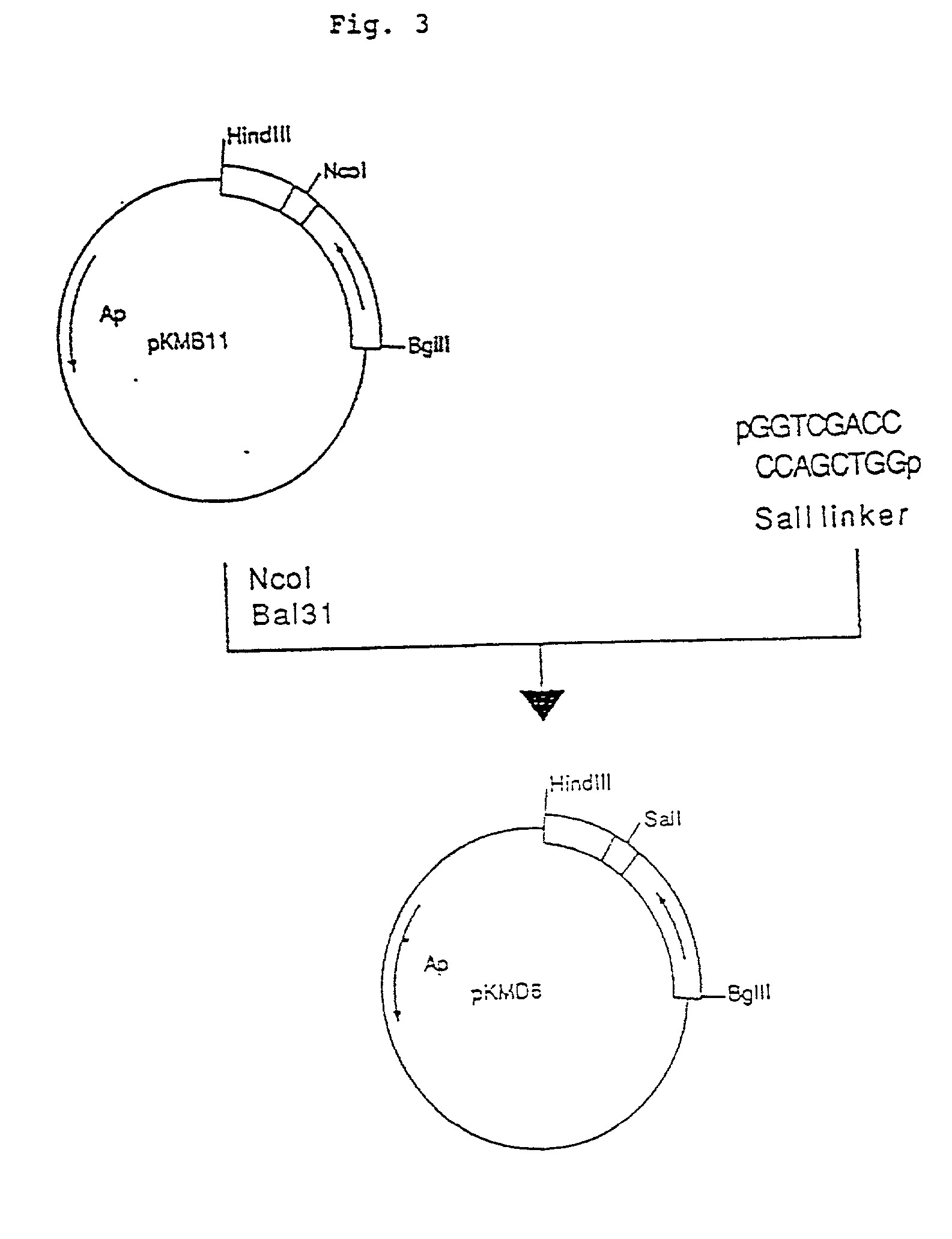
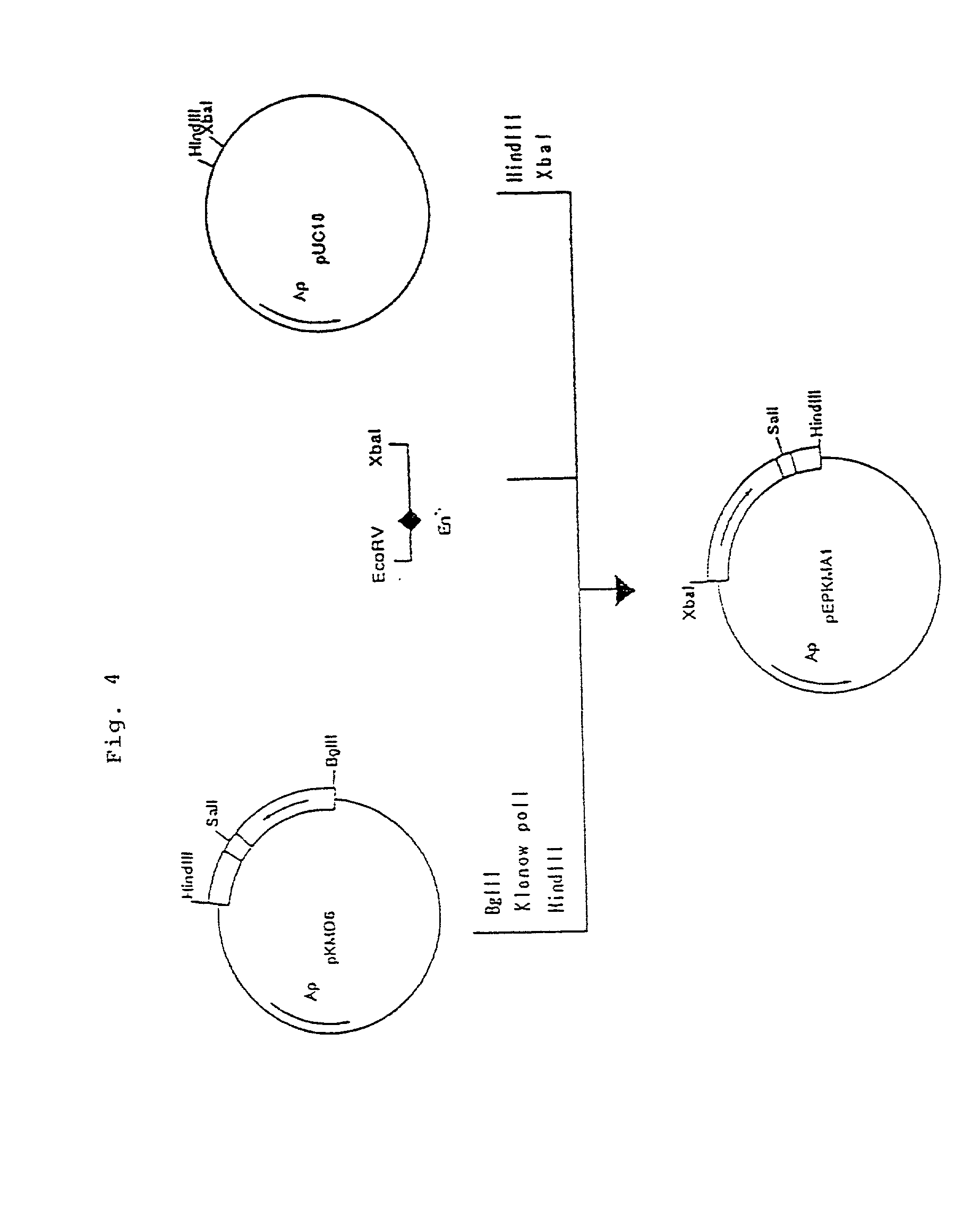
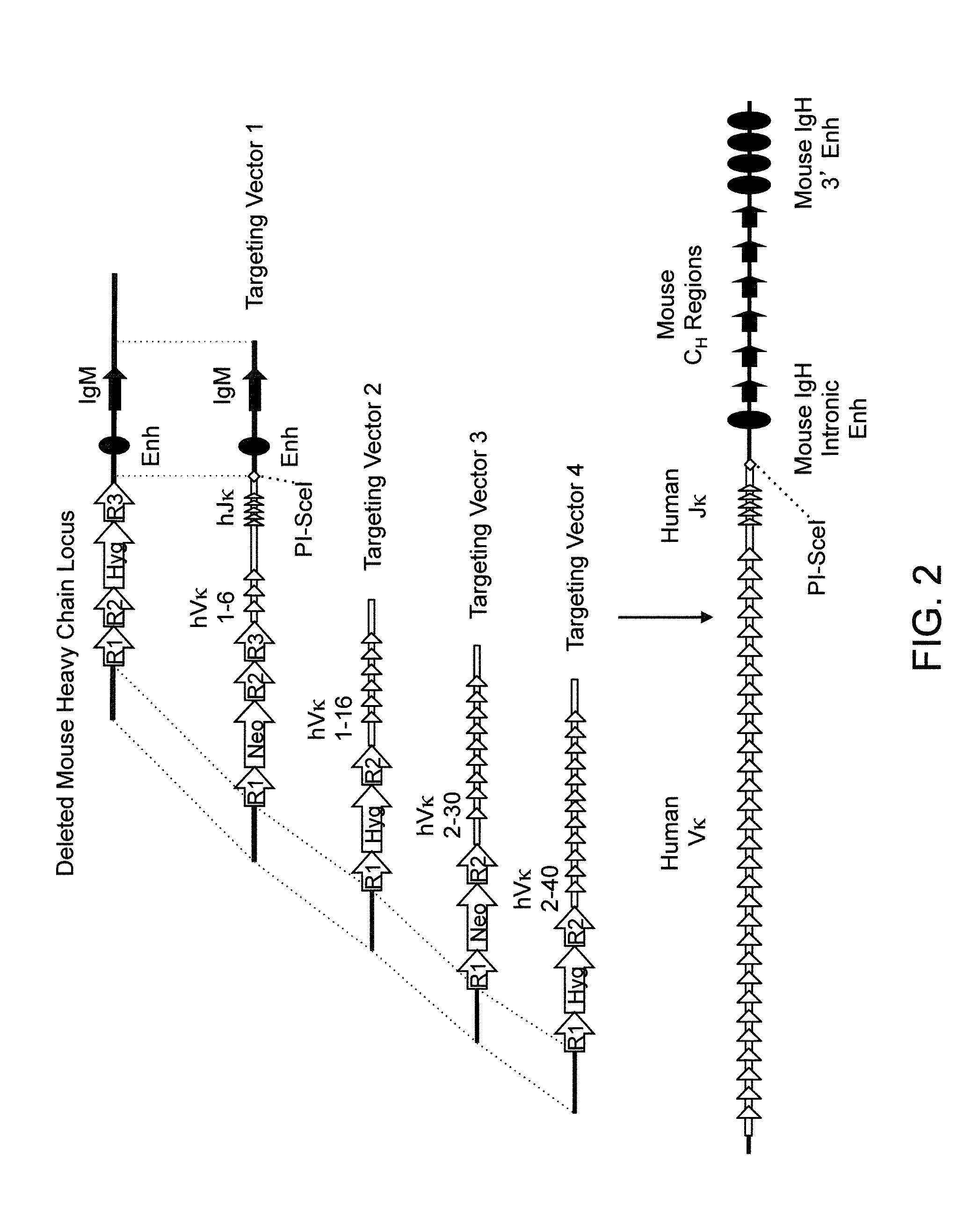
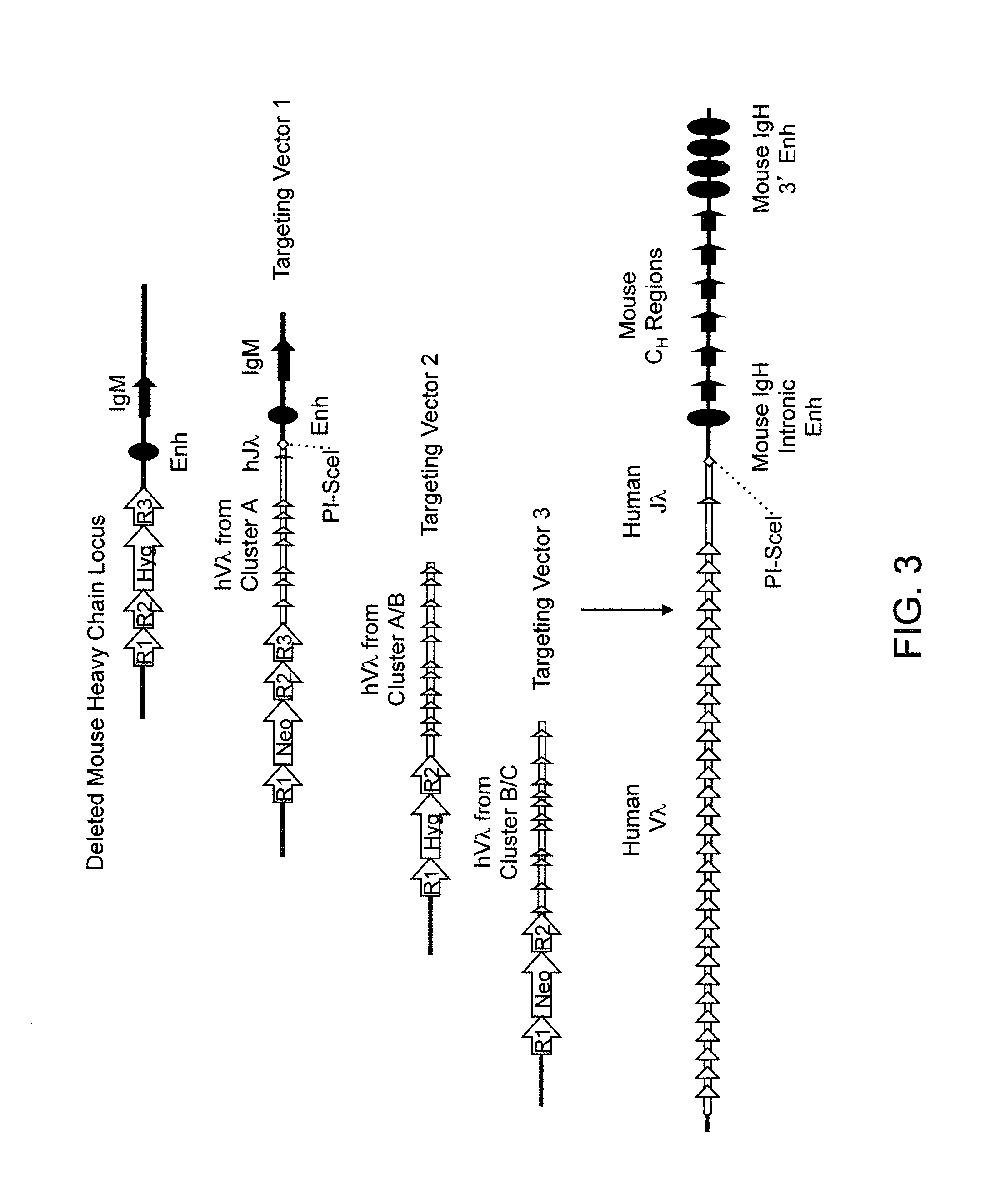
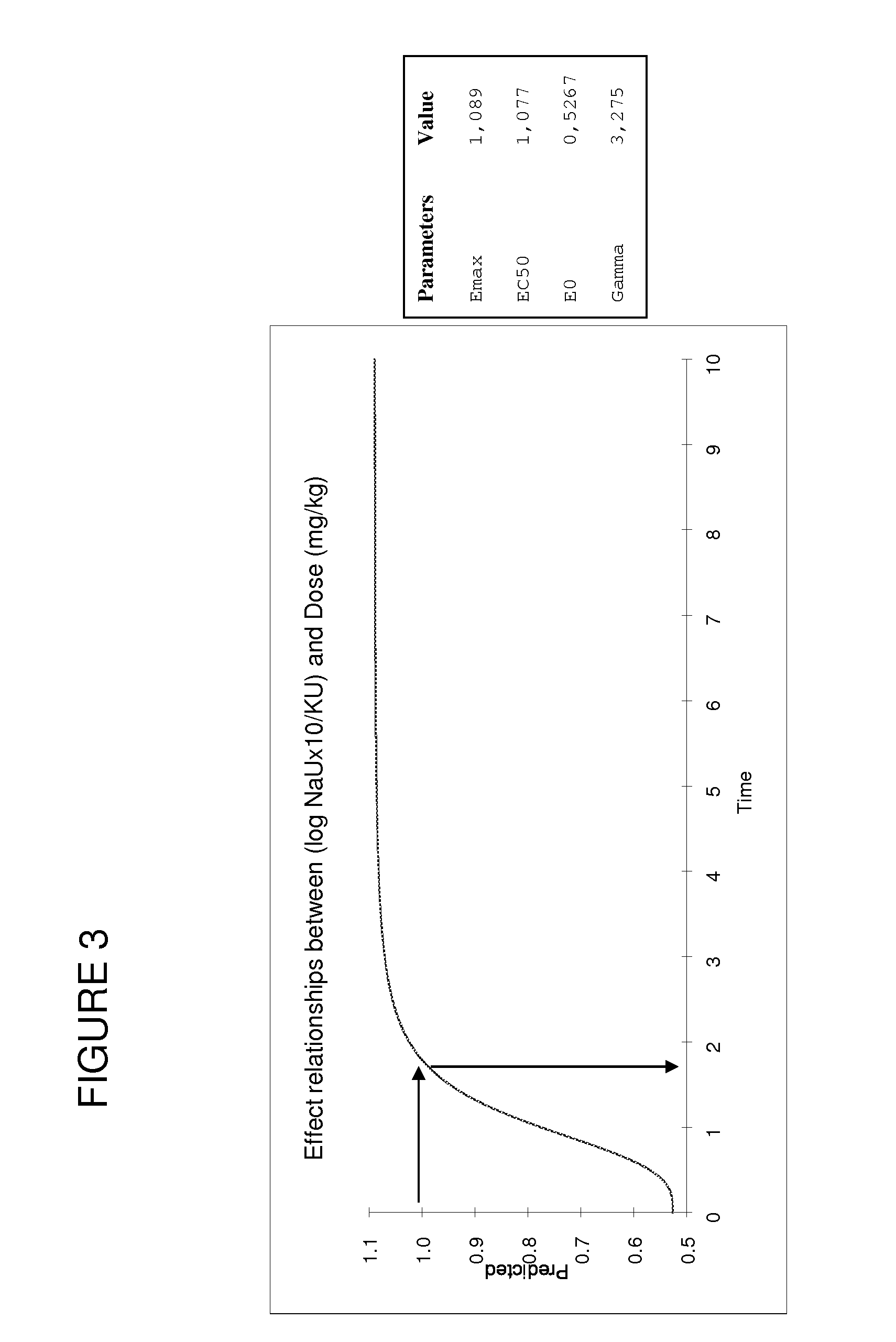
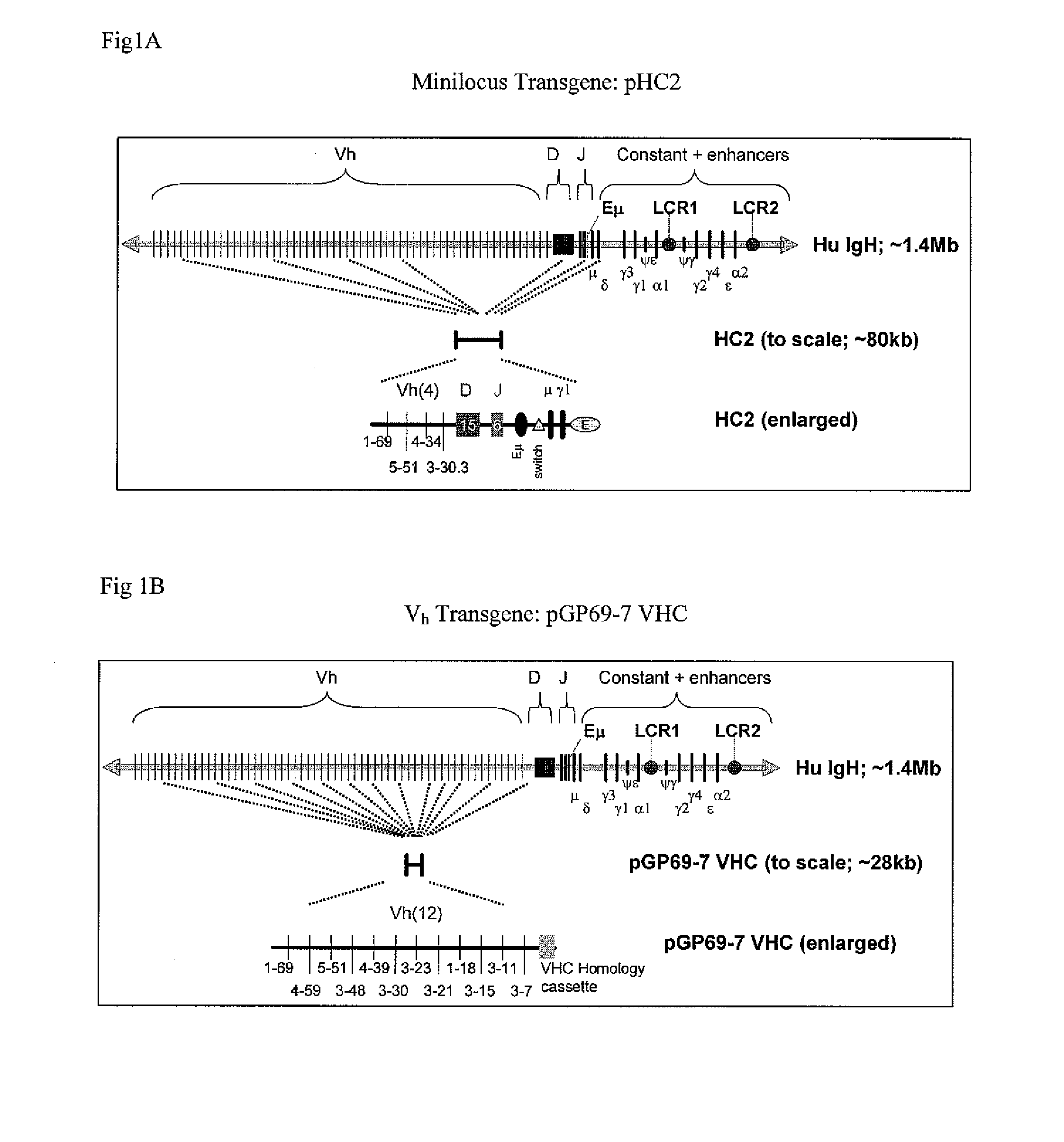
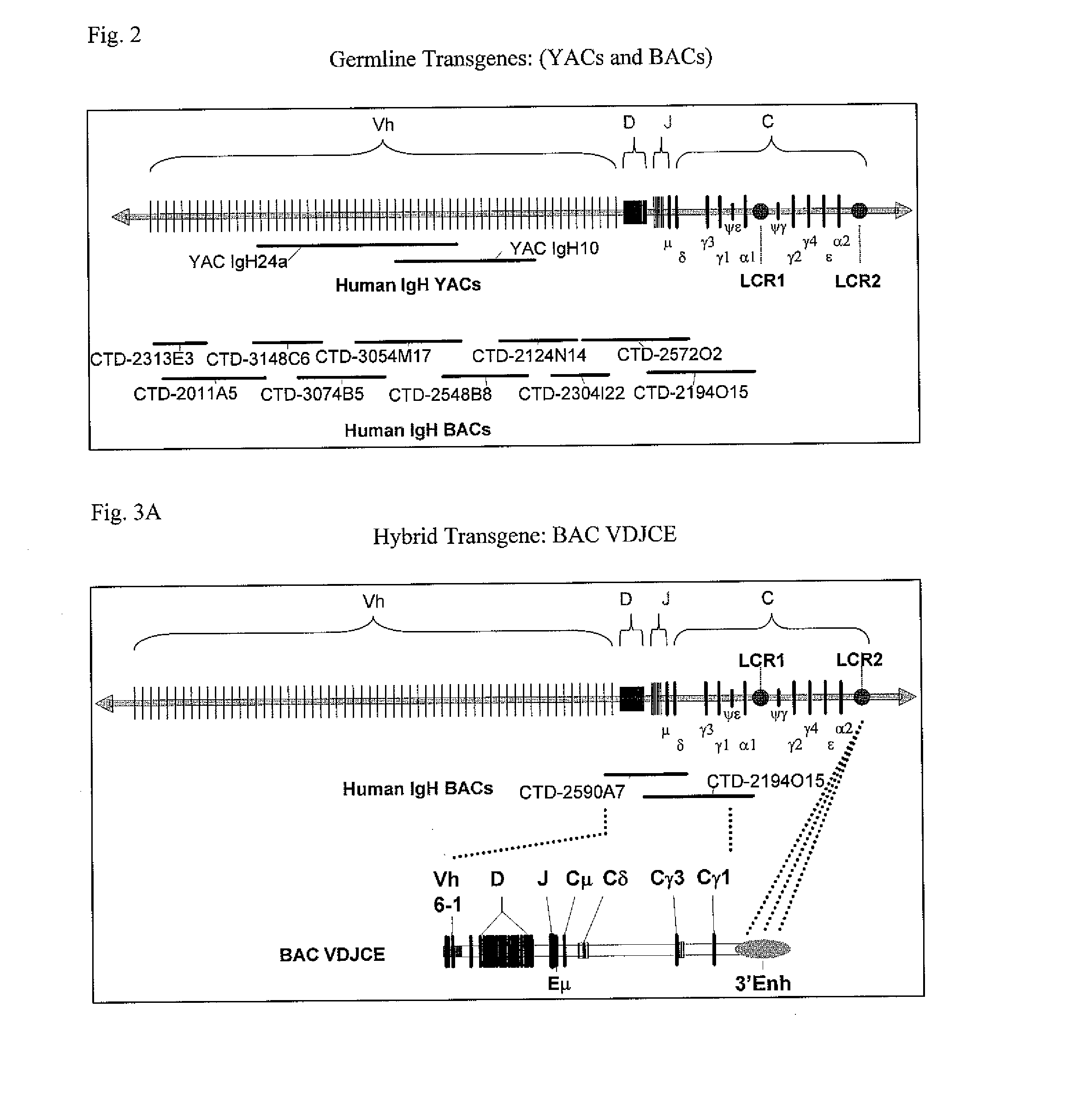
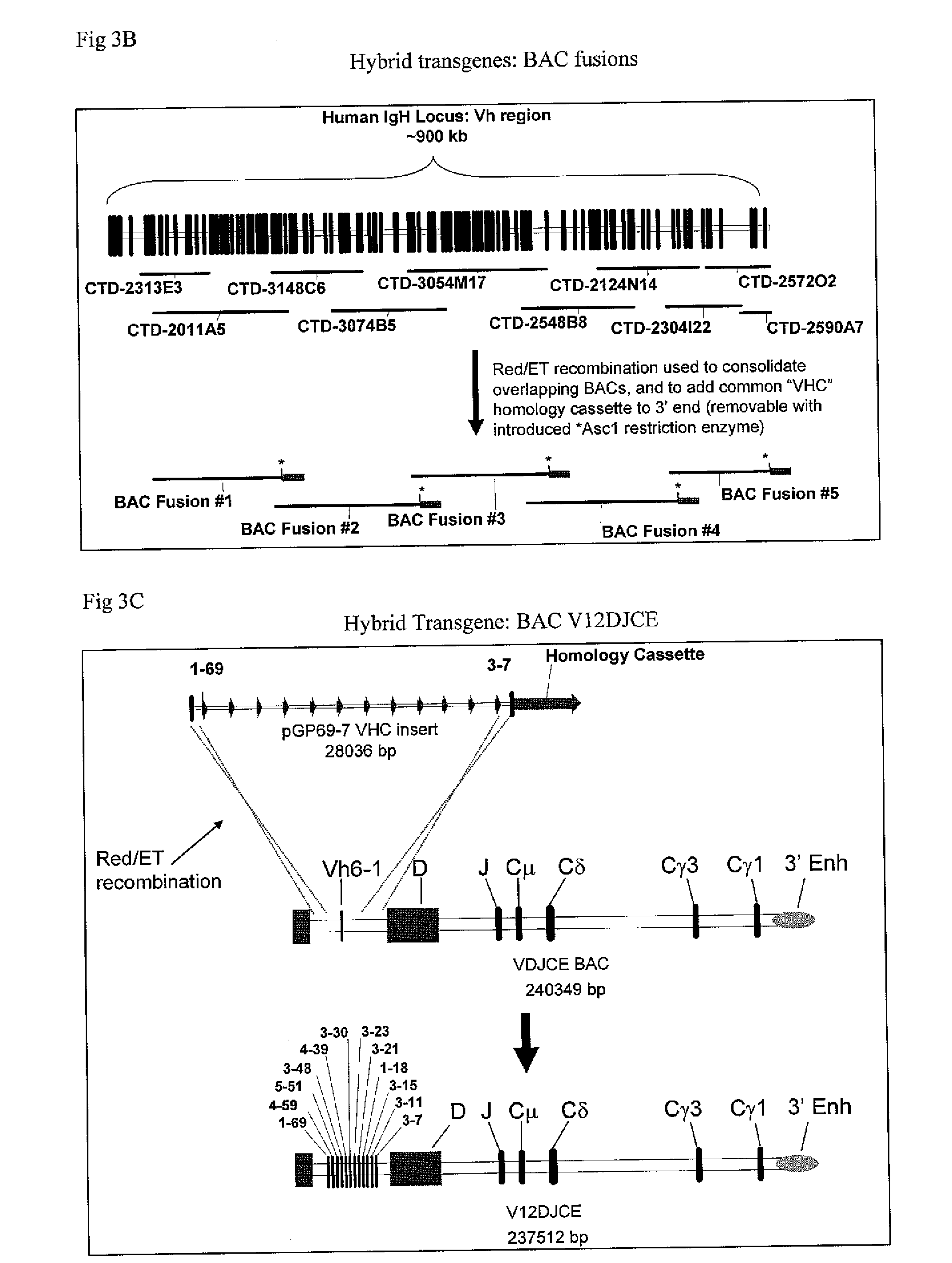
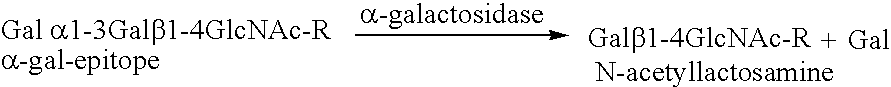Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
334 results about "Nonhuman animal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sleep in non-human animals refers to a behavioral and physiological state characterized by altered consciousness, reduced responsiveness to external stimuli, and homeostatic regulation. Sleep is observed in mammals, birds, reptiles, amphibians, and some fish, and, in some form, in insects and even in simpler animals such as nematodes.
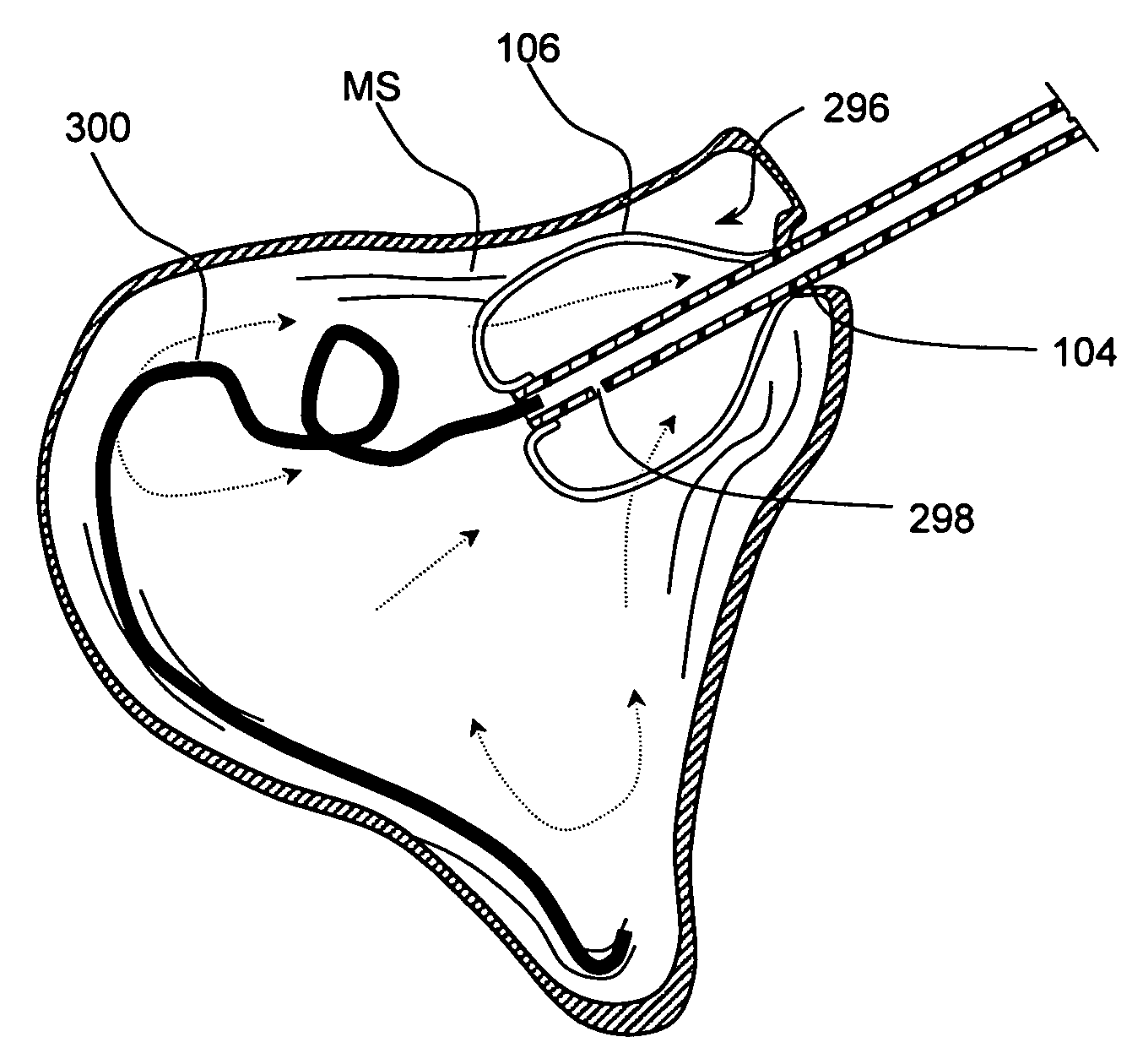
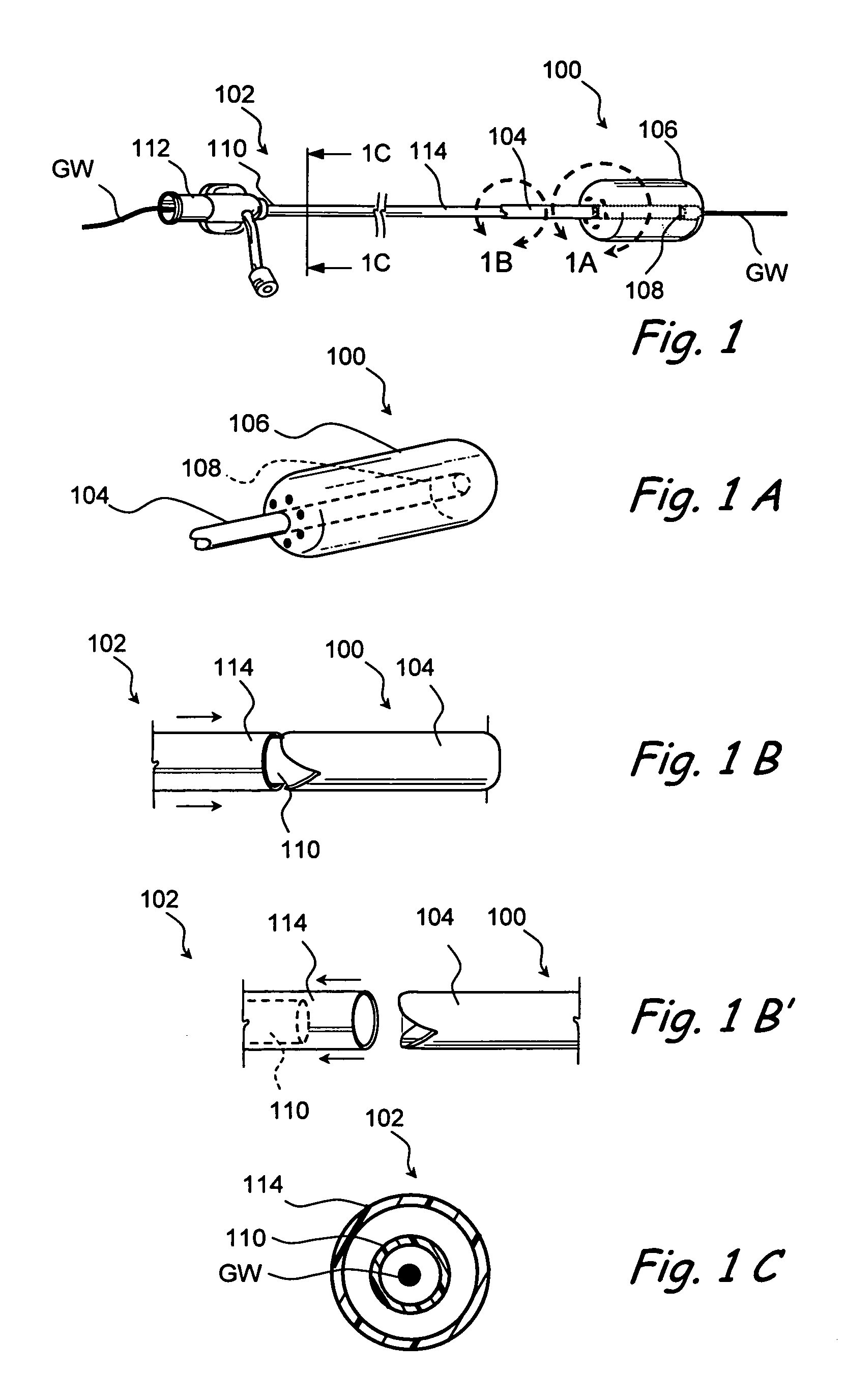
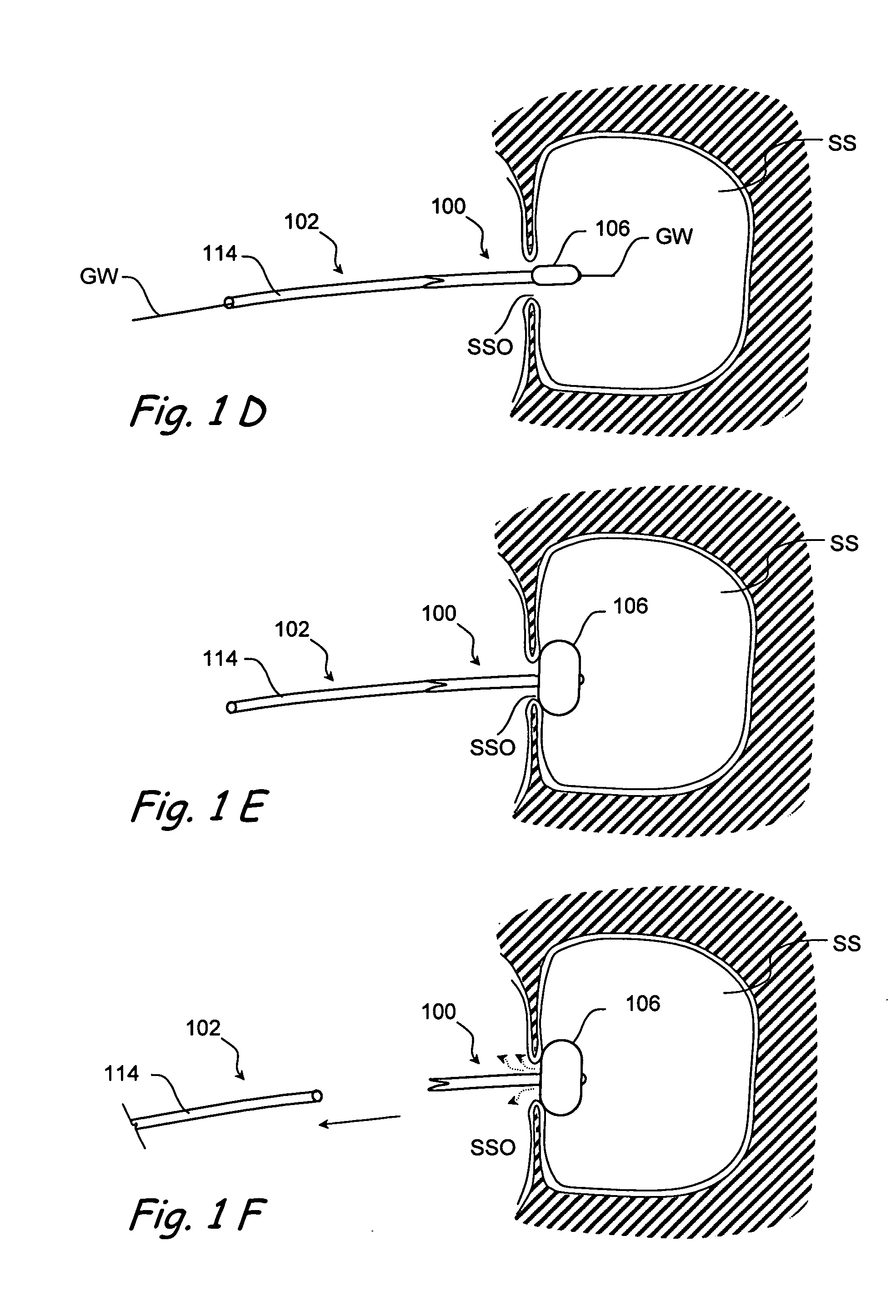
