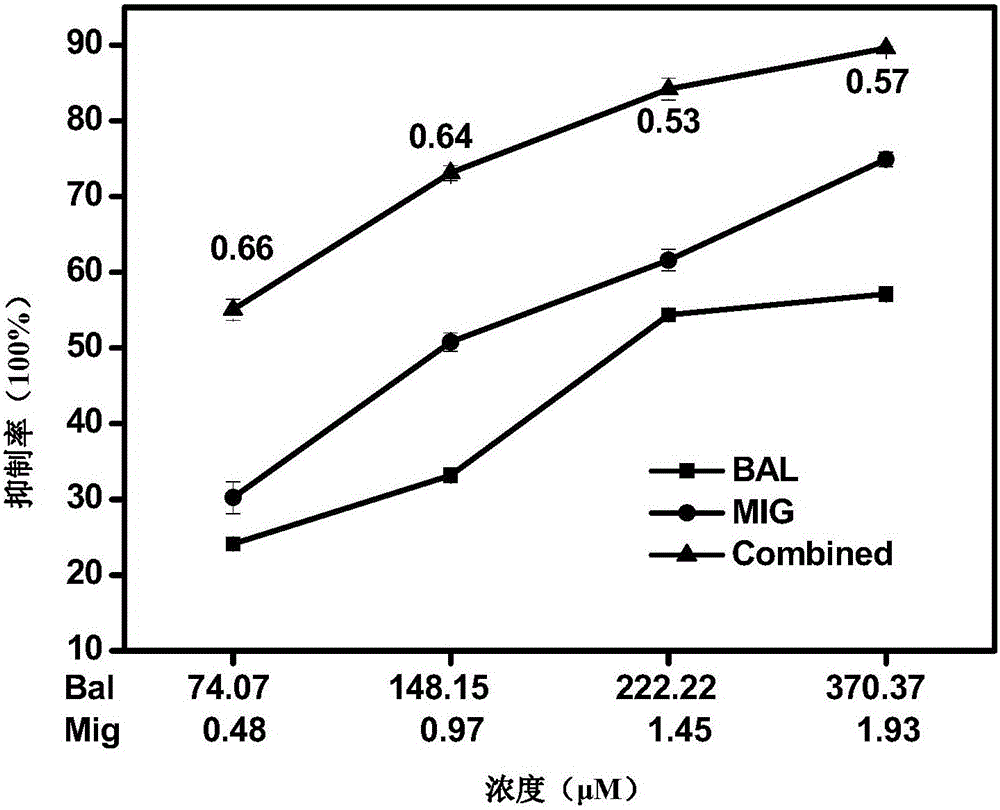
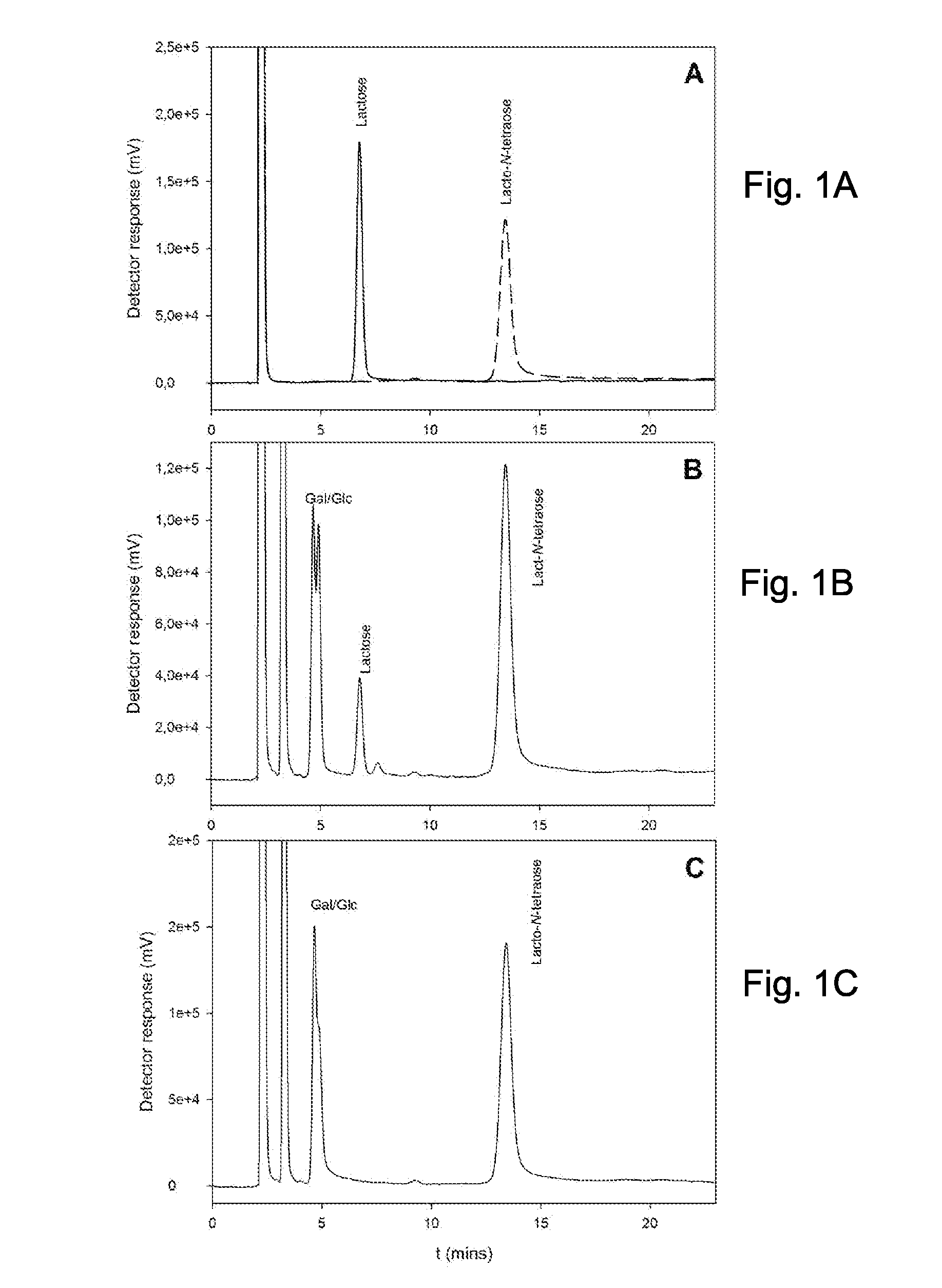
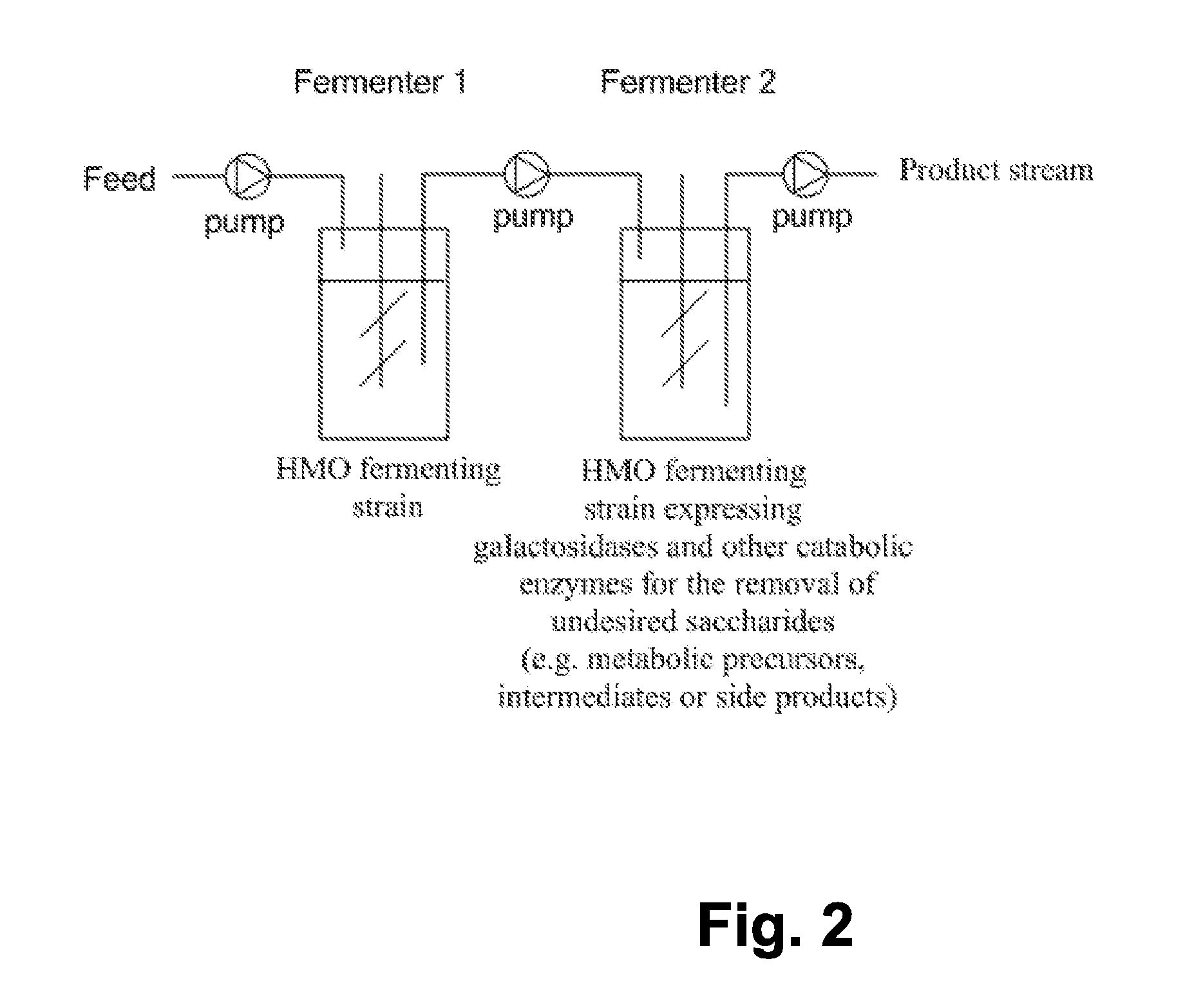
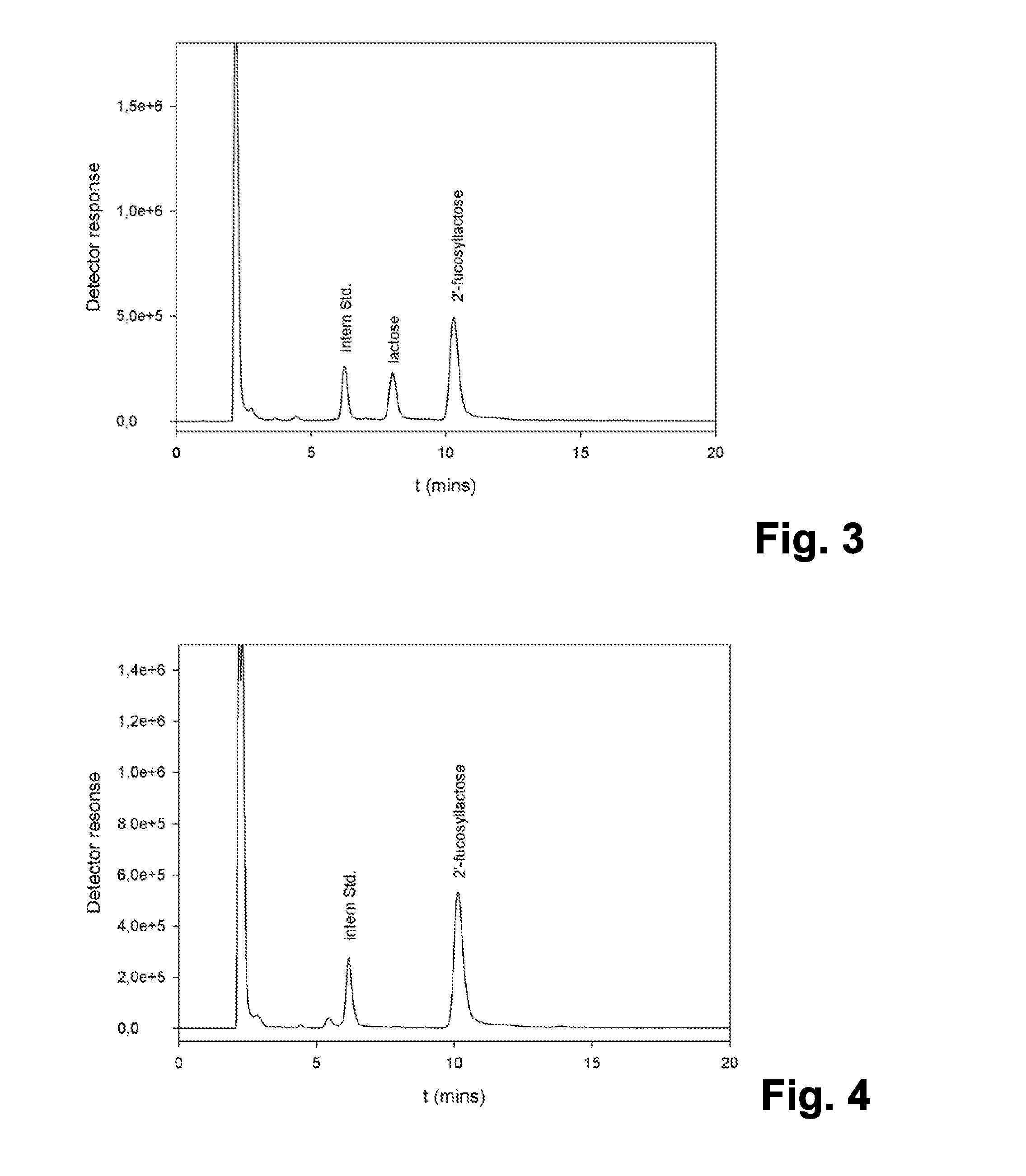
The present invention relates to the use of one or more glycosidases in the process for the production and / or purification of a produced desired oligosaccharide. The process is preferably a microbial fermentation process using a host microorganism, which may also comprise nucleic acids expressing sugar catabolic pathway proteins suitable for the degradation of saccharides otherwise hindering the purification of the desired oligosaccharide.
Owner:CHR HANSEN HMO GMBH
Extraction method of natural anthocyanidin active aglycone
The invention relates to an extraction method of natural anthocyanidin active aglycone, which belongs to the technical field of bioengineering. The method comprises the following steps: performing classification, crushing, water absorption, freezing, heating for unfreezing, and filtering for juice preparation of anthocyanidin-containing plants, performing biodegradation of anthocyanin by glycosidase for decomposing anthocyanin glucosidic bonds, performing combination reaction precipitation / suspension by an anionic surfactant, degrading the precipitates / suspensions by bacterial microbe, and performing sterilization, enzyme and bacterium filtration, and freeze drying of the anthocyanidin aqueous solution. The method of the invention can obtain cationic anthocyanidin, instead of anthocyanin, with a real content of more than 95%. The cationic anthocyanidin prepared by the method of the invention needs no degradation by microbe in human body intestinal tracts, can be absorbed and used directly by human body, or can directly enter human body blood, and is applicable to medicine and health care.
Owner:耿兆翔
Sterilized xenograft tissue
InactiveUS20100196870A1Improve integrityAdvantageous implantabilityHeart valvesDead animal preservationHuman animalDisaccharidase
The invention provides an article of manufacture comprising a substantially non-immunogenic xenograft for implantation into humans. The invention also provides methods for preparing a xenograft by removing at least a portion of a soft tissue from a non-human animal to provide a xenograft; washing the xenograft in saline and alcohol; subjecting the xenograft to cellular disruption treatment; treating the xenograft with crosslinking agents, and digesting the xenograft with a proteoglycan-depleting factor and / or glycosidase. The invention further provides a method for sterilizing xenograft material, having the steps of obtaining substantially non-immunogenic xenograft material; treating the xenograft material with at least one crosslinking agent; and subjecting the crosslinked xenograft material to radiation treatment.
Owner:APERION BIOLOGICS
Selective glycosidase inhibitors, methods of making inhibitors, and uses thereof
Owner:SIMON FRASER UNIVERSITY
Novel Chalcone Derivatives, Pharmaceutically Acceptable Salt, Method for Preparation and Uses Thereof
InactiveUS20090252694A1Easy to useStrong enzyme inhibitory activityBiocideCosmetic preparationsDiseaseM-aminoacetophenone
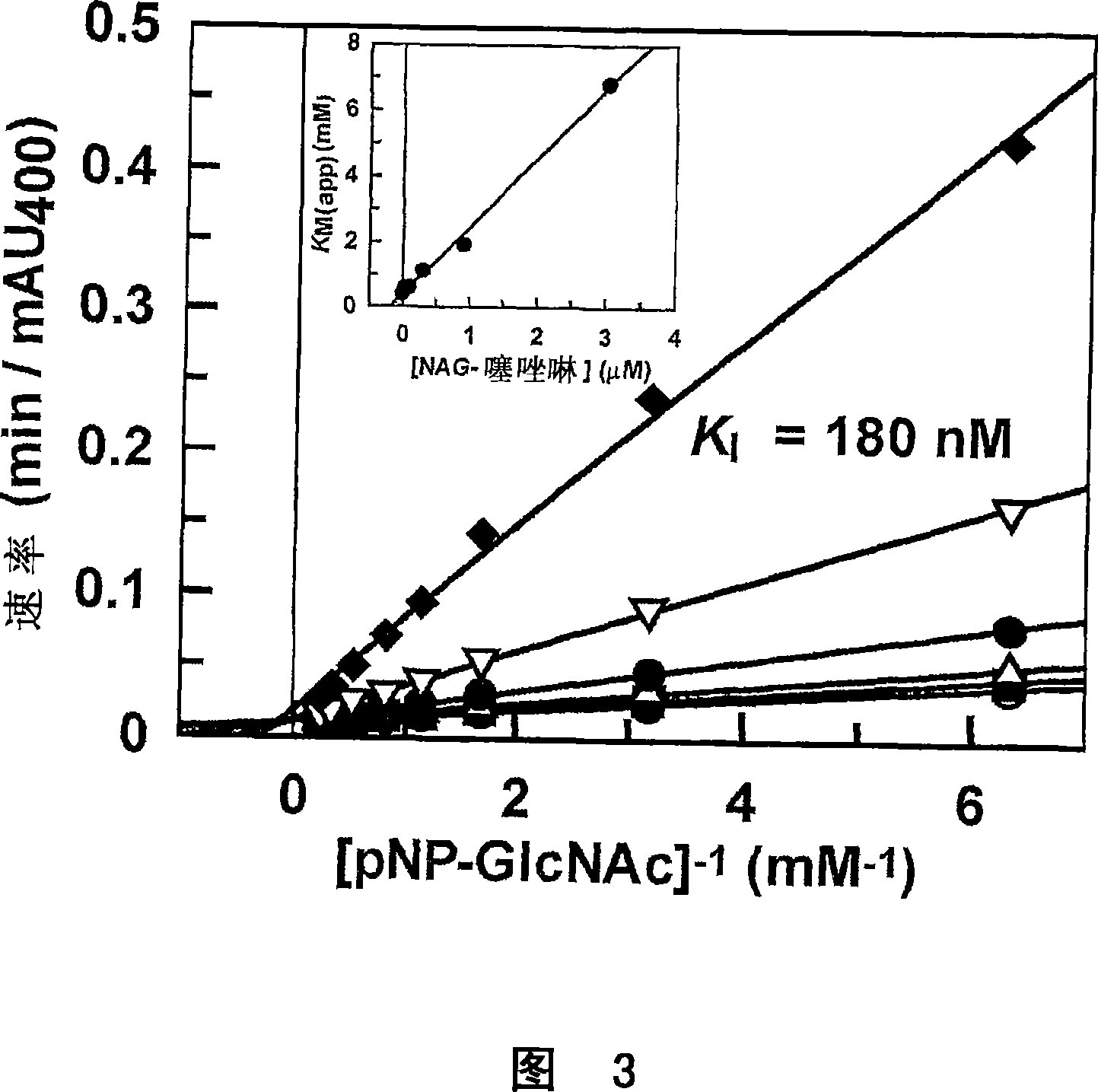
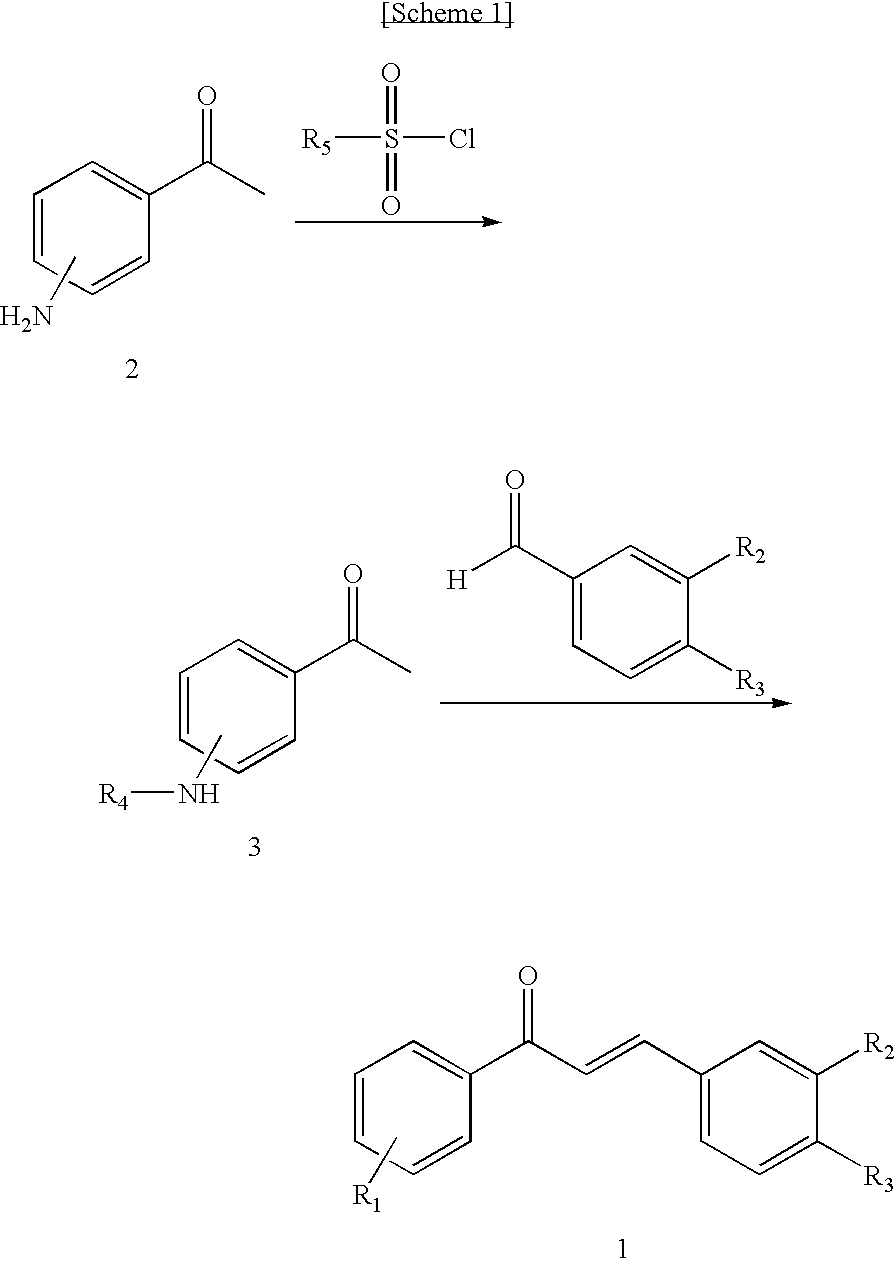