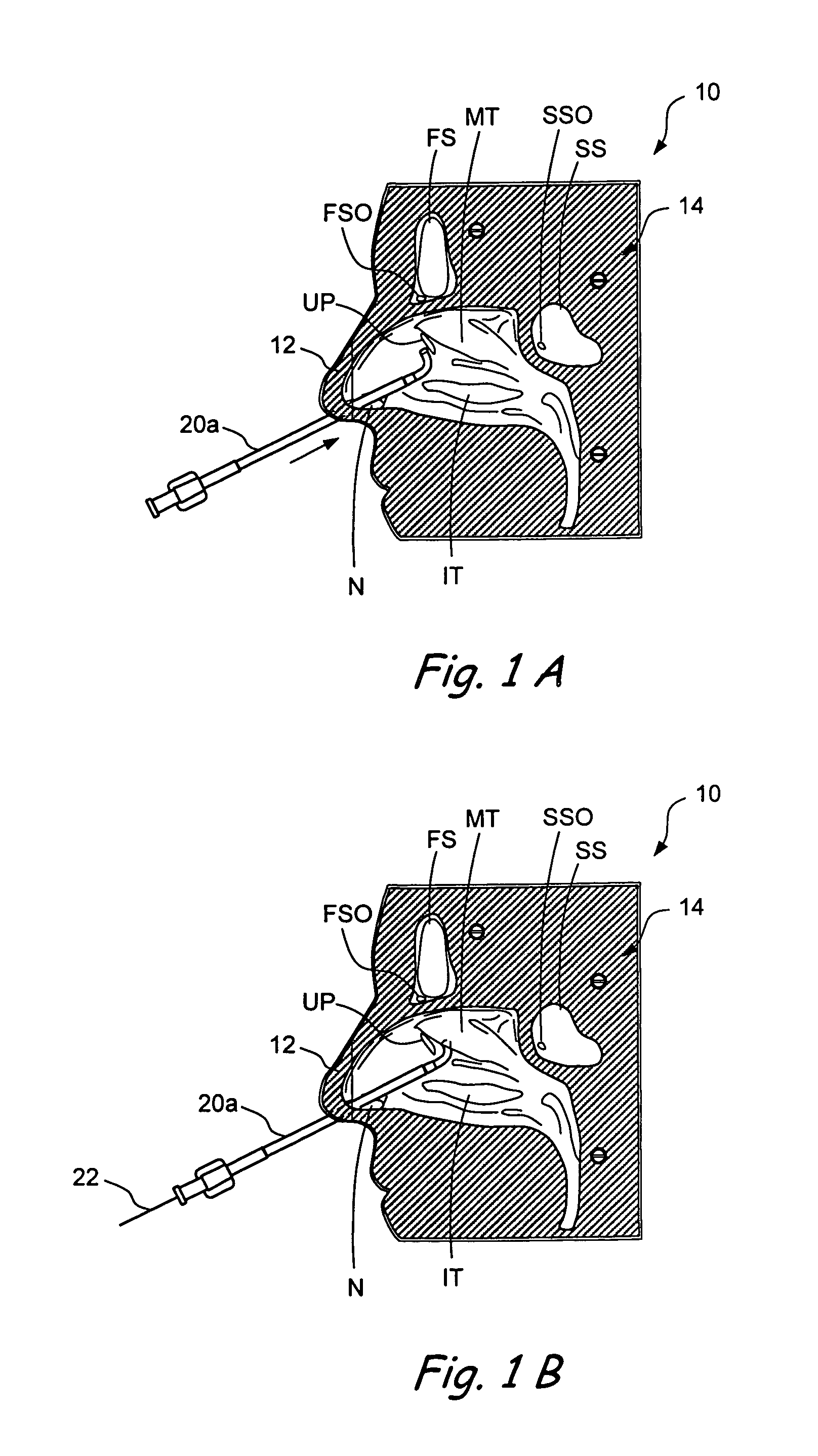
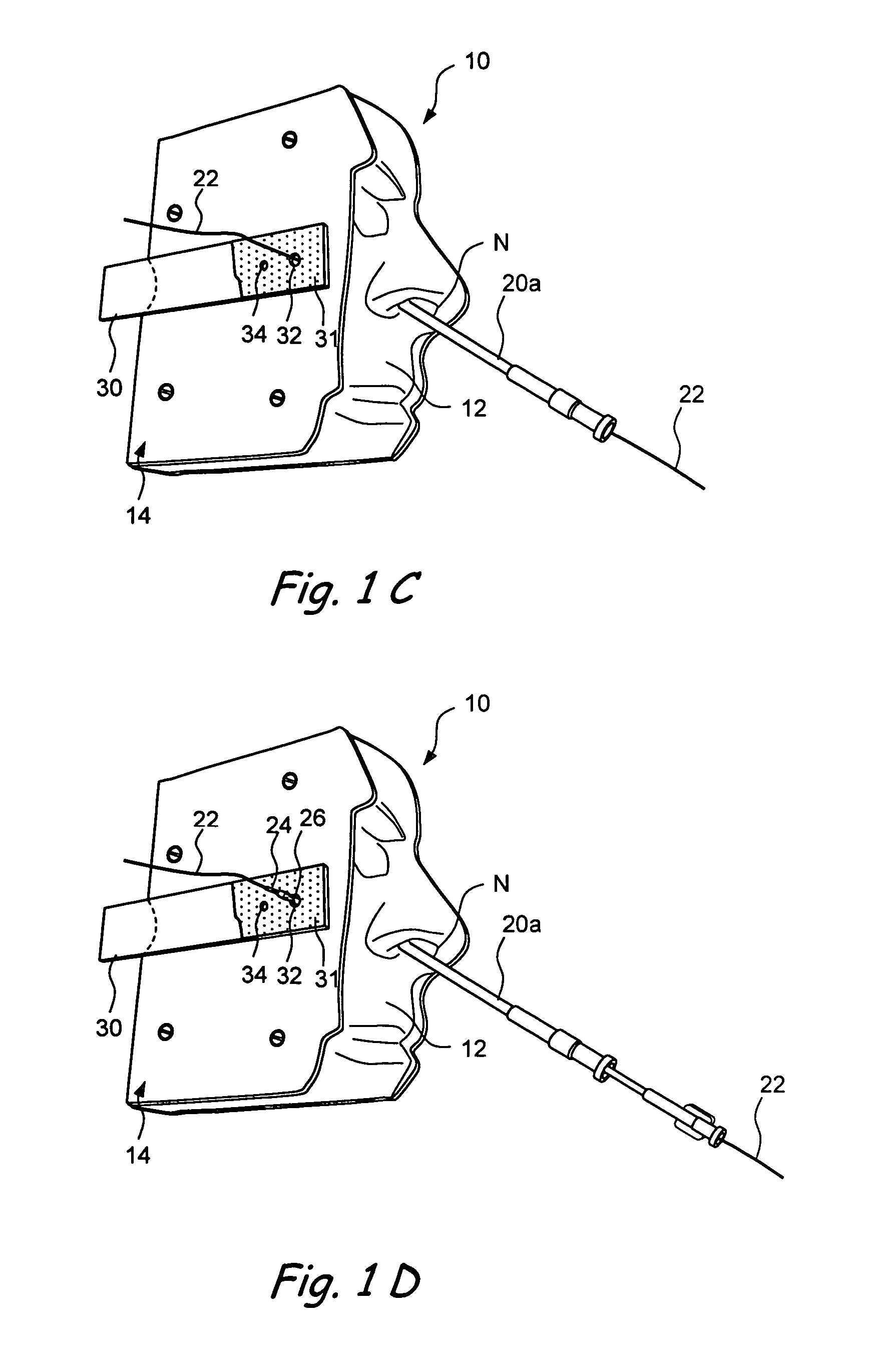
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
ActiveUS20060106361A1Surgical needlesPharmaceutical delivery mechanismDrugUnexpected therapeutic effect
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
Anatomical models and methods for training and demonstration of medical procedures
Anatomical models representing one or more anatomical structures of a human or non-human animal and related methods for demonstrating, training, promotion and sale of medical, cosmetic and surgical products or procedures. A working device is inserted into the anatomical model and used to perform a simulated procedure, thereby causing an indicator apparatus (e.g., a removable business card) to become perceptibly altered. The indicator apparatus may then be removed and provided a potential user, consumer or purchaser of the medical, surgical or cosmetic product.