Disclosed relates to a novel chalcone derivative, pharmaceutically acceptable salt thereof, a method for preparing the same and uses thereof, the chalcone derivative being readily obtained through the steps of: reacting aminoacetophenone with sulfonylchloride under the presence of an appropriate salt; and reacting the compound prepared in the above step with hydroxybenzaldehide under the presence of an appropriate catalyst. The chalcone derivative of formula 1 in accordance with the present invention having strong enzyme inhibitory activities for glycosidase can be effectively used in preventing and treating various diseases induced by glycosidase, and the chalcone derivative of the invention having tyrosinase and melanin synthesis inhibitory activities can be effectively used as a skin-whitening compound.
Owner:JCN FARM CO LTD
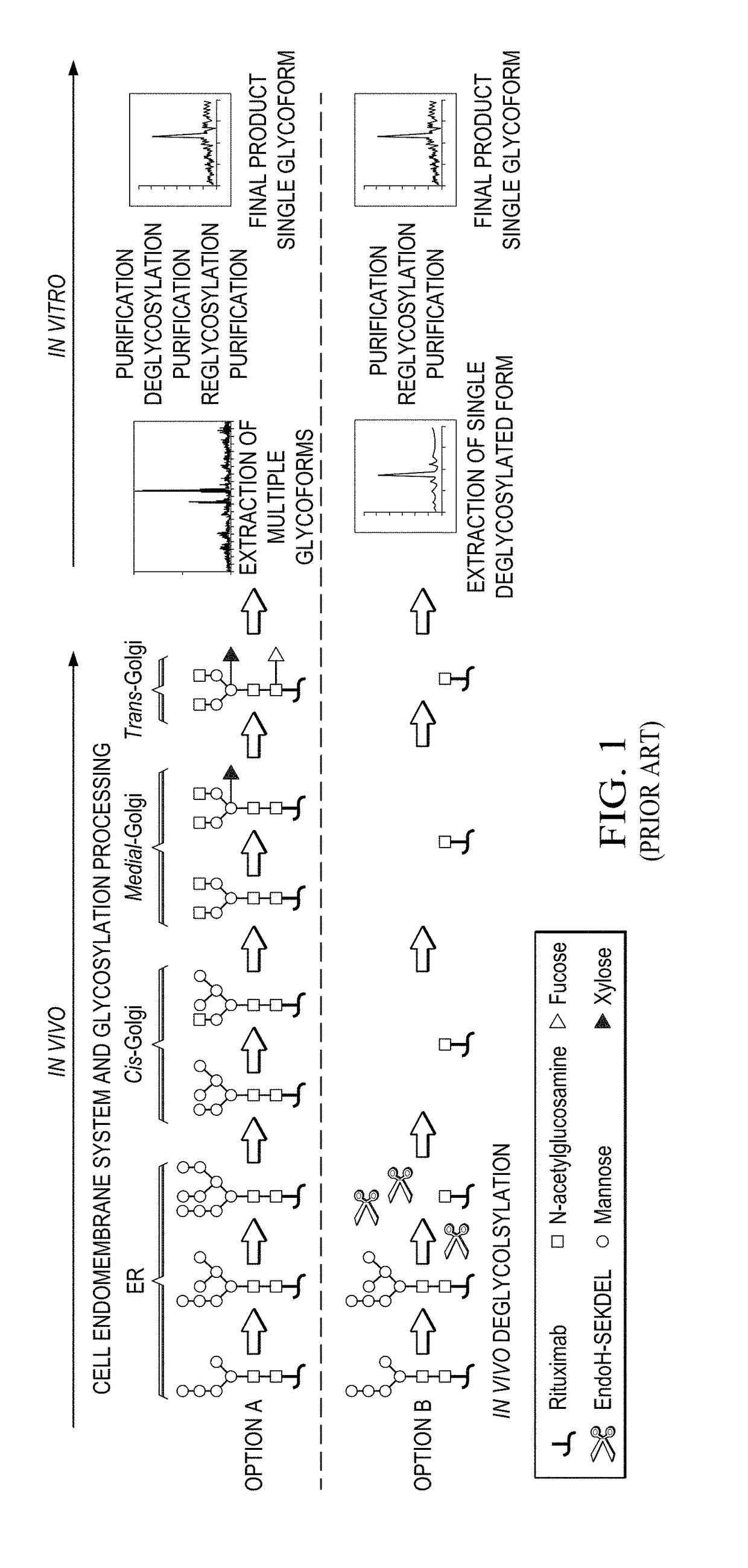
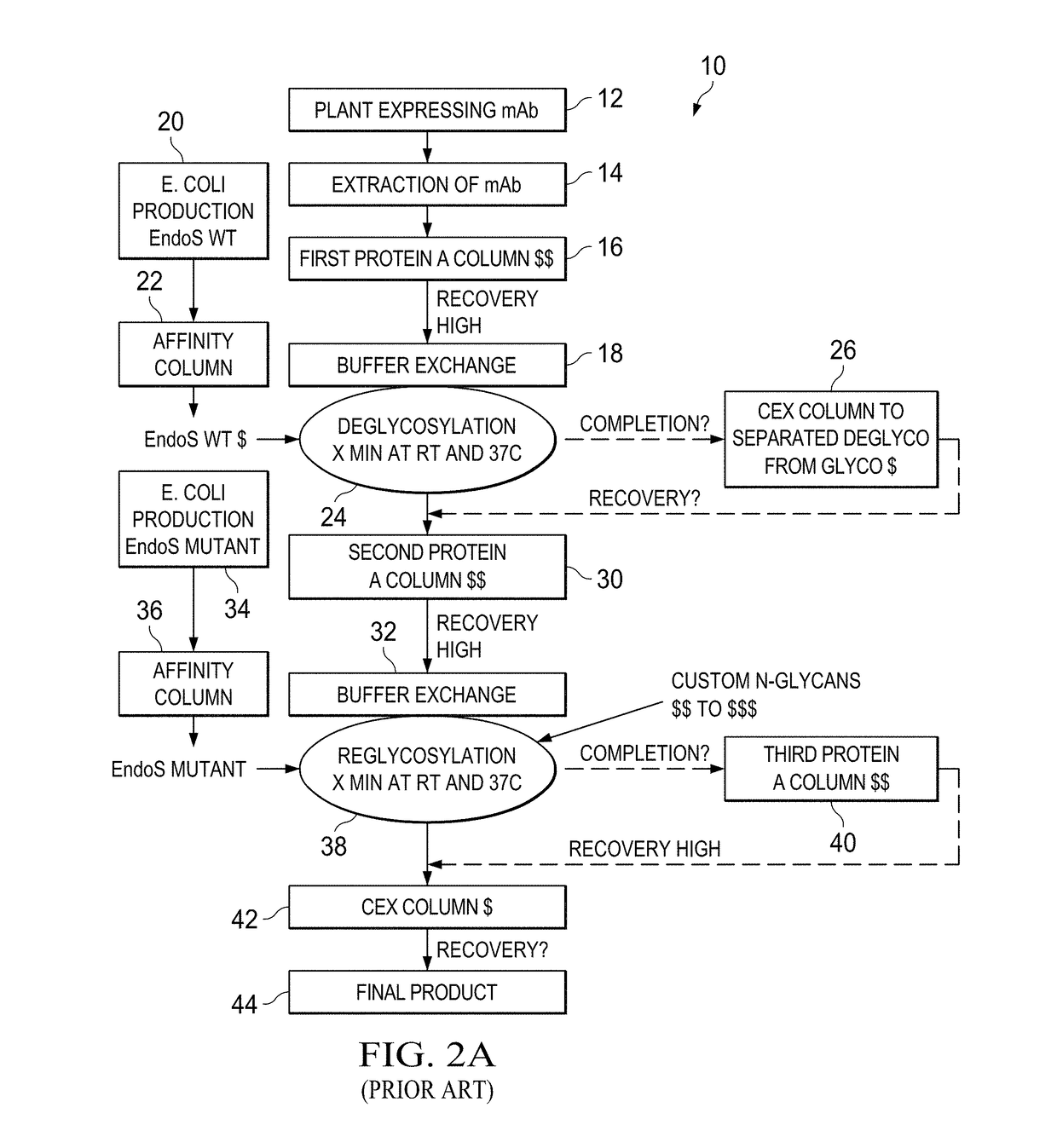
Method for in vivo production of deglycosylated recombinant proteins used as substrate for downstream protein glycoremodeling
The present invention includes compositions and methods of reducing the glycosylation of proteins comprising: obtaining a cell that expresses one or more proteins that comprise one or more glycosylation sites and are glycosylated; expressing in the cell one or more glycosidases that cleaves one or more glycosyl groups from the one or more proteins; and isolating the one or more proteins with reduced glycosylation from the cell.
Owner:CALIBER BIOTHERAPEUTICS
N-containing saccharides and method for the synthesis of N-containing saccharides from amino-deoxy-disaccharides and amino-deoxy-oligosaccharides
InactiveUS6183994B1High selectivityHigh catalytic activitySugar derivativesFermentationDisaccharidaseOligosaccharide synthesis
Synthesis of an amino-disaccharide, amino-oligosaccharide or a derivative thereof, characterized in that a monosaccharide, a disaccharide, an oligosaccharide, a glycoside or a derivative thereof, in the presence of a glycosidase as catalyst, is reacted with an amino-deoxy-saccharide or a glycoside or derivative thereof, and that the amino-saccharide is optionally isolated from the product mixture directly or after chemical / enzymatic modification.
Owner:GLYCOREX TRANSPLANTATION AB PUBL
Applications of four kaurane diterpene compounds in preparation of glycosidase inhibitor medicines
ActiveCN104083348APotent inhibitory activityInhibitory activityMetabolism disorderEster active ingredientsGlycosidase activityMonomer