Owner:ACCLARENT INC
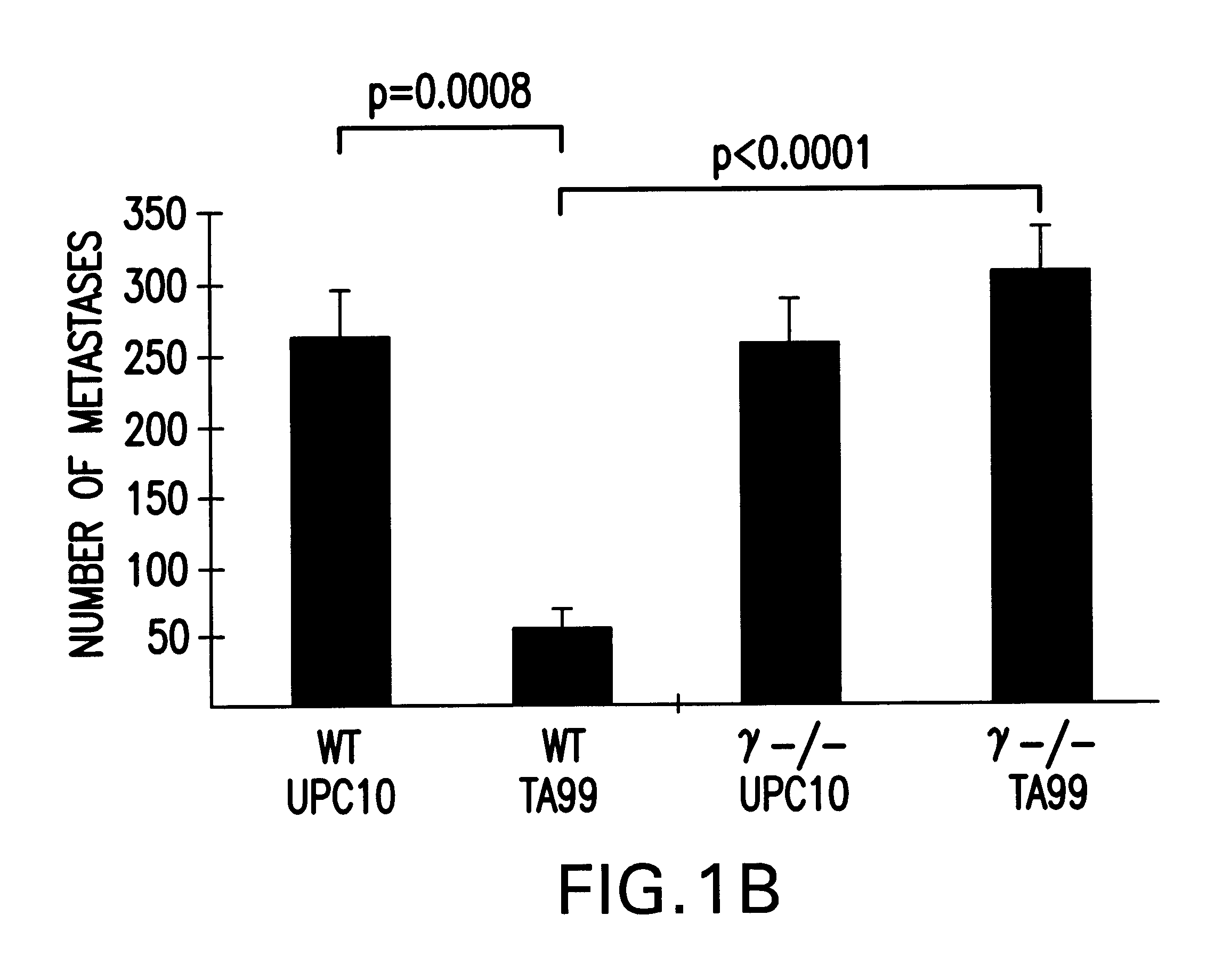
Animal model and methods for its use in the selection of cytotoxic antibodies
InactiveUS6676927B1Reduces antibody efficacyEnhances antibody efficacyCompounds screening/testingAntibody ingredientsReceptor for activated C kinase 1Infectious agent
The present invention relates to non-human animals and in vivo methods for testing the efficacy of antibodies directed to antigens expressed by tumors in such animals. In particular, the invention relates to an animal deficient in the expression of one or more Fc receptors. Additionally, such an animal is also immunodeficient, and thus permits the growth of a xenogeneic tumor implant. Such immunodeficient animals may also express human receptors. The present invention also relates to methods of evaluating the enhanced ability of an existing antibody or Fc-modified antibody to act as an immunotherapeutic to eradicate tumor cells or infectious agents.
Owner:THE ROCKEFELLER UNIV
Anti-HLA-DR antibody
InactiveUS7262278B2Suppress lowering survival ratioSurvival ratioFungiBacteriaCancer cellMonoclonal antibody
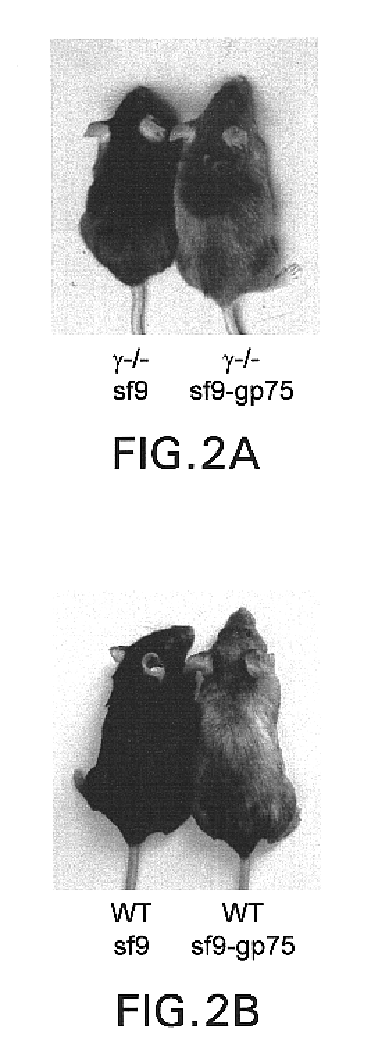
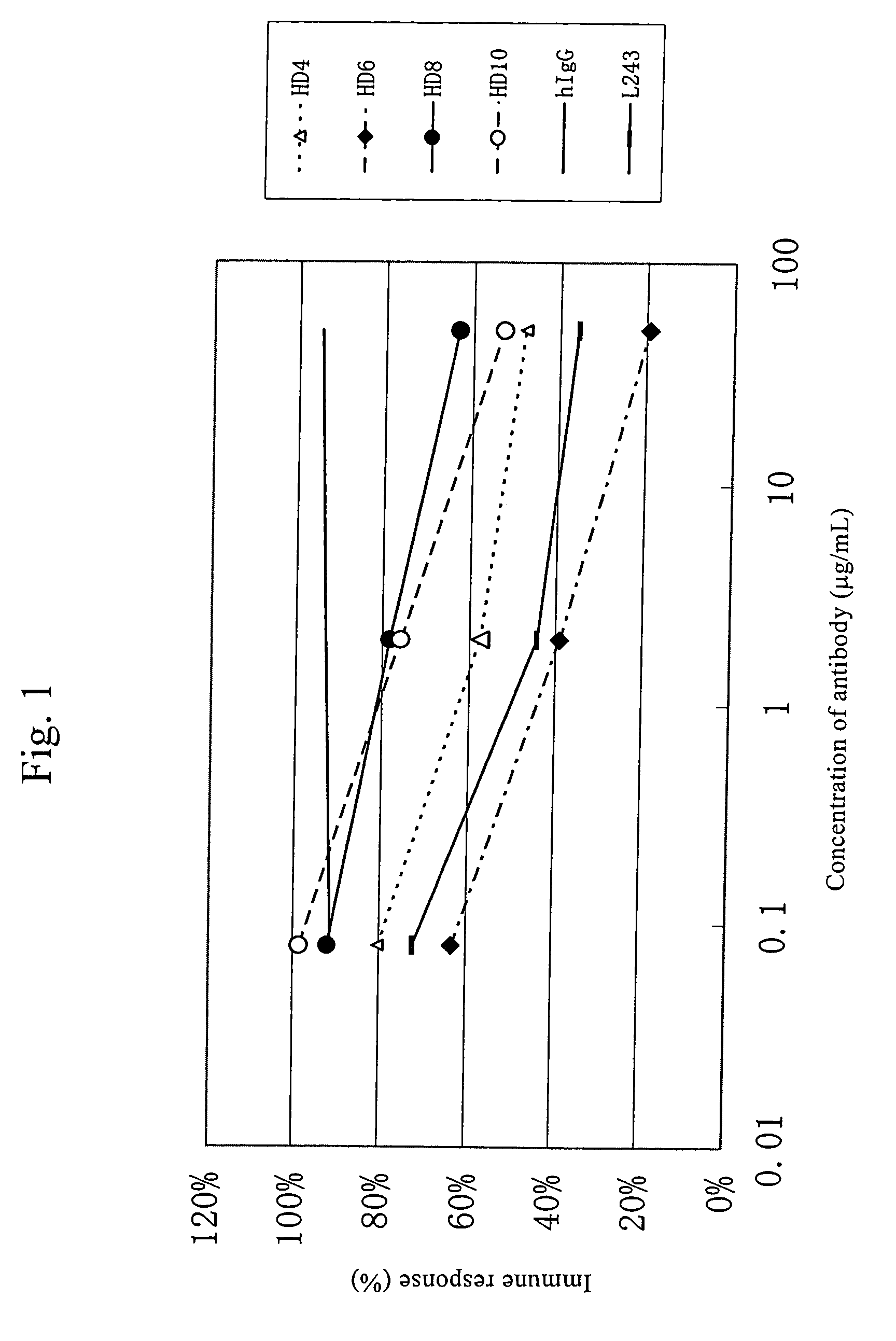
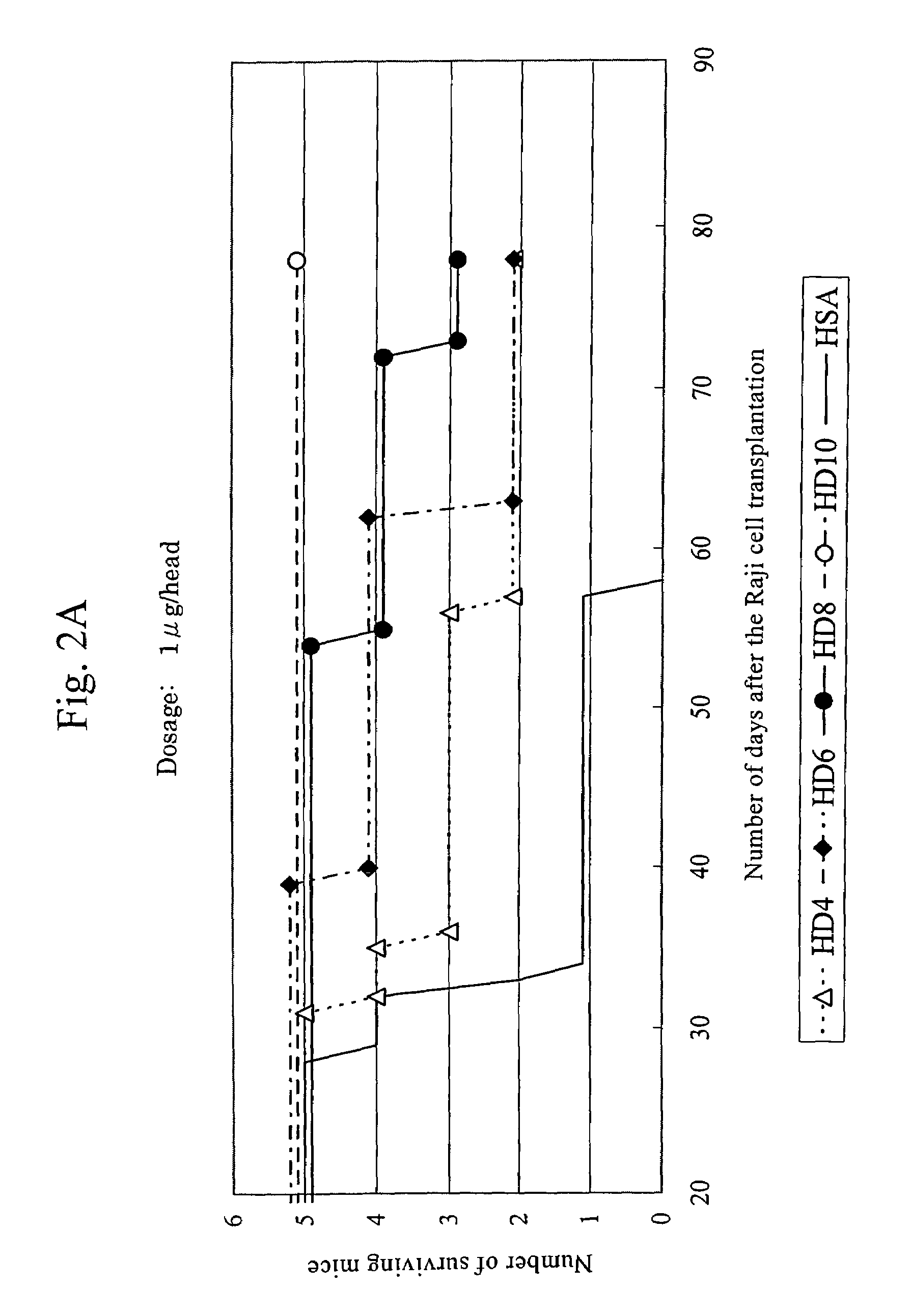
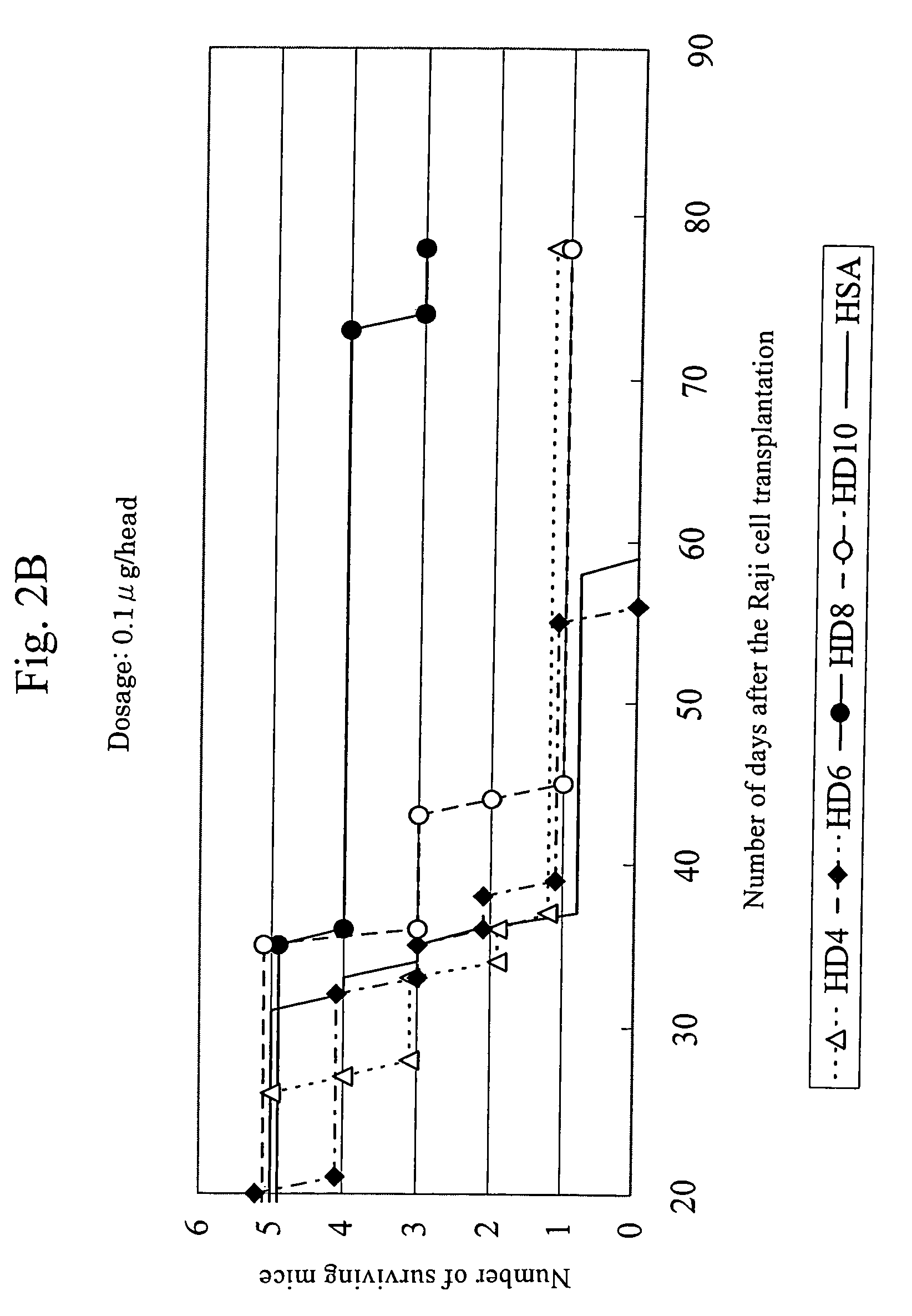
This invention provides an anti-HLA-DR monoclonal antibody. This invention relates to an antibody binding to HLA-DR or a functional fragment thereof having (a) life-extending effects in nonhuman animals bearing HLA-DR-expressing cancer cells and (b) activity of suppressing immune responses lower than that of L243, or an antibody binding to HLA-DR or a functional fragment thereof exhibiting immunosuppressive activity equivalent to or higher than that of the mouse anti-HLA-DR monoclonal antibody L243 (ATCC HB-55).
Owner:KIRIN BREWERY CO LTD
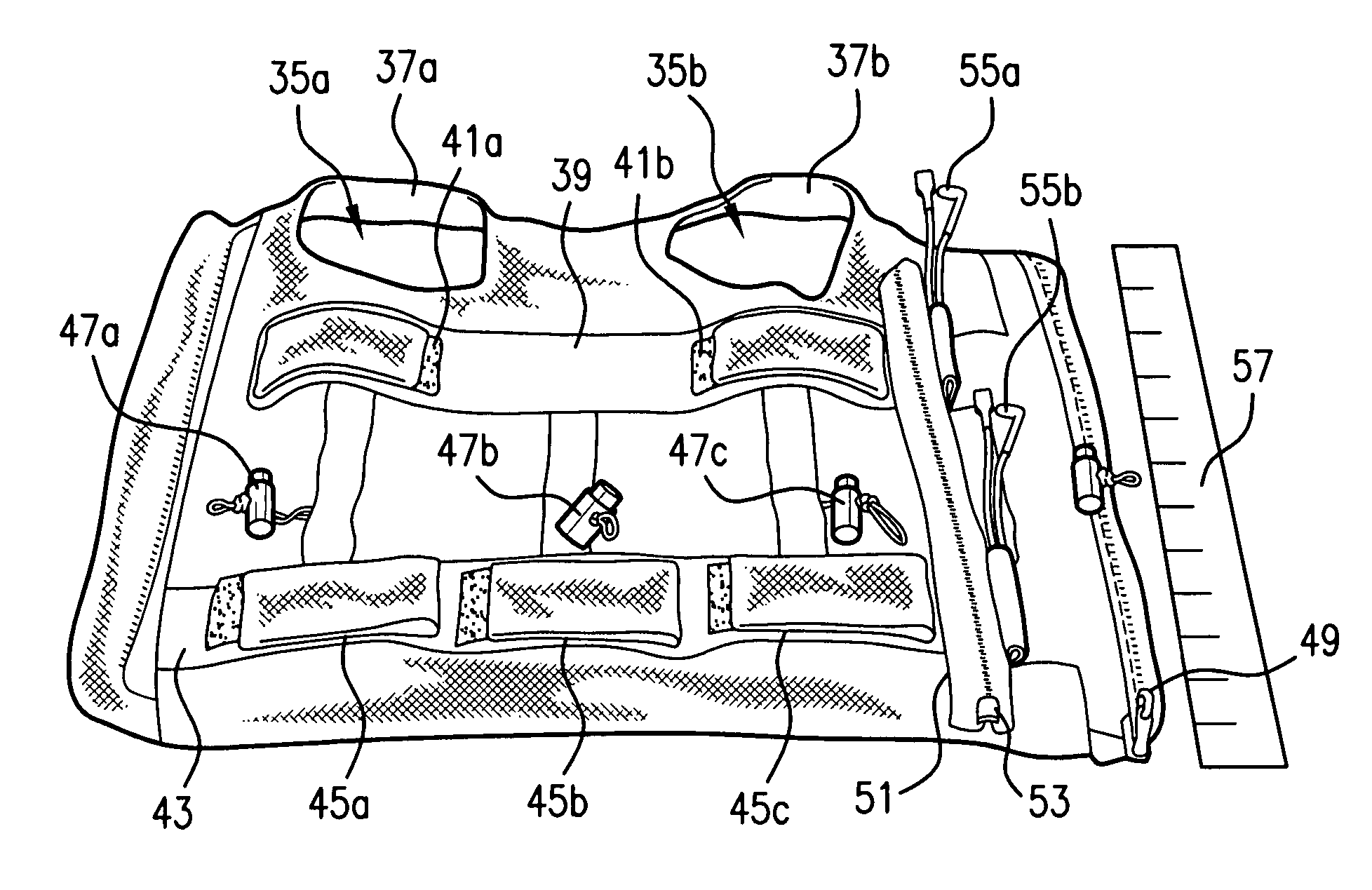
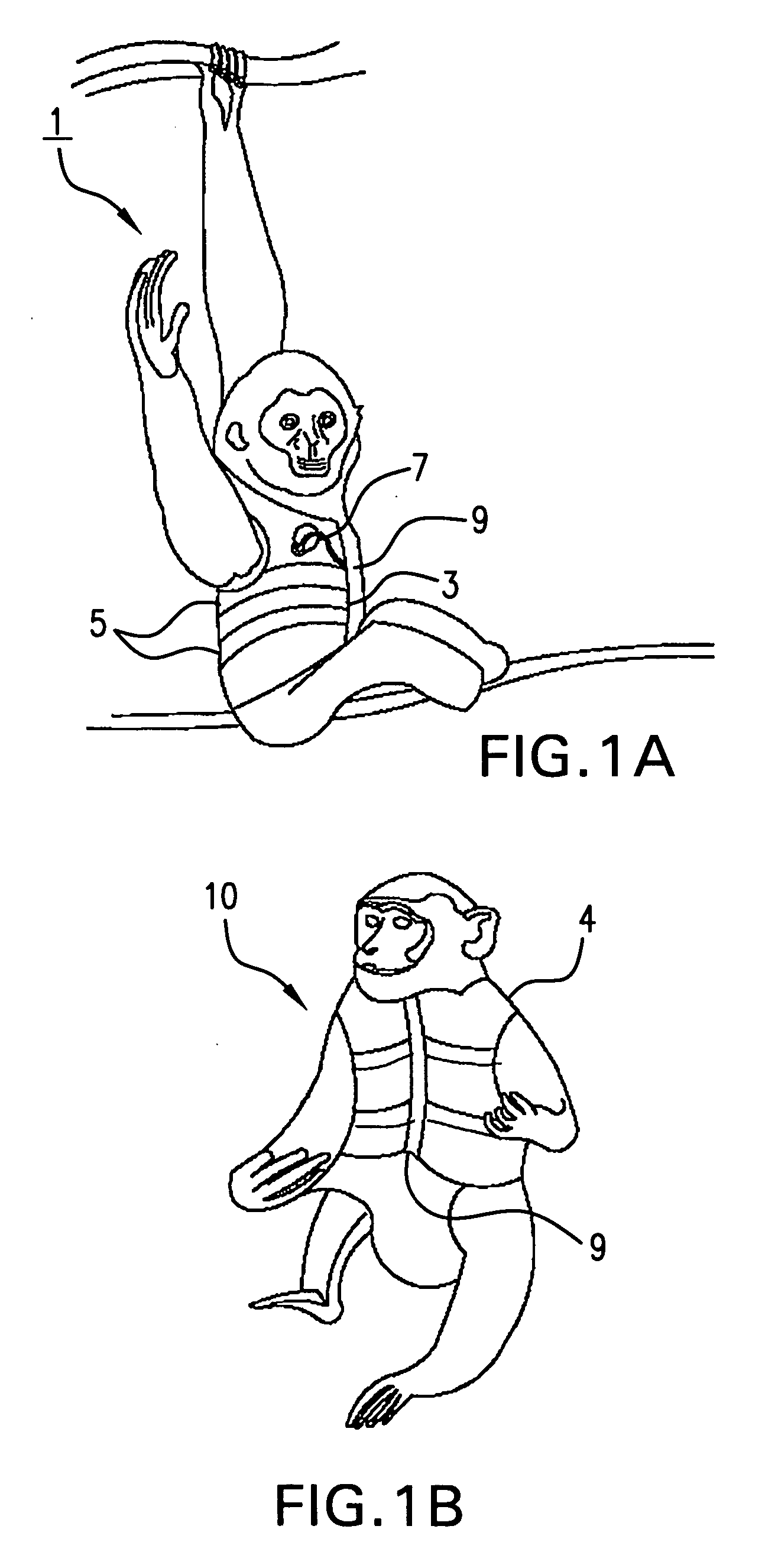
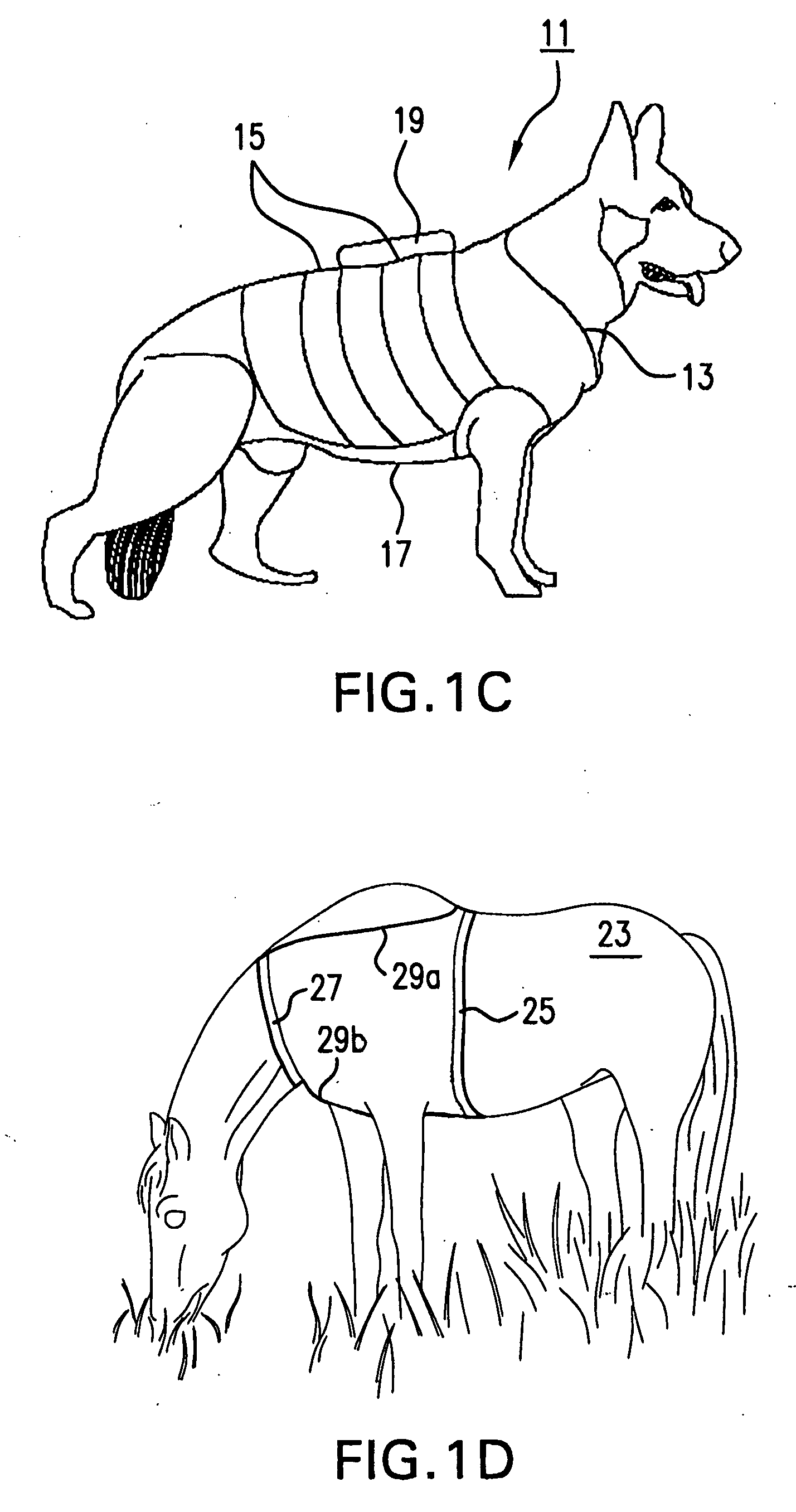
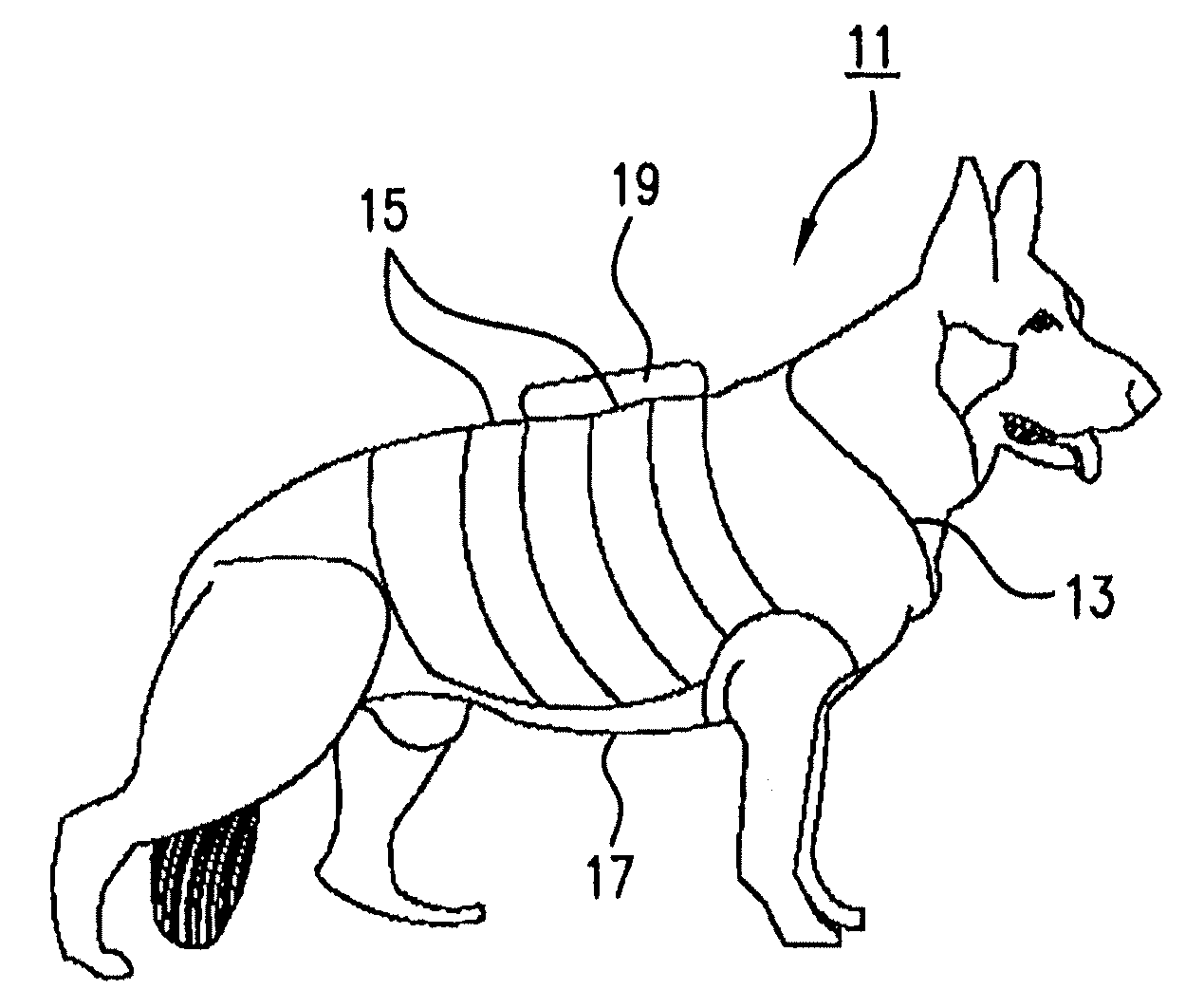
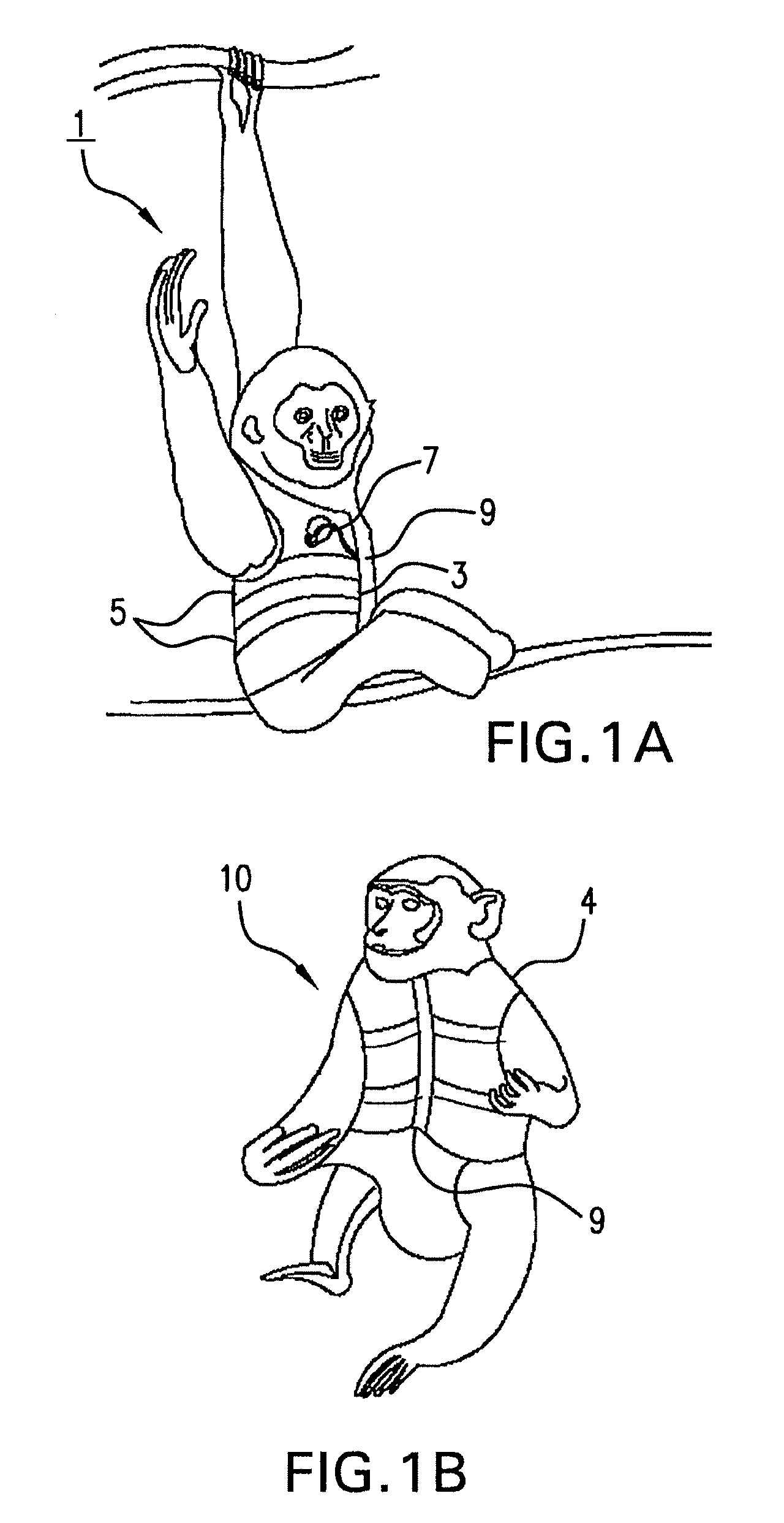
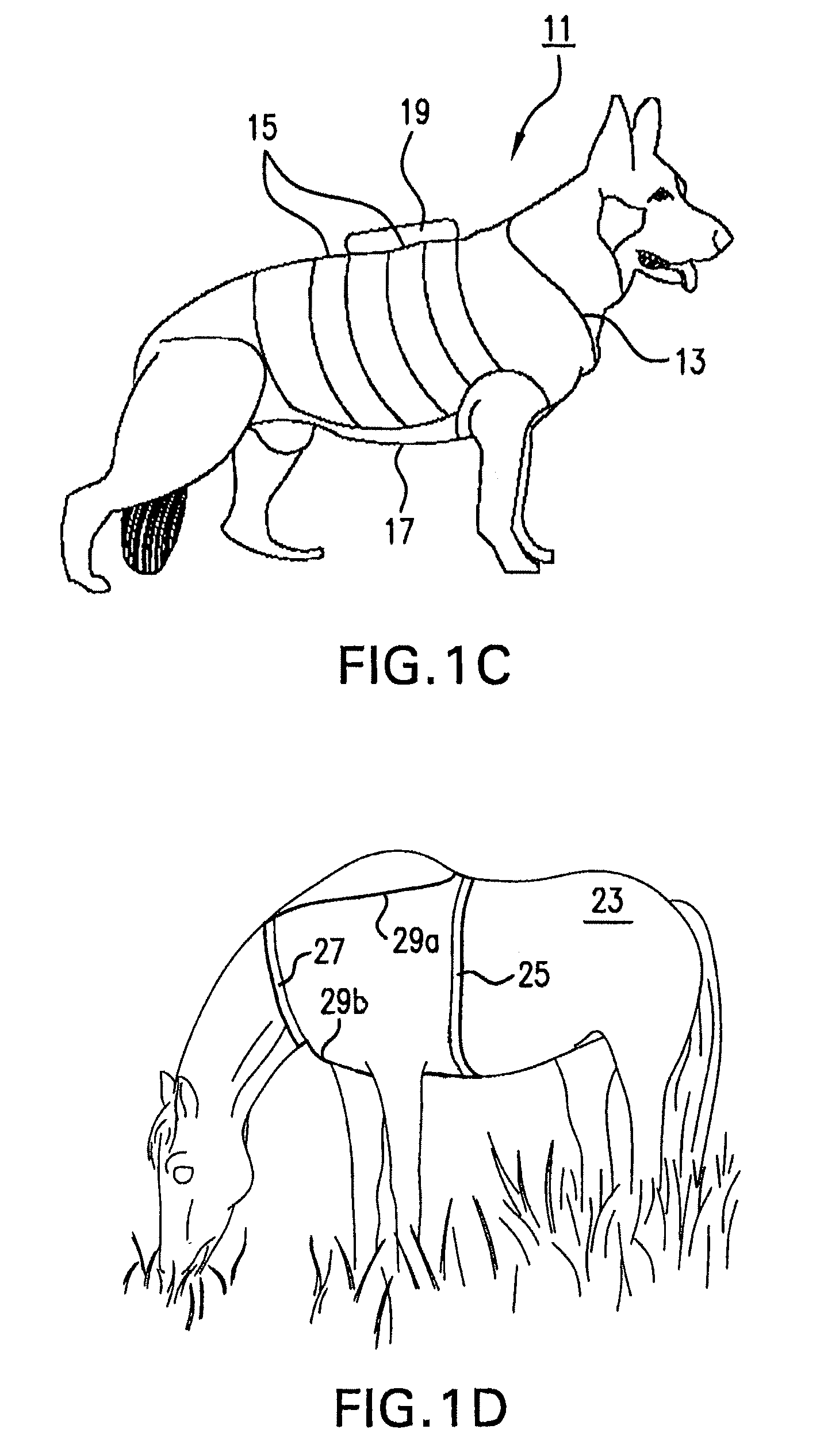
Systems and methods for non-invasive physiological monitoring of non-human animals
InactiveUS20060258914A1Little and no distressNo painElectrocardiographyPerson identificationAnimal scienceMedicine
This invention provides monitoring garments for non-invasively monitoring physiological parameters in un-restrained and / or restrained animals, such as monkeys, rabbits, dogs, horses, and the like. The invention also includes methods and systems for collecting and processing monitoring data.