The invention provides applications of four kaurane diterpene compounds in preparation of glycosidase inhibitor medicines. The four compounds capable of highly inhibiting alpha-glycosidase activity are natural compounds which are high in safety and capable of rapid natural degradation without residue in the environment. The four compounds are obtained by separation from wedelia trilobata, and other plant materials. The plant materials are rich in source. A preparation process is easy in operation. Monomers of the four compounds are stable and liable to store. The alpha-glucosidase inhibition activity of the four compounds is obviously higher than or equivalent to that of acarbose that is a clinic medicine. The four compounds are extremely likely developed into effective and safe alpha-glucosidase inhibitor medicines used for preventing and treating type II diabetes, and have good prospects.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Preparation method of spermacoce latifolia triterpenoids and application of spermacoce latifolia triterpenoid in preparation of glycosidase inhibitor medicine
InactiveCN104490894APotent alpha-glucosidase activityStrong preventionOrganic active ingredientsMetabolism disorderDisaccharidaseGlycosidase inhibitor
The invention provides a preparation method of spermacoce latifolia triterpenoids and an application of the spermacoce latifolia triterpenoids in preparation of a glycosidase inhibitor medicine. Six compounds for intensively inhibiting the activity of alpha-glycosidase provided by the invention are natural compounds which are high in safety, and can be naturally degraded rapidly without a residue in the environment. The compounds can be separated from plant materials such as spermacoce latifolia; the plant materials are abundant in source; and the preparation process is easy to operate. Monomers of the six compounds are relatively stable and easy to store; the alpha-glycosidase inhibiting activity is obviously higher than that of clinical medicine acarbose; and the compounds are extremely likely to be further developed into the effective and safe alpha-glycosidase inhibitor medicines for preventing and treating type 2 diabetes mellitus, and have relatively good prospects.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Icaritin preparation method
ActiveCN104711301AHigh purityEfficient removalOrganic chemistryFermentationDisaccharidaseEnzymatic hydrolysis
The invention provides an icaritin preparation method which comprises the following steps: a, performing the enzymatic hydrolysis reaction of an epimedium extract under the action of Beta-glycosidase; b, filtering an enzymatic hydrolysis reaction product obtained in step a; c, dissolving filter cake obtained after filtration into acetone; and d, adding water to an acetone solution, performing reflux dissolution, keeping the solution standing, performing filtration, and collecting a crystal substance to obtain icaritin. According to the icaritin preparation method, the yield of icaritin is up to 20% relative to the epimedium extract, and the purity of icaritin is up to 99.8%.