Owner:ADIDAS
Pharmaceutical compositions comprising an oligonucleotide as an active agent
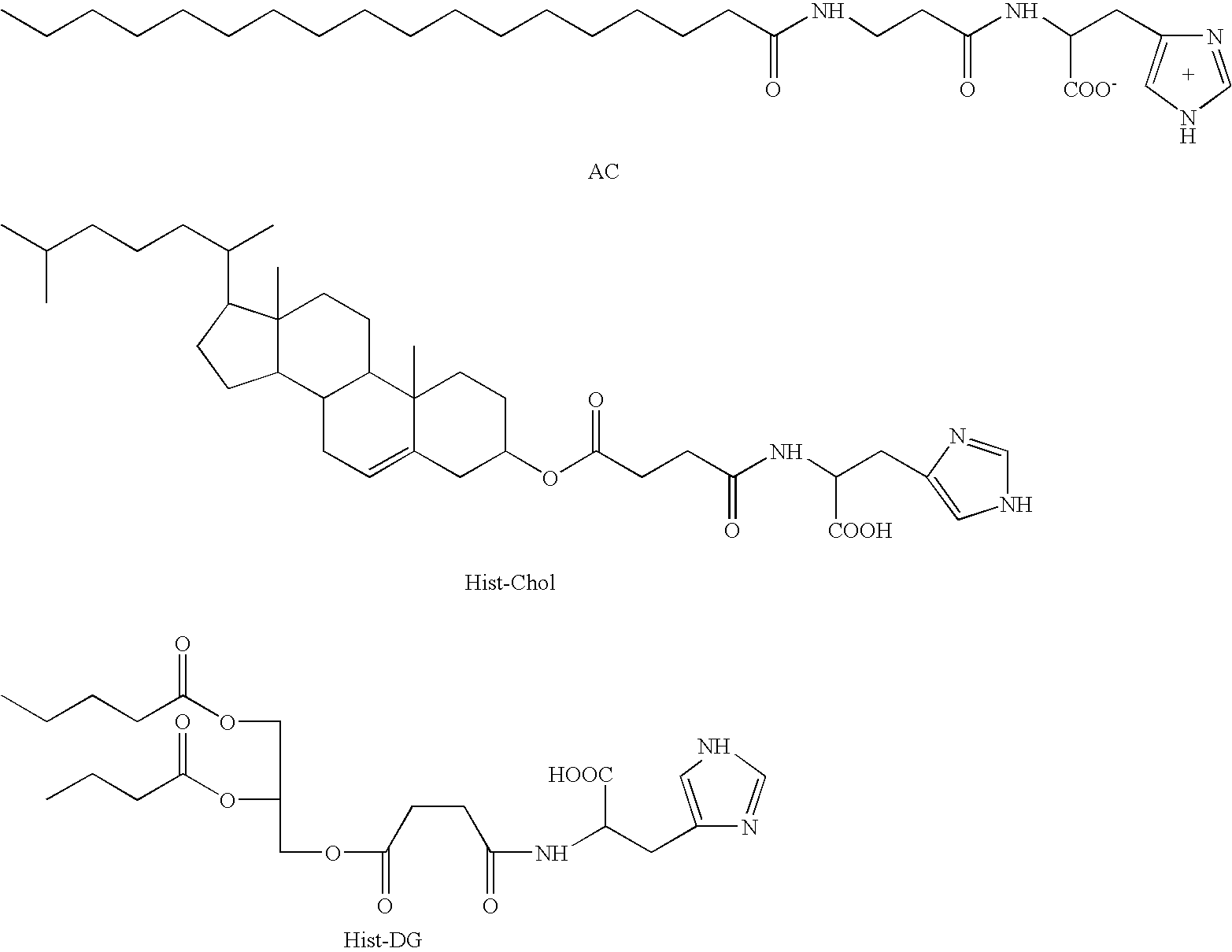
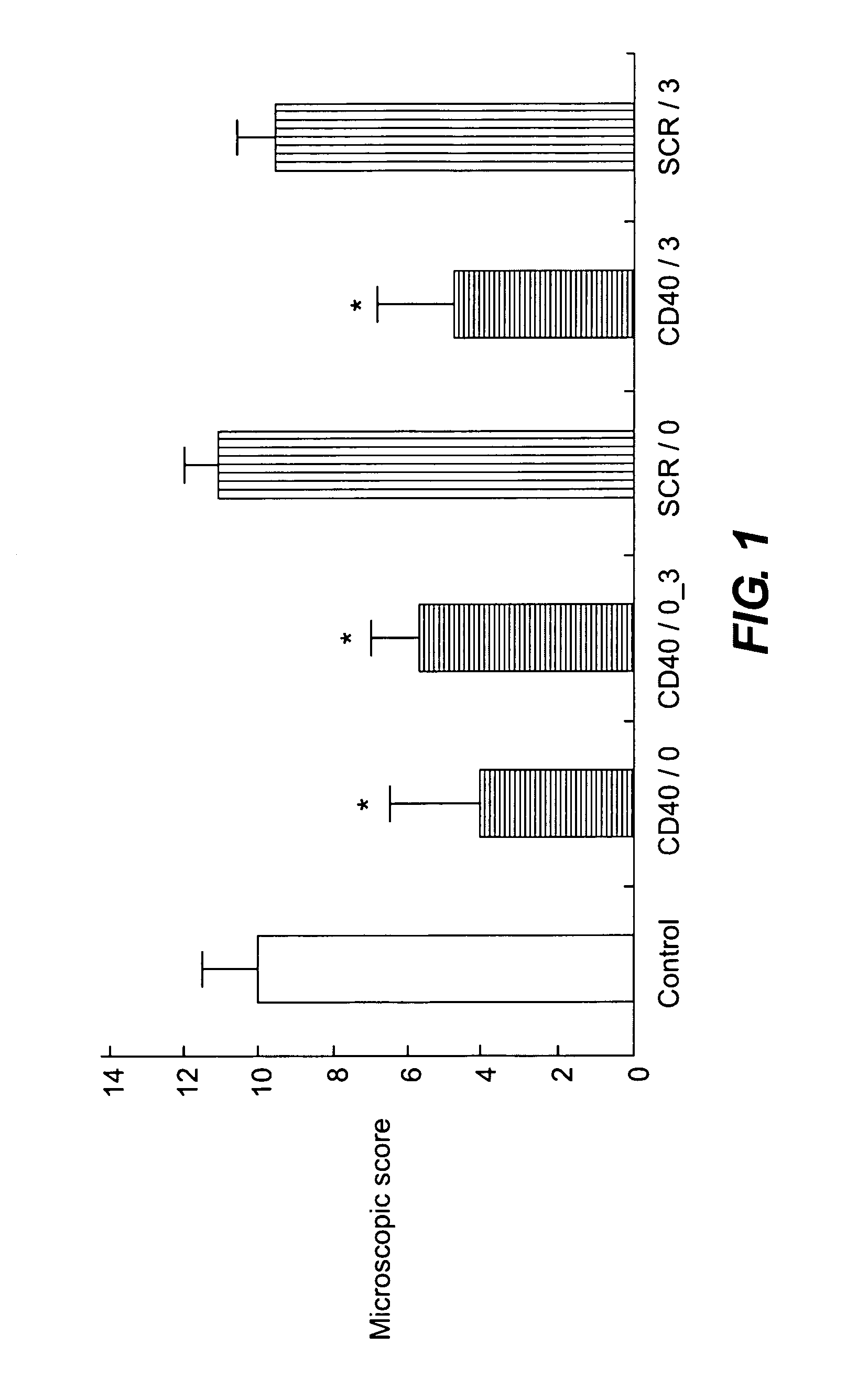
A pharmaceutical composition is disclosed, which composition comprises an oligonucleotide as an active agent, the oligonucleotide being adapted to target nucleic acids encoding CD40 thereby to modulate the expression of CD40 in mammalian cells, and a liposome as an excipient. Said liposome is an amphoteric liposome. Also disclosed is a method for the treatment or prophylaxis of a disease or condition associated with the expression of CD40 in a human or non-human animal patient by administering to said patient a therapeutically or prophylactically effective amount of such a composition.
Owner:NOVOSOM
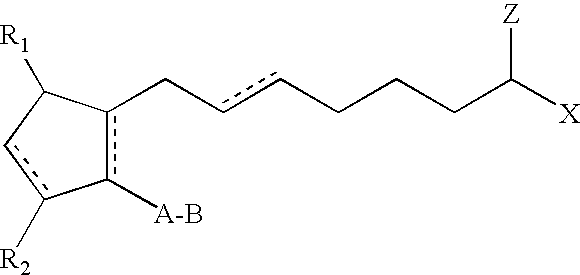
Method of Enhancing Hair Growth
Methods and compositions for stimulating the growth of hair are disclosed wherein said compositions include a cyclopentane heptanoic acid, 2-cycloalkyl or arylalkyl compound represented by the formula I wherein the dashed bonds represent a single or double bond which can be in the cis or trans configuration, A, B, Z, X, R1 and R2 are as defined in the specification. Such compositions are used in treating the skin or scalp of a human or non-human animal. Bimatoprost is preferred for this treatment.
Owner:ALLERGAN INC
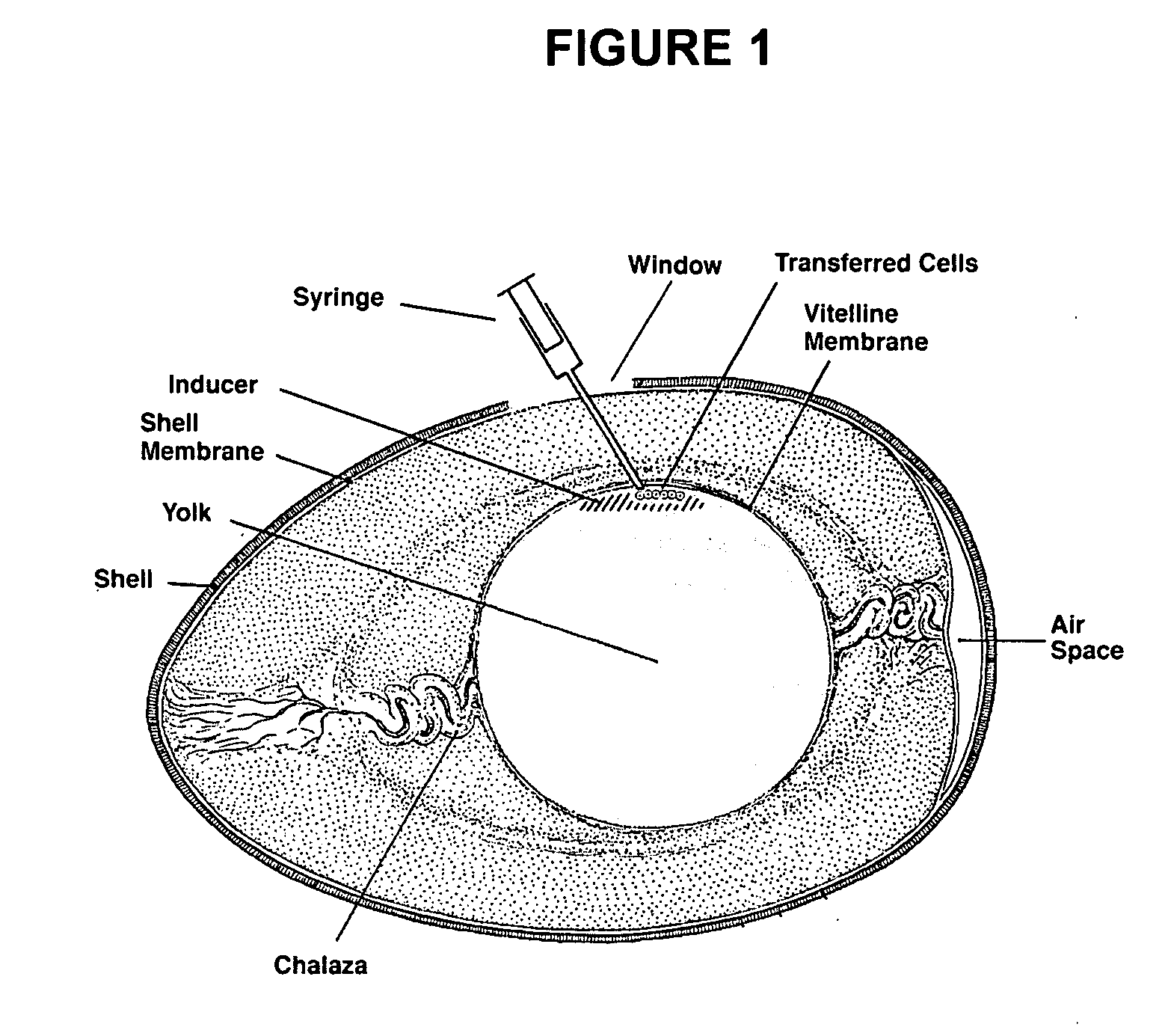
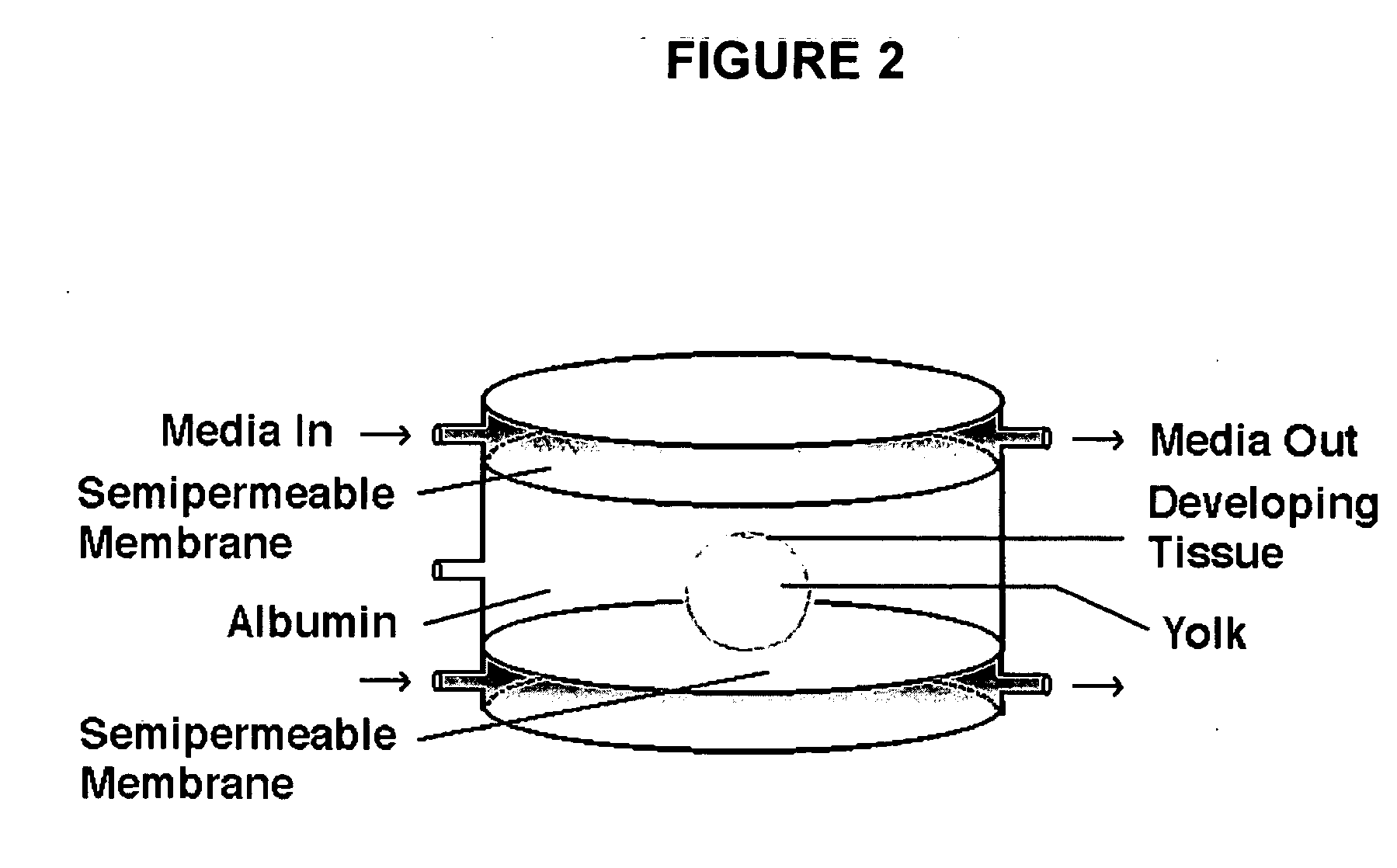
Novel culture systems for EX vivo development
ActiveUS20060031955A1Improve efficiencyIncreased riskArtificial cell constructsEmbryonic cellsEmbryoEx vivo
The present invention provides methods for the culture of animal pluripotent stem cells and their differentiated progeny cells, tissues, and organs, and nonhuman animal embryos and fetuses.
Owner:ADVANCED CELL TECH INC
Process for producing humanized chimera antibody
InactiveUS20020026036A1Easy to produceEasy constructionPeptide/protein ingredientsAntibody mimetics/scaffoldsRestriction enzyme digestionSynthetic DNA
A process for the production of humanized chimera antibody, wherein the chimera antibody is produced easily without changing any of the amino acids of its mouse antibody variable region, which comprises the steps of: (1) constructing a cassette vector by inserting a cDNA coding for a heavy chain constant region of human antibody into an expression vector for animal cell use and establishing a cloning site in the upstream region of the heavy chain constant region of said cassette vector for inserting a cDNA which encodes a heavy chain variable region of nonhuman animal antibody; (2) digesting a cDNA coding for the heavy chain variable region of nonhuman animal antibody with restriction enzymes; (3) inserting said cDNA coding for the heavy chain variable region of nonhuman animal antibody into the cassette vector, using a synthetic DNA which comprises a base sequence corresponding to the 5'-end side of said heavy chain constant region of human antibody and a base sequence corresponding to the 3'-end side of said heavy chain variable region of nonhuman animal antibody and is possessed of the restriction enzyme recognition sites on both of its ends, thereby constructing a humanized chimera antibody heavy chain expression vector in which said cDNA coding for the heavy chain constant region of human antibody and said cDNA coding for the heavy chain variable region of nonhuman animal antibody are linked together through said synthetic DNA; (4) constructing a cassette vector by inserting a cDNA coding for a light chain constant region of human antibody into an expression vector for animal cell use and establishing a cloning site in the upstream region of the light chain constant region of said cassette vector for inserting a cDNA which encodes a light chain variable region of nonhuman animal antibody; (5) digesting a cDNA coding for the light chain variable region of nonhuman animal antibody with restriction enzymes; (6) inserting said cDNA coding for a light chain variable region of nonhuman animal antibody into the cassette vector using a synthetic DNA which comprises a base sequence corresponding to the 5'-end side of said light chain constant region of human antibody and a base sequence corresponding to the 3'-end side of said light chain variable region of nonhuman animal antibody and is possessed of the restriction enzyme recognition sites on both of its ends, thereby constructing a humanized chimera antibody light chain expression vector in which said cDNA coding for the light chain constant region of human antibody and said cDNA coding for the light chain variable region of nonhuman animal antibody are linked together through said synthetic DNA; (7) introducing these expression vectors into host cells to obtain a transformant; and (8) culturing said transformant in an appropriate culture medium, thereby allowing the transformant to produce and accumulate a humanized chimera antibody, and collecting said humanized chimera antibody from the resulting culture broth.
Owner:KYOWA HAKKO KIRIN CO LTD
Humanized Rodents that Express Heavy Chain Containing VL Domains
InactiveUS20130212719A1Reduced fertilityImprove fertilityAnimal cellsHybrid immunoglobulinsGenetic MaterialsVariable domain
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a rodent (e.g., a mouse), wherein the non-human animals rearrange human immunoglobulin light chain gene segments in the context of heavy chain constant regions and express immunoglobulin-like molecules comprising human immunoglobulin light chain variable domains fused to heavy chain constant domains that are cognate with human immunoglobulin light chain variable domains fused to light chain constant domains.