Owner:BEIJING SHENOGEN PHARMA GRP
Production of oligosaccharides
The present invention relates to the use of one or more glycosidases in the process for the production and / or purification of a produced desired oligosaccharide. The process is preferably a microbial fermentation process using a host microorganism, which may also comprise nucleic acids expressing sugar catabolic pathway proteins suitable for the degradation of saccharides otherwise hindering the purification of the desired oligosaccharide.
Owner:CHR HANSEN HMO GMBH
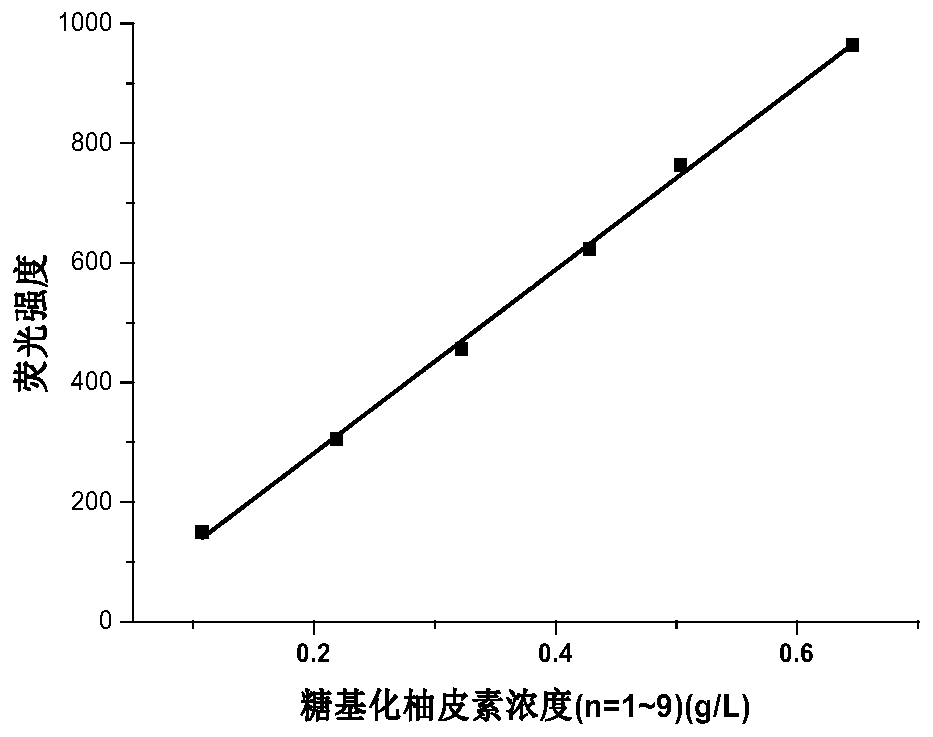
Preparation method and application of glycosylated hesperetin
PendingCN110105409AImprove solubilitySolve the problem of decomposition and absorptionOrganic active ingredientsSugar derivativesSolubilityDisaccharidase
The invention provides a preparation method of glycosylated hesperetin. The preparation method comprises the specific steps that raw materials and a glycosyl donor are put into a reaction buffer solution; after the materials and the solution are mixed evenly, glycosidase and glycosyl transferase are added in a reaction system, wherein the raw materials are hesperidin, hesperetin, an extract or a conversion product of neohesperidin and a derivative thereof. According to the prepared glycosylated hesperetin, 1-9 glycosyl groups are added on a flavonoid parent, so that the solubility of the hesperetin is greatly improved, the problem of poor solubility of the hesperetin is solved, the problems about decomposition and absorption of the hesperetin in the human body are also solved, and the bioavailability of the hesperetin in citrus is greatly improved.
Owner:FOSHAN GOLDEN HEALTH TECH CO LTD
Aspergillus niger JH-2 and application to biotransformation and synthesis of asiatic acid
ActiveCN104357332AIncrease contentWon't breakFungiMicroorganism based processesDrug biotransformationDisaccharidase
The invention provides a glycosidase producing strain, that is, Aspergillus niger JH-2 and an application of the Aspergillus niger JH-2 to biotransformation and increasing of the content of asiatic acid in centella asiatica. The Aspergillus niger is a common strain in the fermentation industry and is non-toxic, harmless and safe to use, the converting culture medium is simple in component, only five salt components are needed, and the production cost is low; the Aspergillus niger JH-2 grows fast and is high in infectious microbe resistance, easy to culture and stable in batch. Dry centella asiatica powder is taken as a raw material and fed into the culture medium for conversion, asiaticoside separation and extraction operations are omitted, separation and purification steps of enzyme are also omitted, the process is simple, and industrial application is facilitated; the content of the asiatic acid can be increased by 3 times at most after the centella asiatica powder is subjected to biotransformation treatment.
Owner:ZHEJIANG UNIV OF TECH
Method for applying pretreatment of amylase to swine waste marsh gas fermentation
InactiveCN101289266AShorten fermentation timeIncrease productionBio-organic fraction processingBiological substance pretreatmentsDisaccharidaseMethane yield
Owner:KUNMING UNIV OF SCI & TECH
Preparation method and application for glycosylated naringenin
PendingCN110205351AImprove solubilitySolve the problem of decomposition and absorptionOrganic active ingredientsSugar derivativesSolubilityDisaccharidase
The present disclosure provides a preparation method for glycosylated naringenin, wherein the preparation method is characterized by particularly comprising the steps: putting raw materials and glycosyl donors into a reaction buffer solution, mixing the feed liquid evenly, and then adding glycosidase and glycosyltransferase into the reaction system, wherein the raw materials are naringin and naringenin extracts or conversion products and derivatives thereof. The glycosylated naringenin prepared by the method can have the solubility of naringenin greatly improved by adding 1-9 glycosyl groups to a flavone matrix, not only solves the problem of poor solubility of naringenin, but also solves the problem of decomposition and absorption of naringenin in a human body, and greatly improves the bioavailability of naringenin.