Owner:REGENERON PHARM INC
Dielectric properties models and methods of using same
ActiveUS8137110B2Avoid product qualityLengthened product timelinesAdditive manufacturing apparatusDiagnostic recording/measuringAnatomical structuresElectricity
Disclosed herein are dielectric properties models that are designed to enable simulated use testing by medical device companies, medical device designers, individual inventors, or any other entity interested in the performance of medical devices. These models are unique in possessing a level of correlation to dielectric properties of human or nonhuman animal tissues that allows them to be substituted for either a live animal, an animal cadaver, or a human cadaver in the testing of these devices. These models are further characterized by a similarity of geometry, individual component physical properties, and component-to-component interfacial properties with the appropriate target tissue and anatomy.
Owner:SAKEZLES CHRISTOPHER
Novel culture systems for ex vivo development
InactiveUS20060112438A1Improve efficiencyIncreased riskArtificial cell constructsEmbryonic cellsEmbryoEx vivo
The present invention provides methods for the culture of animal pluripotent stem cells and their differentiated progeny cells, tissues, and organs, and nonhuman animal embryos and fetuses.
Owner:WEST MICHAEL D +2
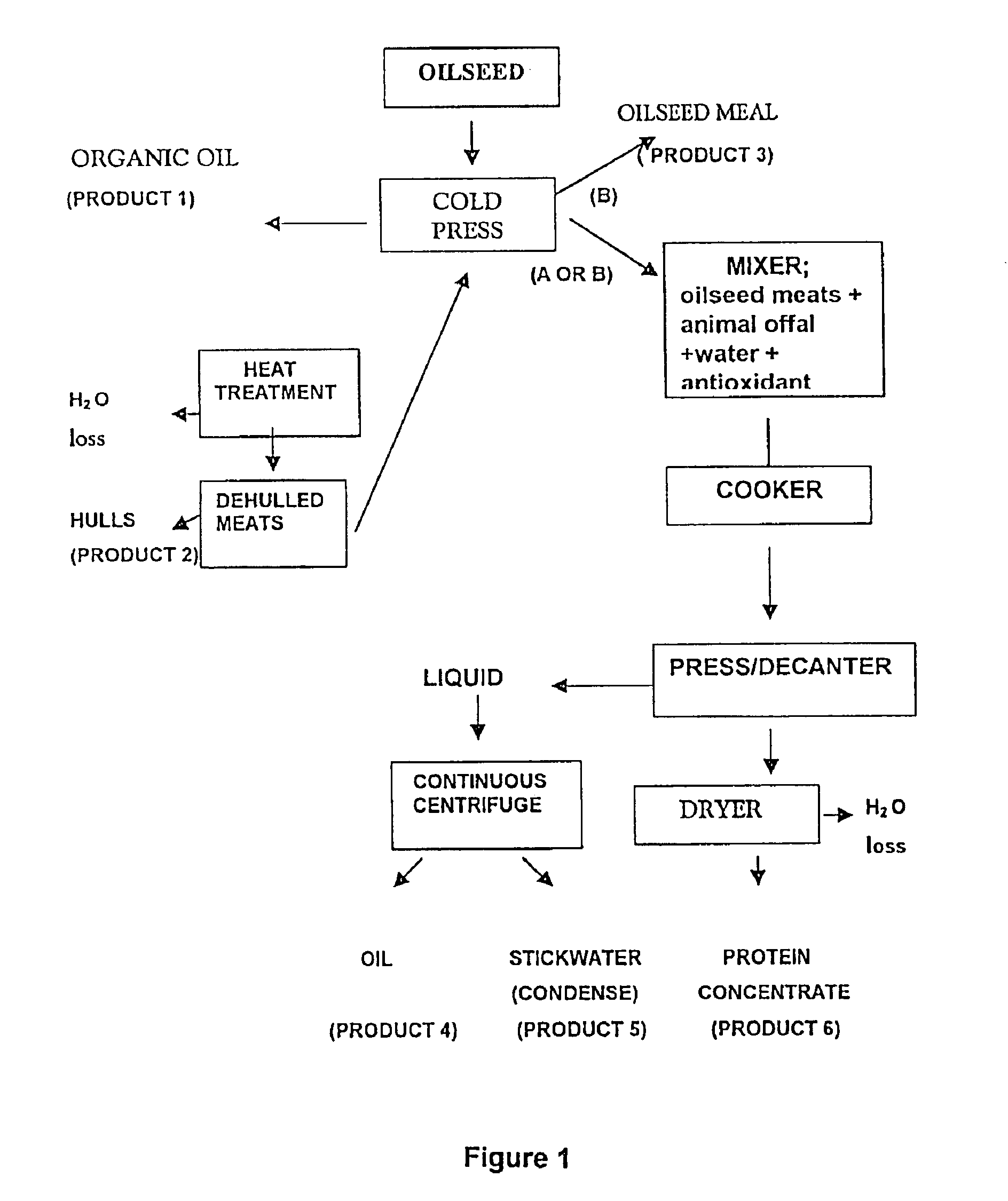
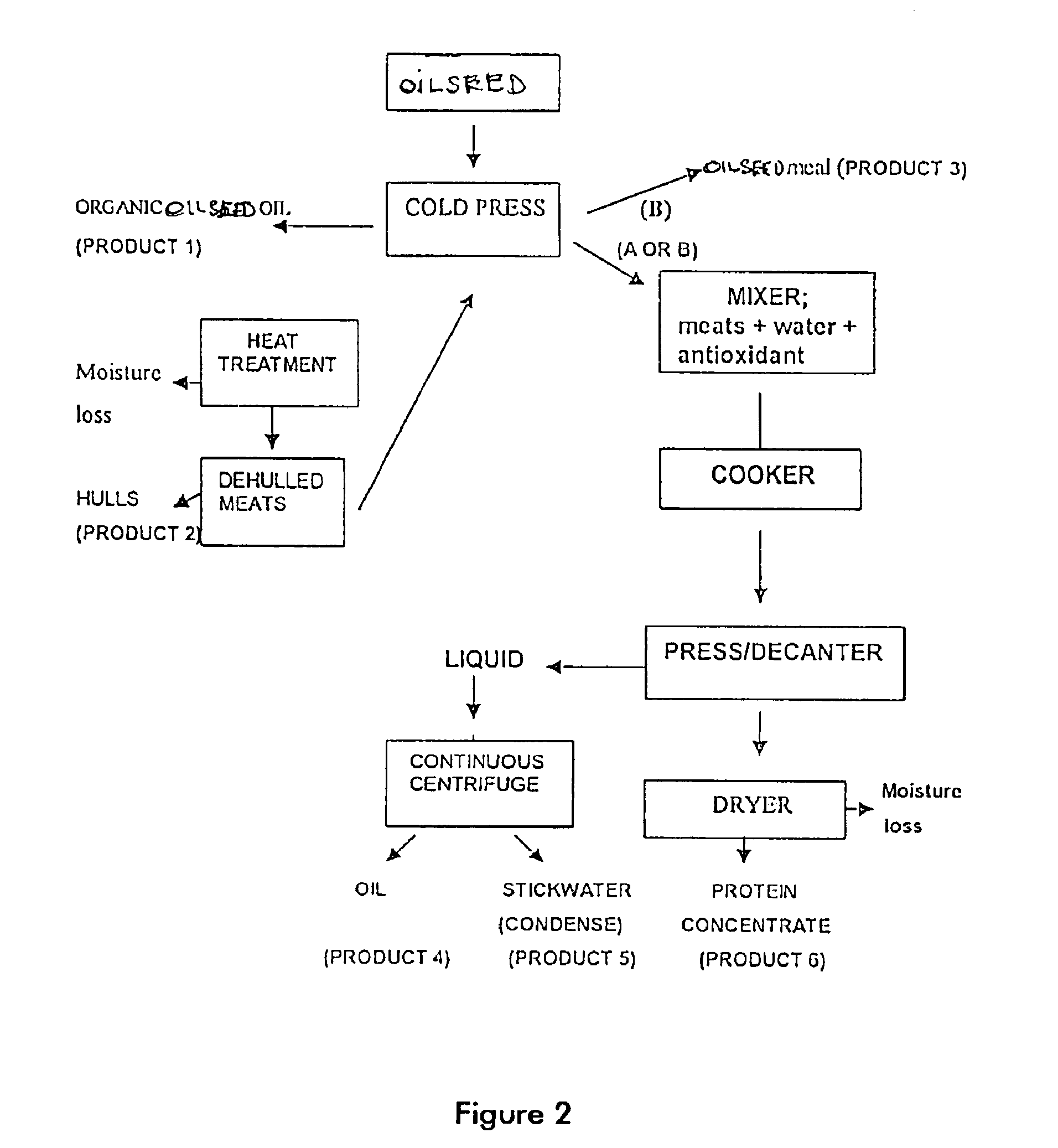
Protein and lipid sources for use in aquafeeds and animal feeds and a process for their preparation
InactiveUS6955831B2Increased digestible energy contentAdditional componentBioloigcal waste fertilisersEdible oils/fatsFiberHuman animal
A process for preparation of nutritionally upgraded oilseed meals which are protein and lipid-rich and have a reduced fiber content, and plant oils from oilseeds for use in fish or other non-human animal diets or human foods comprising the steps of: providing a source of oilseed; subjecting the oilseed to heat treatment to substantially reduce the concentration of at least some antinutritional components normally present in the oilseed to obtain heat-treated seed; dehulling the heat-treated seed to produce a meat fraction, a hull fraction or a mixture thereof; and cold pressing the meat fraction or the mixture to yeild the plant oils and the protein and lipid-rich meals.
Owner:HER MAJESTY THE QUEEN IN RIGHT OF CANADA AS REPRRESENTED BY THE MINIST OF FISHERIES & OCEANS
Systems and methods for non-invasive physiological monitoring of non-human animals
ActiveUS7762953B2Little and no distressAccurate monitoringElectrocardiographyInertial sensorsHuman animalMedicine
This invention provides monitoring garments for non-invasively monitoring physiological parameters in un-restrained and / or restrained animals, such as monkeys, rabbits, dogs, horses, and the like. The invention also includes methods and systems for collecting and processing monitoring data and methods for recognizing apneas and other respiratory events, periods of restfulness and wakefulness, stereotypical behavior and other indicators of dysphoric states, periods of emesis, and occurrence of barking and coughing.
Owner:ADIDAS
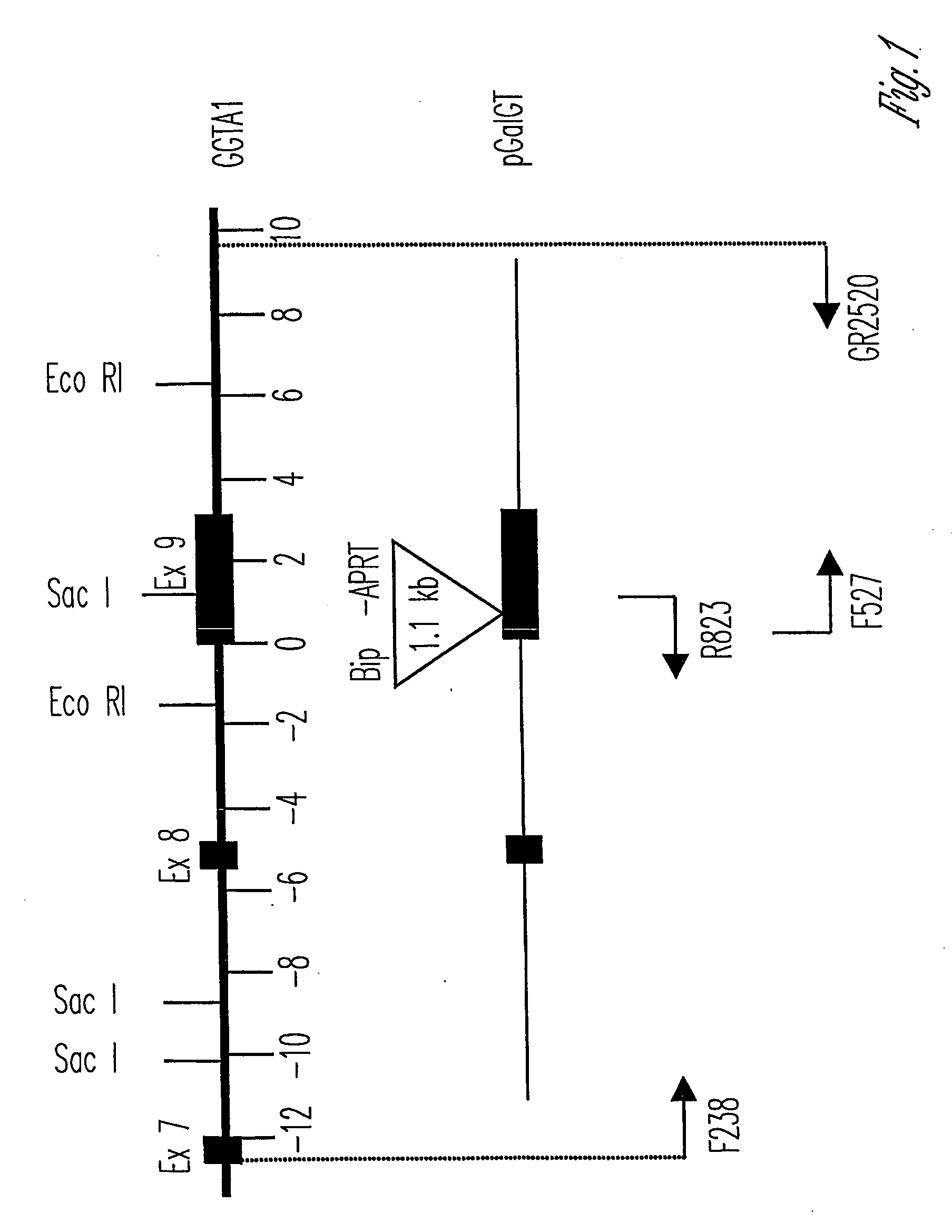
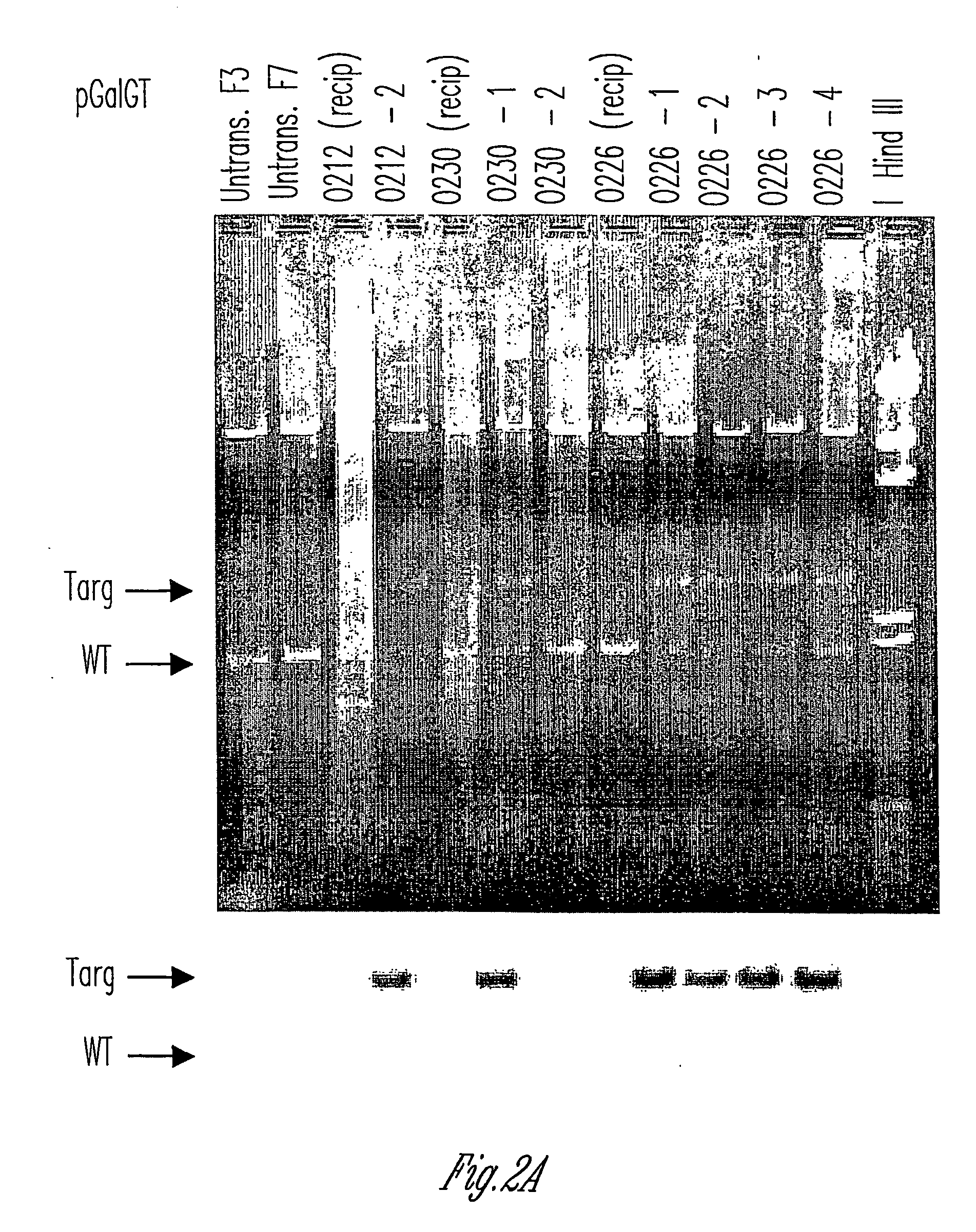
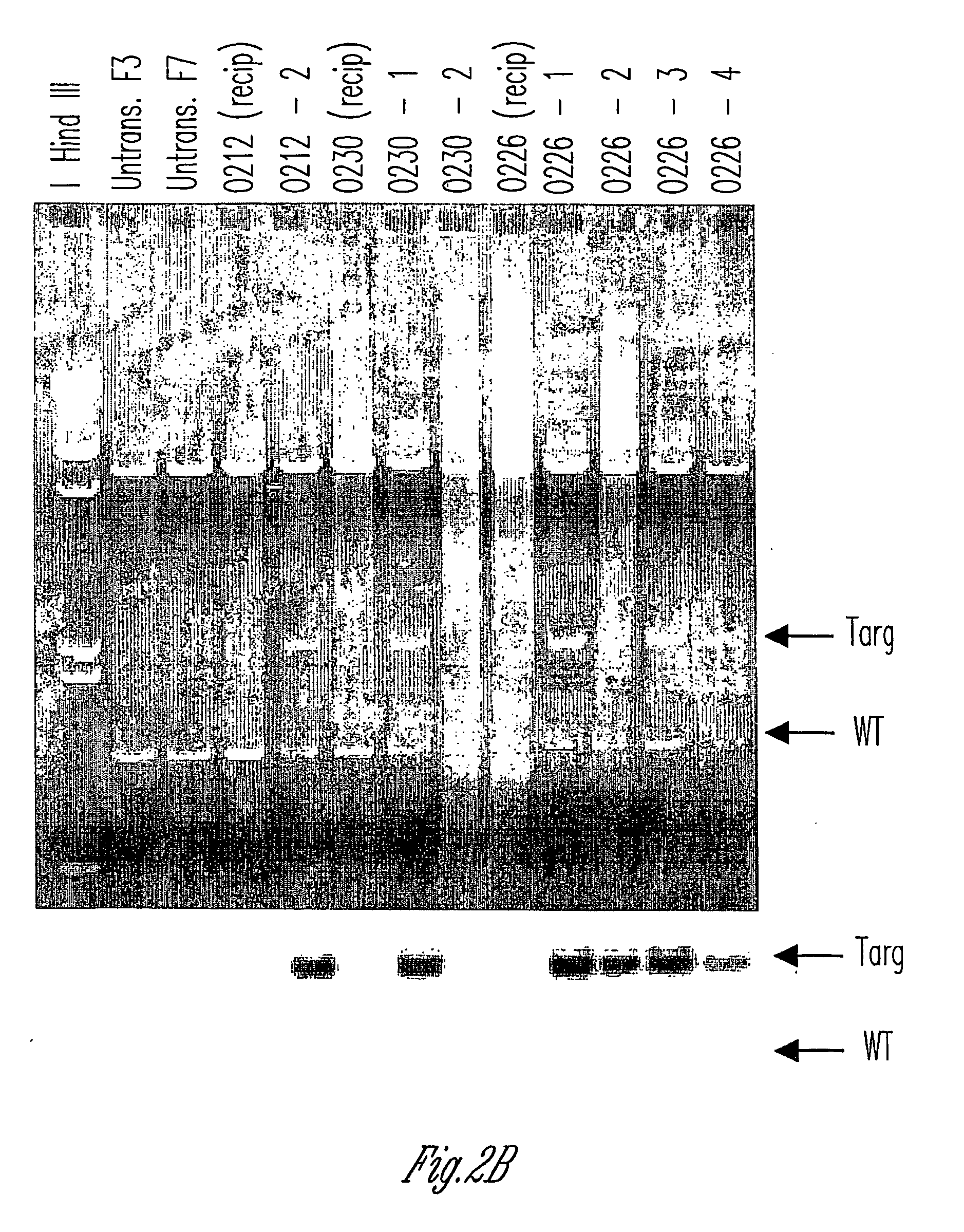
Knockout swine and methods for making the same
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides viable gene knockout swine including swine in which the α(1,3)-galactosyltransferase gene has been disrupted, methods for making such swine, and methods of using the tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS +1
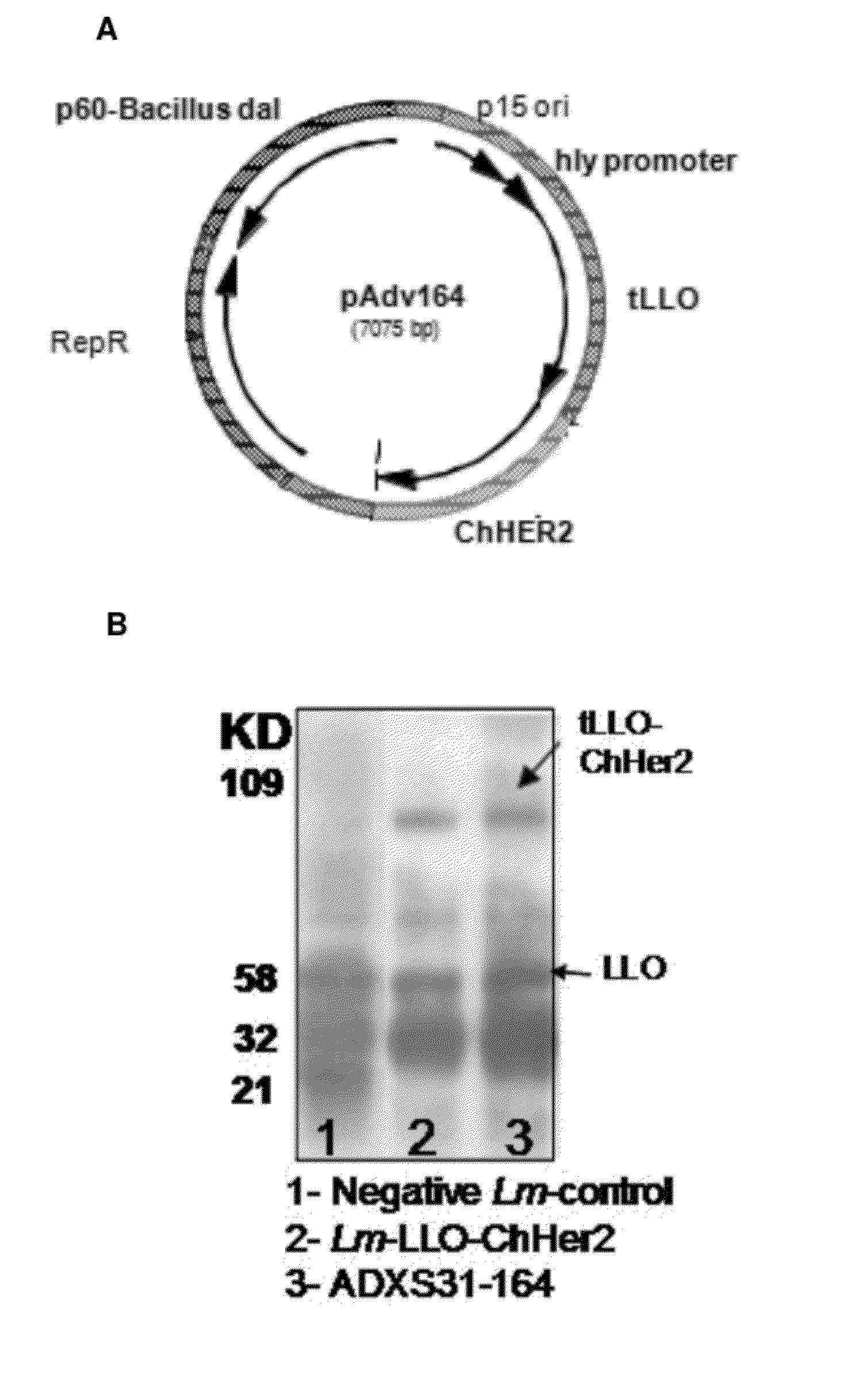
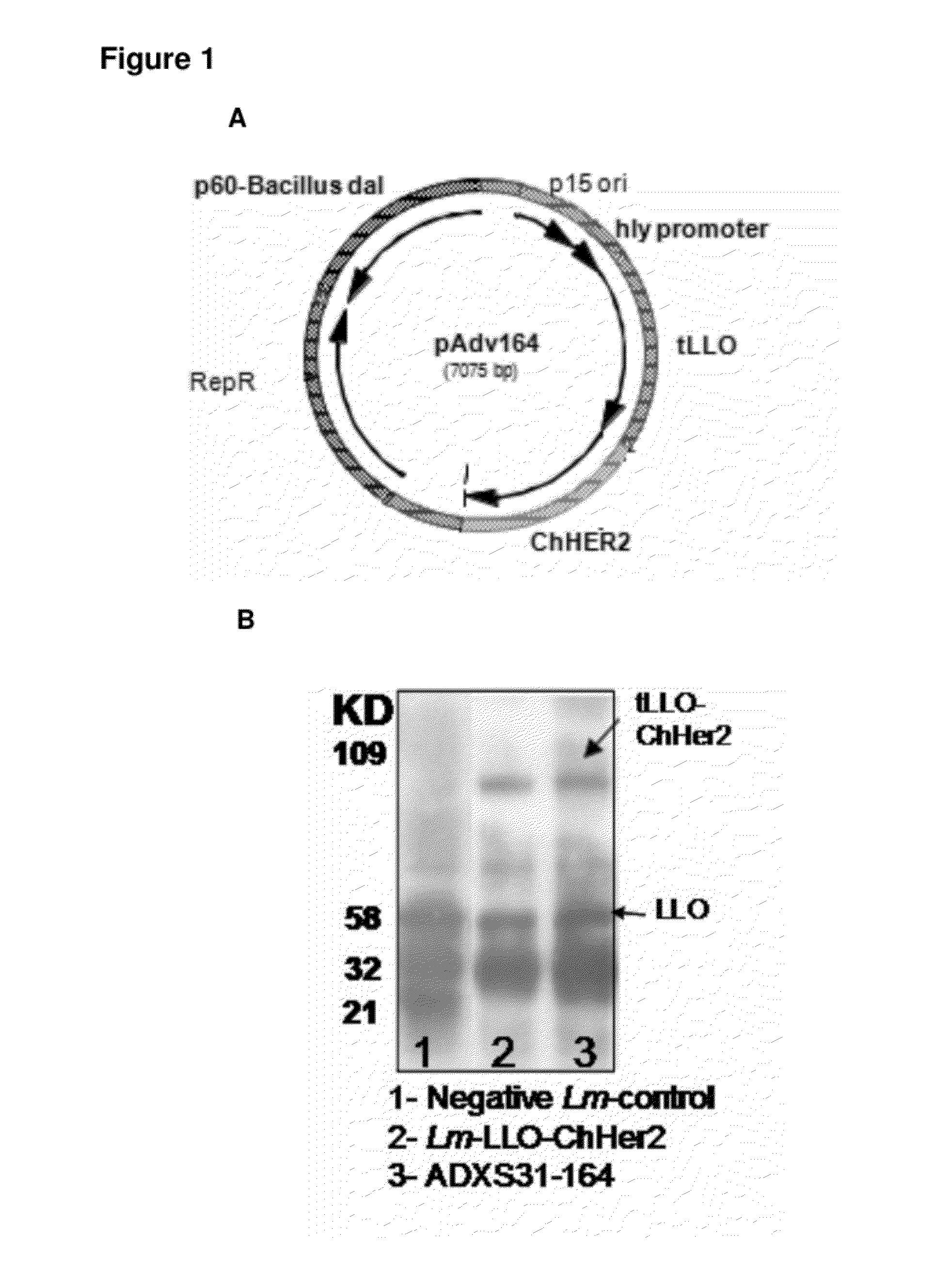
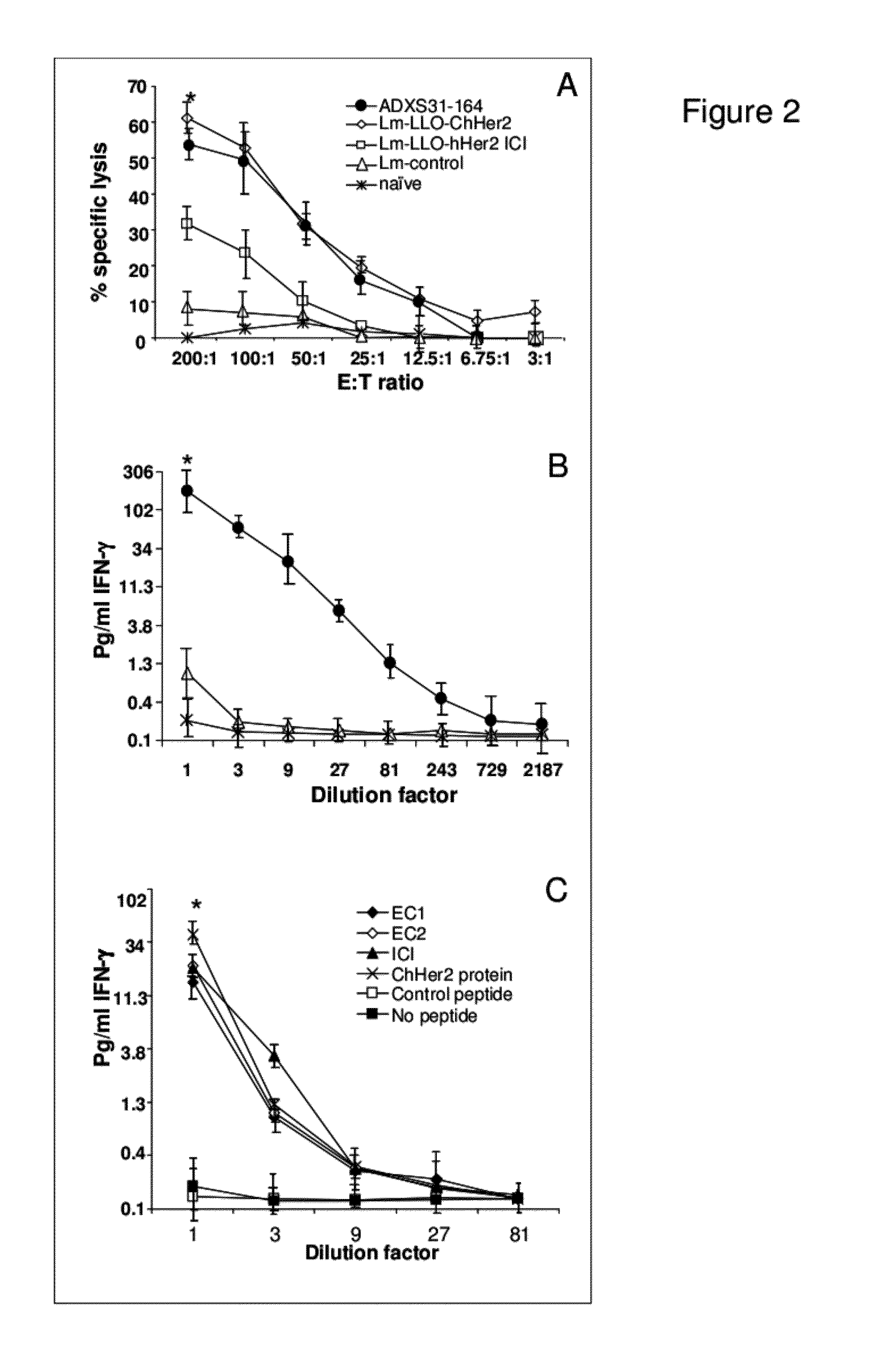
Compositions and methods for prevention of escape mutation in the treatment of her2/neu over-expressing tumors
This invention provides compositions and methods for treating and vaccinating against an Her2 / neu antigen-expressing tumor and inducing an immune response against dominant in a non-human animal.
Owner:ADVAXIS +1
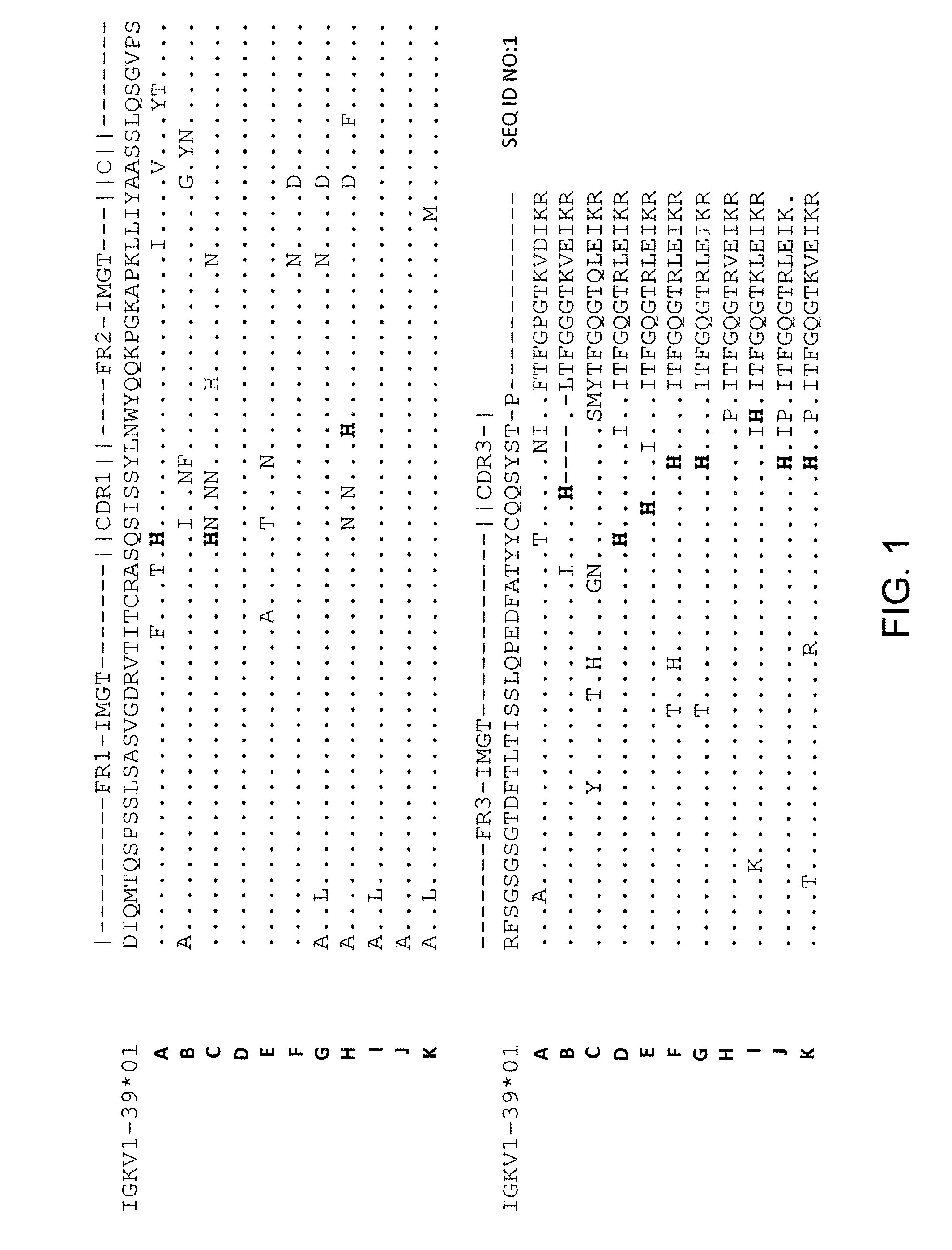
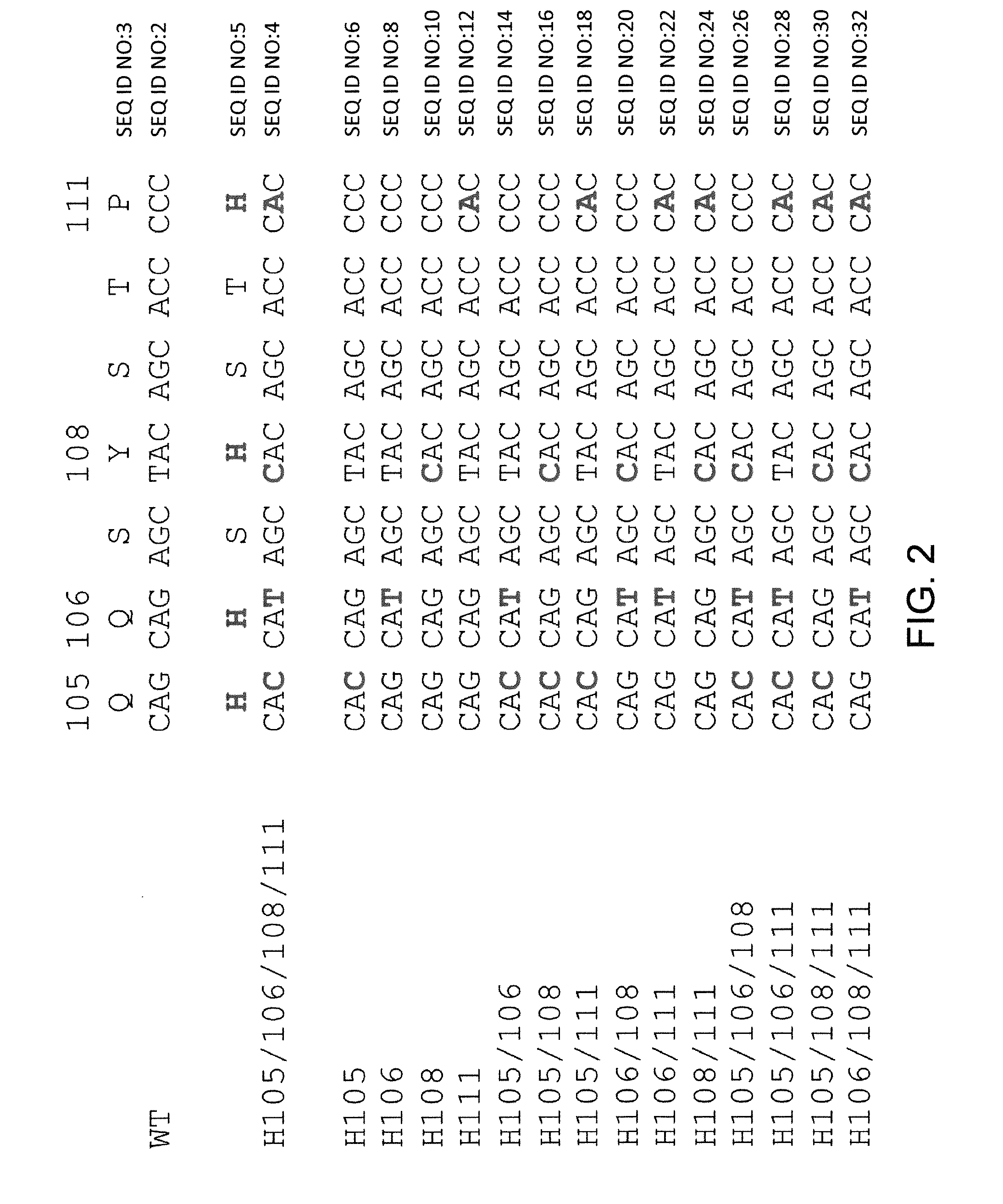
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
ActiveUS20130247234A1Reduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman animalVariable domain
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses a single light chain variable domain derived from a single rearranged light chain variable region gene in the germline of the non-human animal, wherein the single rearranged light chain variable region gene comprises a substitution of at least one non-histidine encoding codon with a histidine encoding codon. Methods of making non-human animals that express antibodies comprising a histidine-containing universal light chain are provided.