Owner:FOSHAN GOLDEN HEALTH TECH CO LTD
Deglycosylated holothurian secondary saponin and preparation method thereof
The invention provides deglycosylated holothurian secondary saponin and a preparation method of the deglycosylated holothurian secondary saponin. For the deglycosylated holothurian secondary saponin, two rear glycosyl radicals on carbohydrate chains of holothurian A (HA) and Echinoside A (EA) are removed through the enzymolysis of glycosidase, and thus the secondary saponin is obtained. The preparation method of the secondary saponin comprises the following steps: alcohol digestion, crude extraction adopting macroporous resin, and purification adopting a silicagel column, thus HA and EA are obtained, finally, enzymolysis is carried out by adopting glycosidase, and thus deglycosylated HA secondary saponin and EA secondary saponin are obtained respectively. According to the deglycosylated holothurian secondary saponin and the preparation method of the deglycosylated holothurian secondary saponin, through the used glycosidase, holothurian saponin forms secondary saponin through enzymolysis under the mild condition, so that the toxicity of holothurian saponin is reduced, and the biological activity of holothurian saponin is improved; meanwhile, glycosidase is adopted for enzymolysis of holothurian saponin, so that the side reaction and by-products brought by micro-biological degradation or degradation adopting crude enzyme extracted from microbes are avoided, the purity and yield of the enzymolysis products are improved, the single product can be obtained, and the purity can achieve 100%.
Owner:OCEAN UNIV OF CHINA
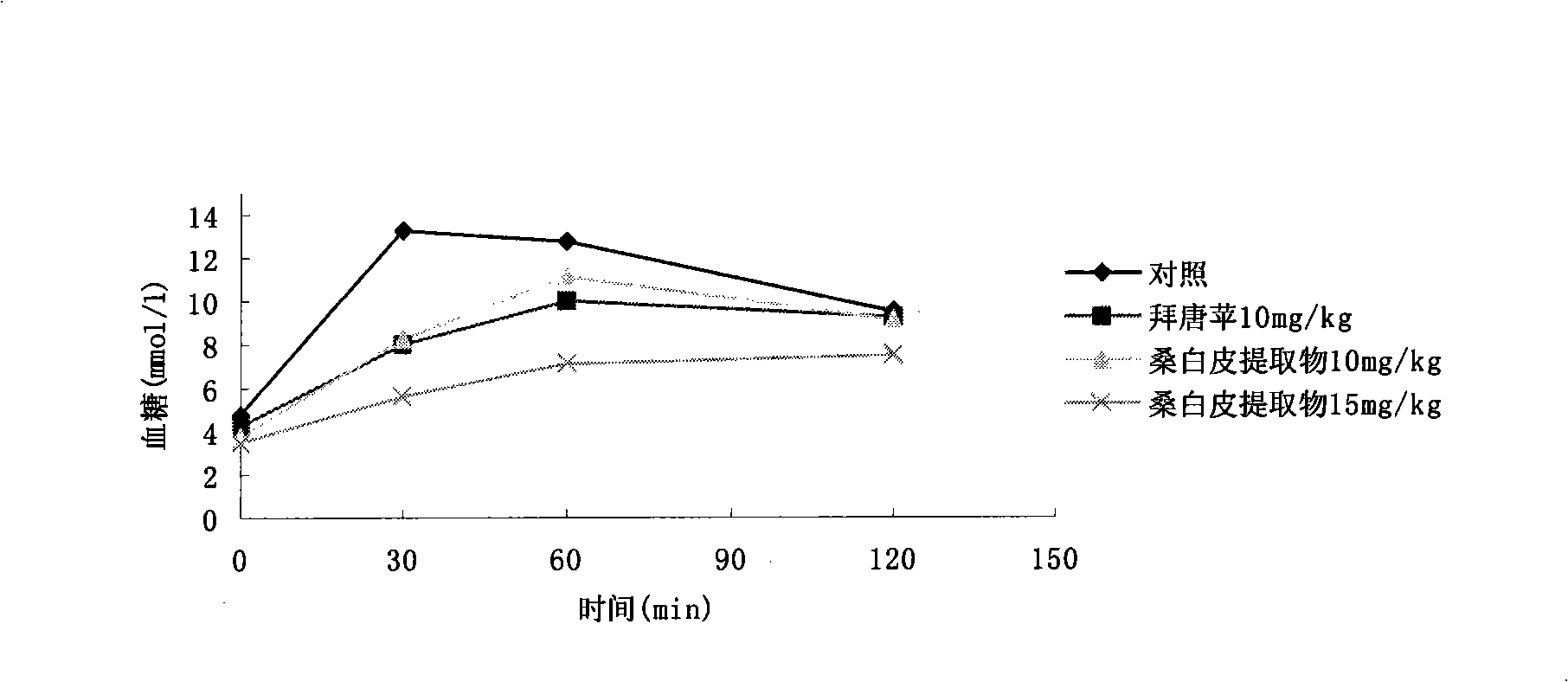
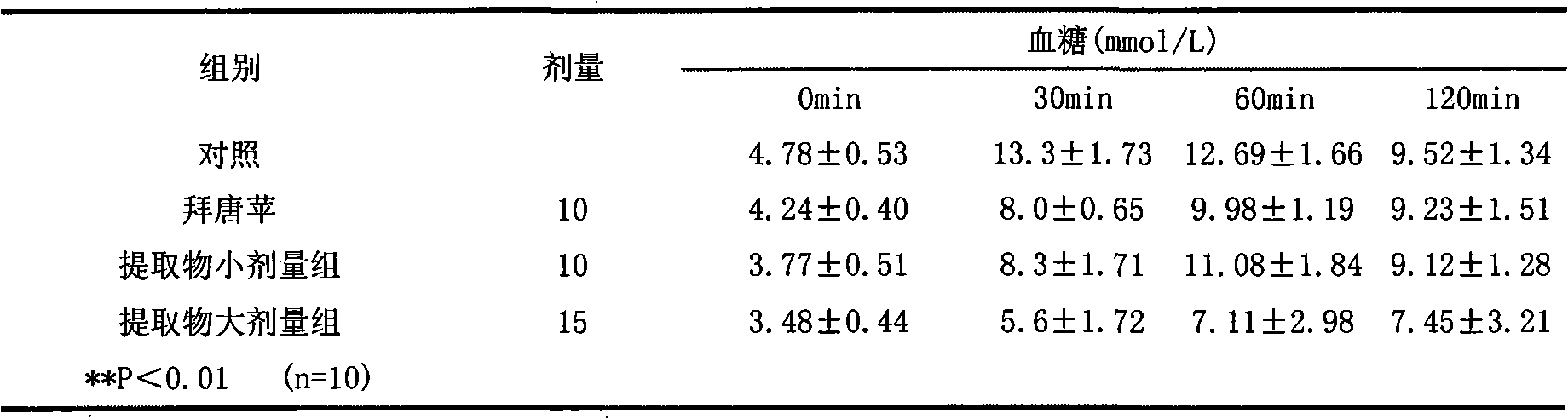
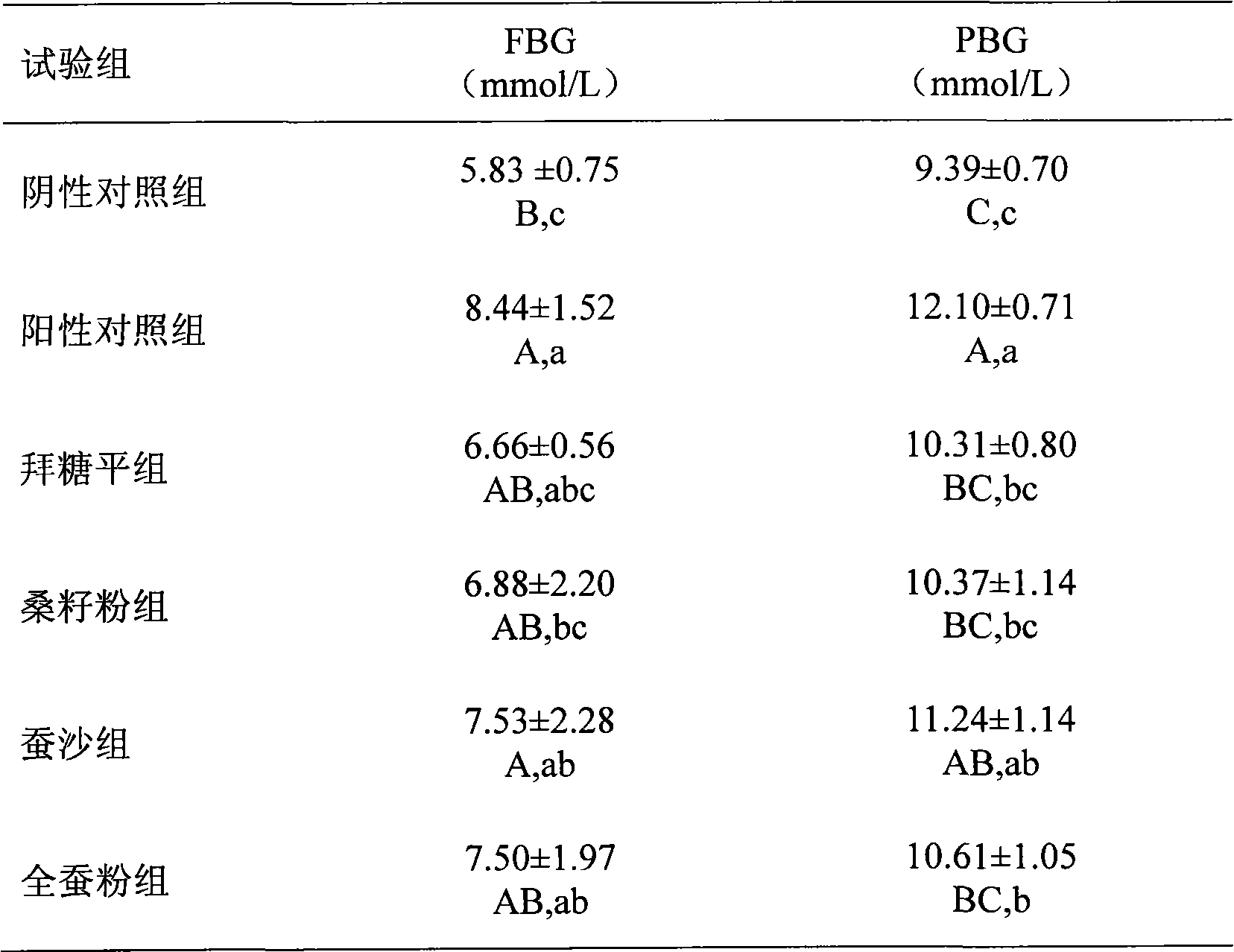
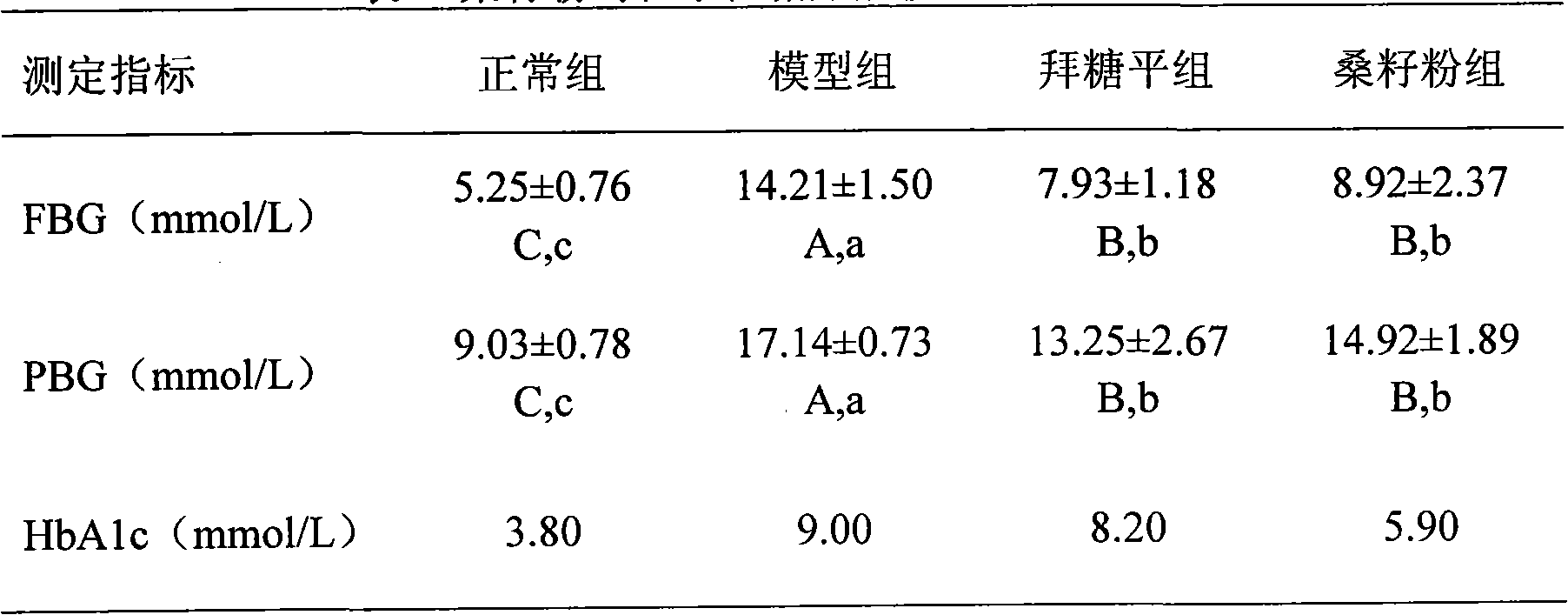
Blood glucose adjusting health food taking mulberry seeds as effective component
InactiveCN101773248AGood hypoglycemic effectGive full consideration to balanceFood preparationHigh concentrationSide effect
The invention relates to a blood glucose adjusting health food taking mulberry seed powder as an effective component, which comprises the following components in parts by weight: 4-99.9 parts of mulberry seed powder, 0-10 parts of flavor agent and 0.1-96 parts of accessory substance. The mulberry seed contains 1-deoxynojirimycin (DNJ) with high concentration, can inhibit the activity of small intestine alpha-glycosidase and can adjust blood glucose, thereby having obvious effect on treating human diabetes and obesity. In the invention, the mulberry seed powder is combined with the flavor agent, the accessory substance and natural products are processed into different types of health foods with the function of adjusting human blood glucose without any toxic or side effect, the safety is high, the taste flavor is good, the edibility is strong so as to control the blood glucose of a diabetic patient in the normal diet process, and simultaneously, natural resources are developed.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com