Owner:REGENERON PHARM INC
Electronic estrus detection device
InactiveUS7083575B1Improve water resistanceGood for observationAnimal reproductionVaccination/ovulation diagnosticsHuman animalEstrus Detection
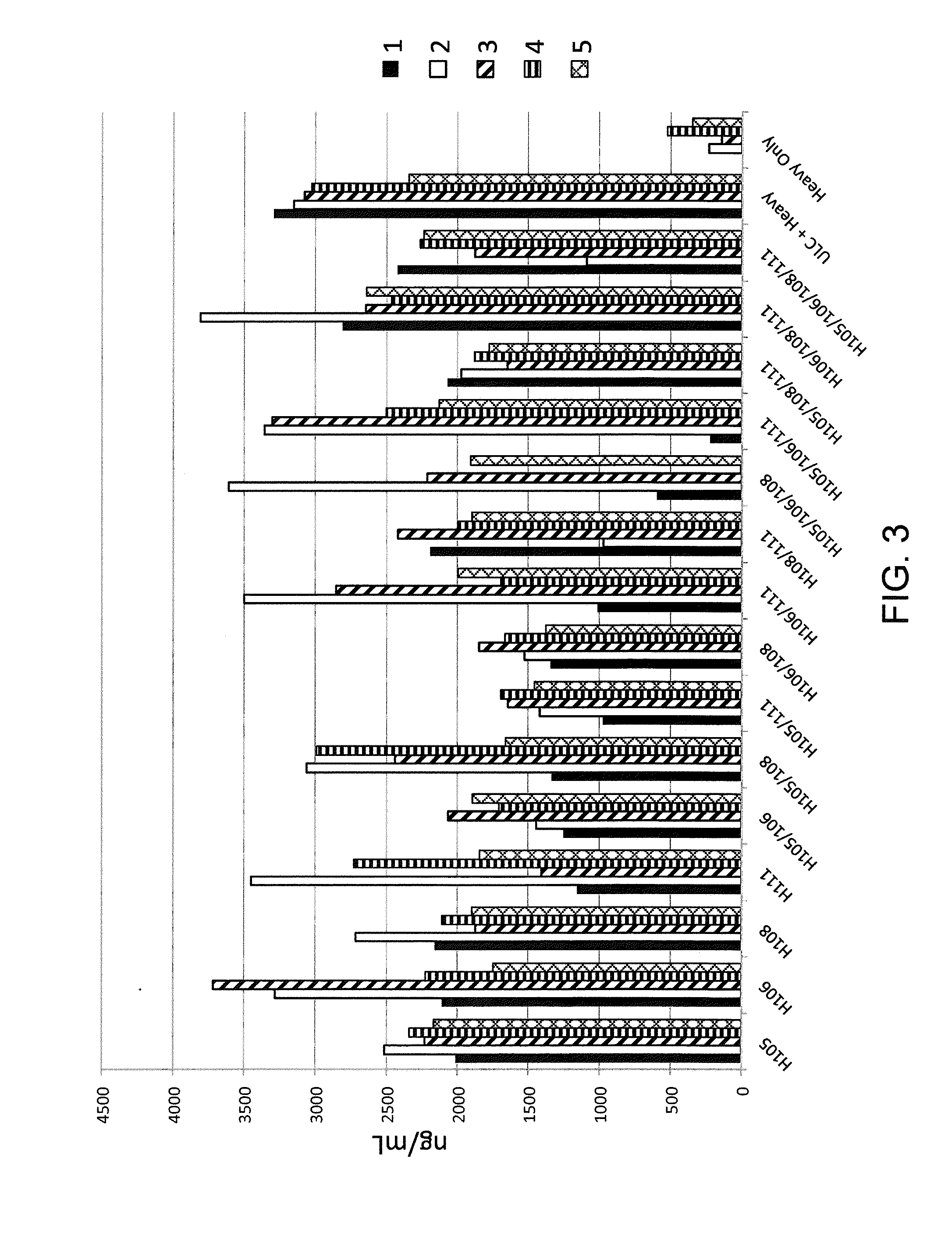
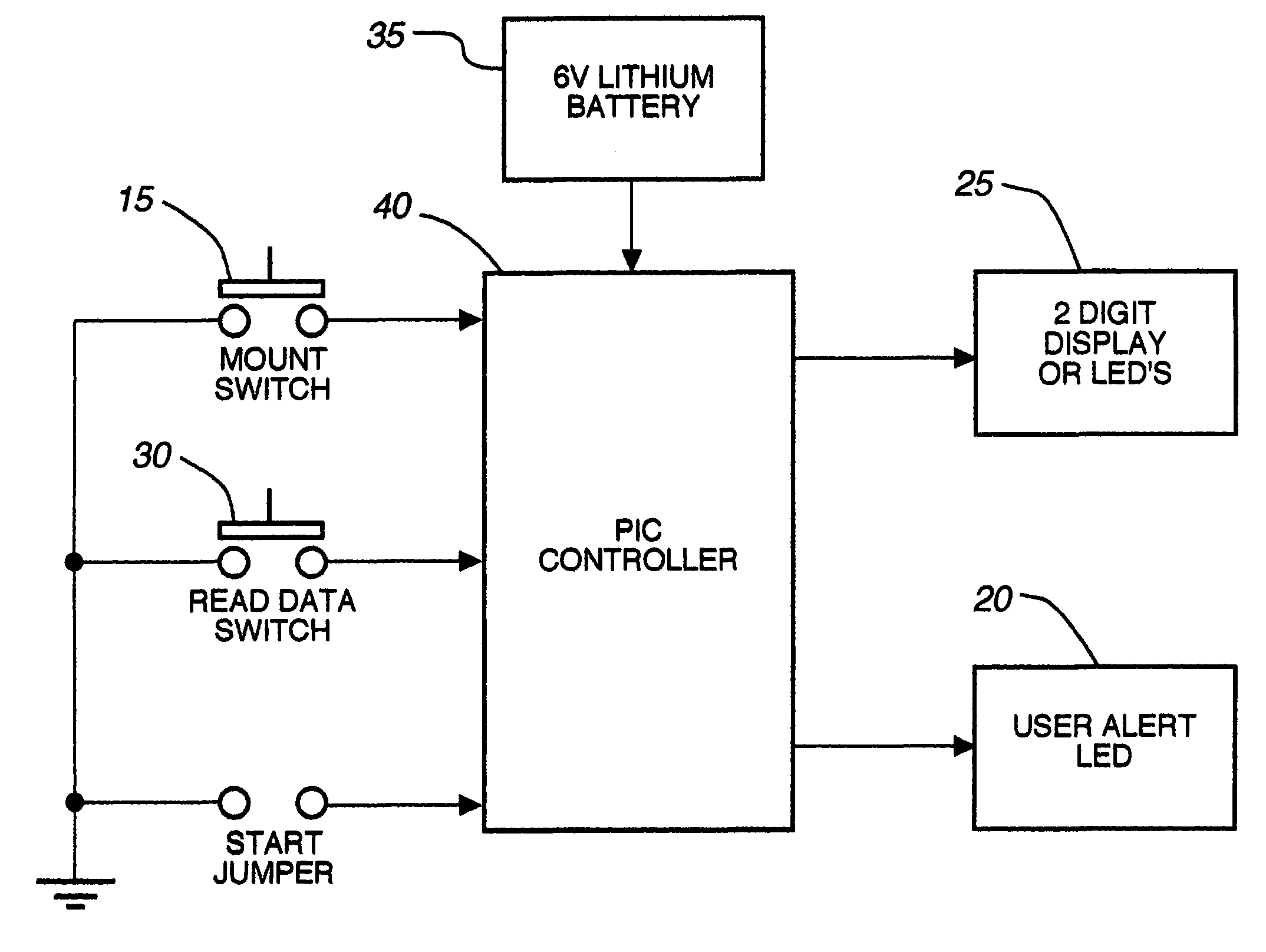
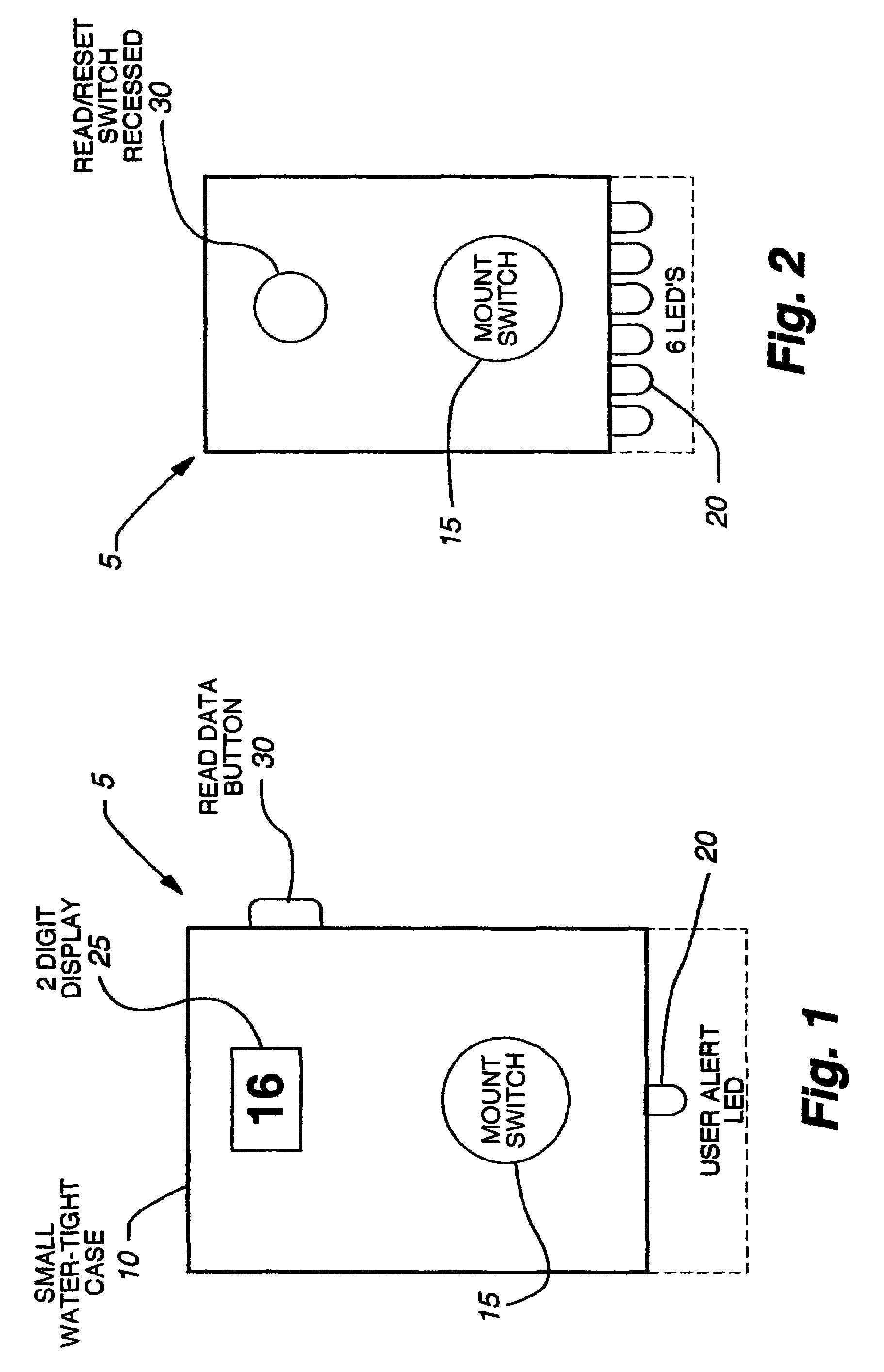
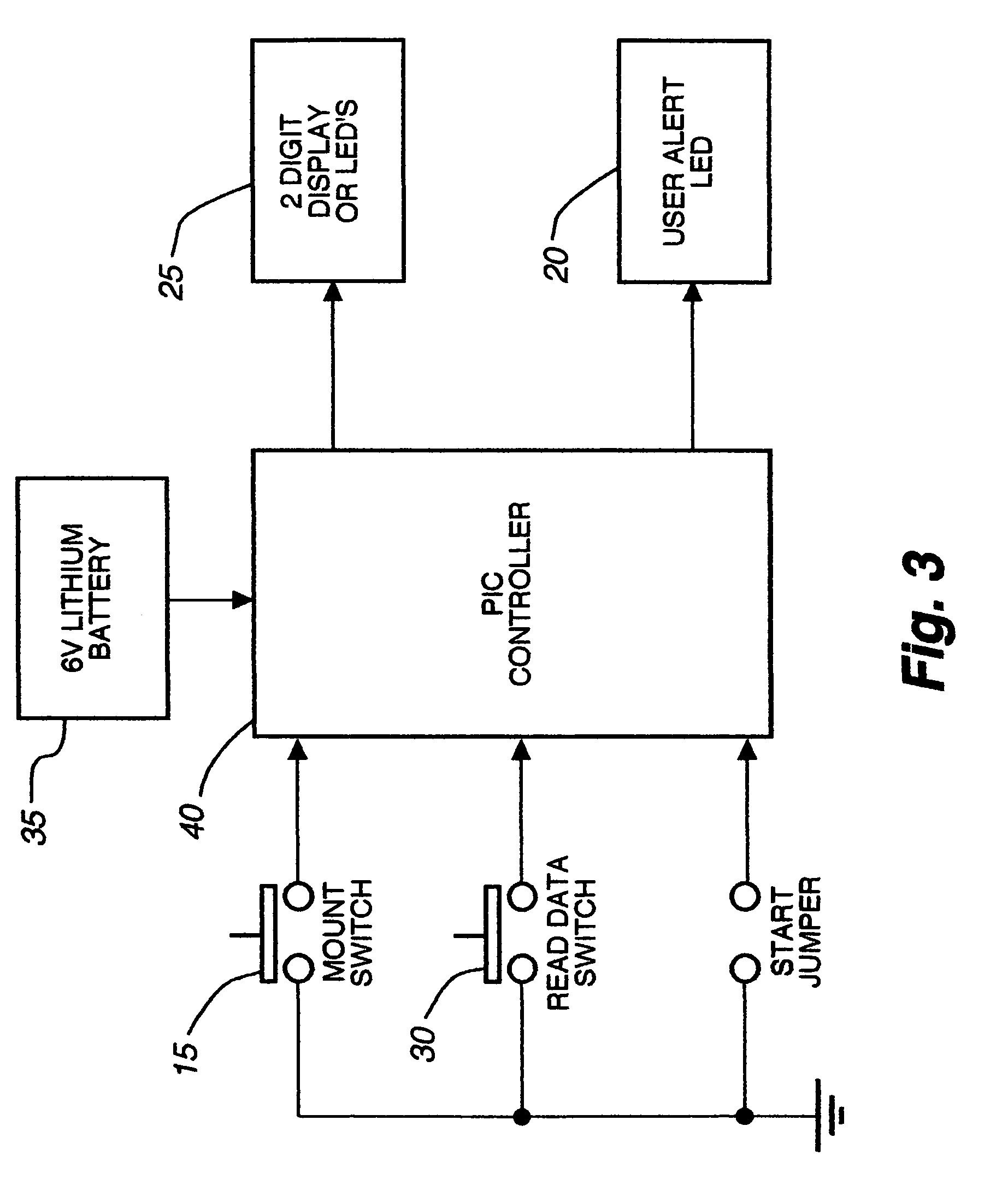
An electronic device for detecting estrus that may be affixed to the tail-head area of a cow is described. This device permits the accurate determination of optimal breeding time, natural or artificial, based on mounting activity. The device includes a water resistant housing, within which is contained the electronic portion of the device consisting of a controller means, a power means, an activation means, a read data means, and at least one display means; said device determining and subsequently indicating suspected and confirmed estrus based on an algorithm. The invention may be used, for example, with any non-human animal exhibiting standing heats and / or mounting behavior indicative of an estrus cycle for making mounting data determinations related to the estrus cycle and analyzing such data to determine optimal time to breed.
Owner:COWCHIPS
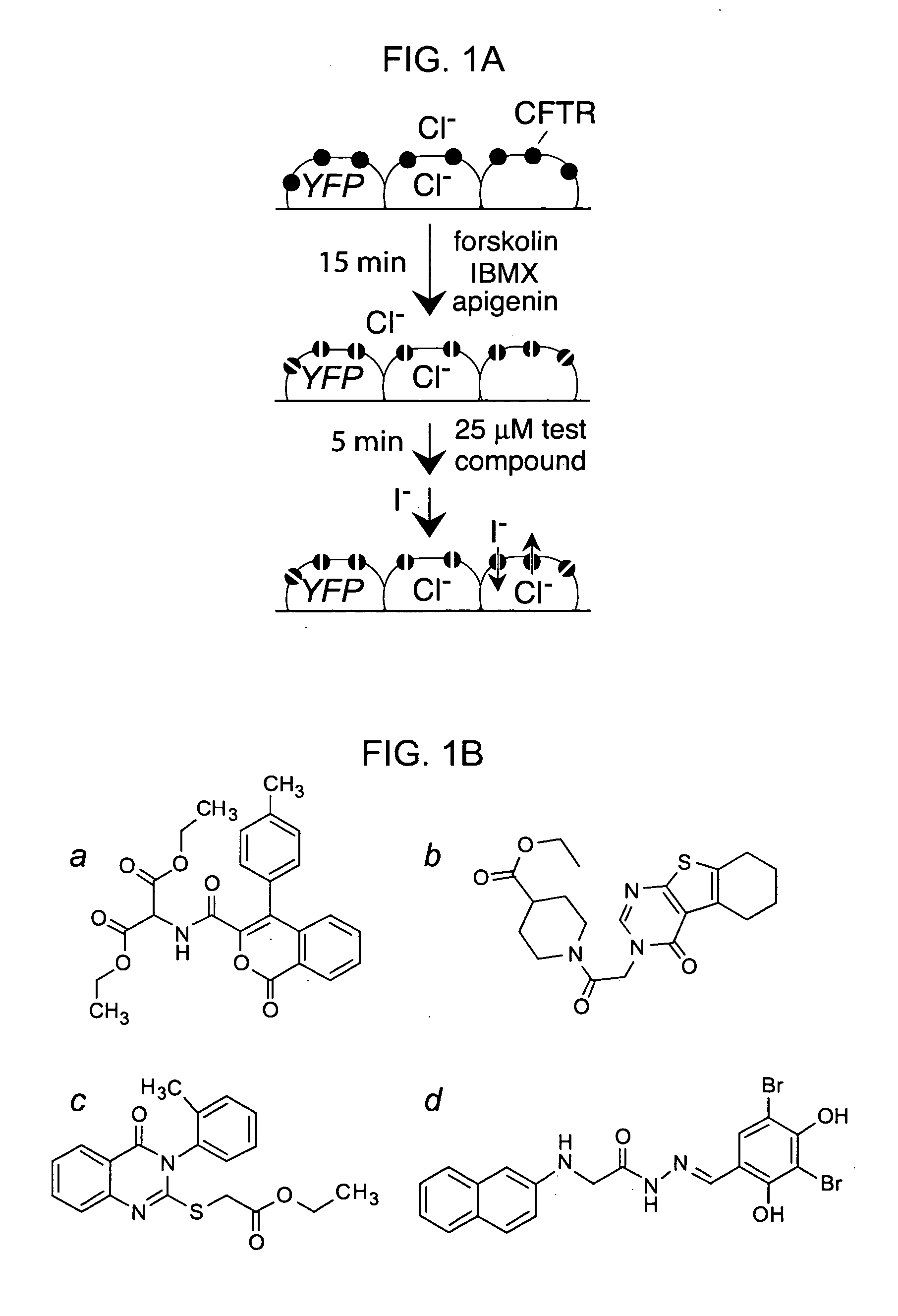
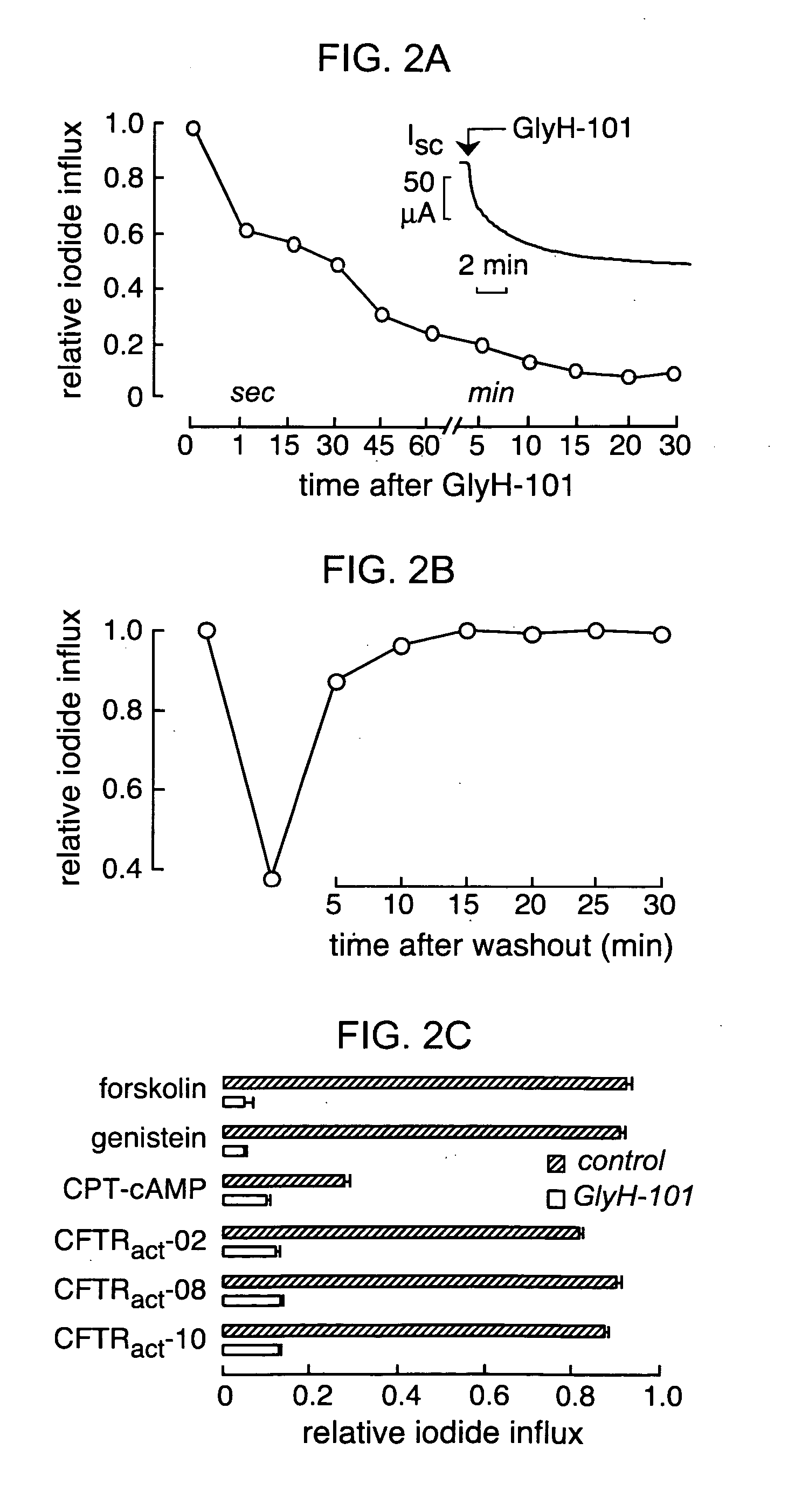
Hydrazide-containing CFTR inhibitor compounds and uses thereof
The invention provides compositions, pharmaceutical preparations and methods for inhibition of cystic fibrosis transmembrane conductance regulator protein (CFTR) that are useful for the study and treatment of CFTR-mediated diseases and conditions. The compositions and pharmaceutical preparations of the invention may comprise one or more hydrazide-containing compounds, and may additionally comprise one or more pharmaceutically acceptable carriers, excipients and / or adjuvants. The methods of the invention comprise, in certain embodiments, administering to a patient suffering from a CFTR-mediated disease or condition, an efficacious amount of a hydrazide-containing compound. In other embodiments the invention provides methods of inhibiting CFTR that comprise contacting cells in a subject with an effective amount of a hydrazide-containing compound. In addition, the invention features a non-human animal model of CFTR-mediated disease which model is produced by administration of a hydrazide-containing compound to a non-human animal in an amount sufficient to inhibit CFTR.
Owner:RGT UNIV OF CALIFORNIA
Compositions and treatment of heart deficiency in non-human animals
InactiveUS20100183718A1Reduce morbidityImprove heart functionBiocideDipeptide ingredientsHuman animalPhysiology
The invention relates to novel compositions including an aldosterone antagonist and administered according to a predetermined dosage for treating heart deficiency in non-human mammals.
Owner:CEVA SANTE ANIMALE
Hco32 and hco27 and related examples
The instant invention relates to transgenic non-human animals capable of producing heterologous antibodies, transgenes used to produce such transgenic animals, transgenes capable of functionally rearranging a heterologous D gene in V-D-J recombination, immortalized B-cells capable of producing heterologous antibodies, methods and transgenes for producing heterologous antibodies of multiple isotypes, methods and transgenes for producing heterologous antibodies wherein a variable region sequence comprises somatic mutation as compared to germline rearranged variable region sequences, transgenic nonhuman animals which produce antibodies having a human primary sequence and which bind to human antigens, hybridomas made from B cells of such transgenic animals, and monoclonal antibodies expressed by such hybridomas.
Owner:ER SQUIBB & SONS INC
Genetically modified major histocompatibility complex mice
The invention provides genetically modified non-human animals that express a humanized MHC II protein (humanized MHC II α and β polypeptides), as well as embryos, cells, and tissues comprising the humanized MHC II protein. Also provided are constructs for and methods of making the genetically modified non-human animals. Methods of using the genetically modified non-human animals to study various aspects of the human immune system are provided.
Owner:REGENERON PHARM INC
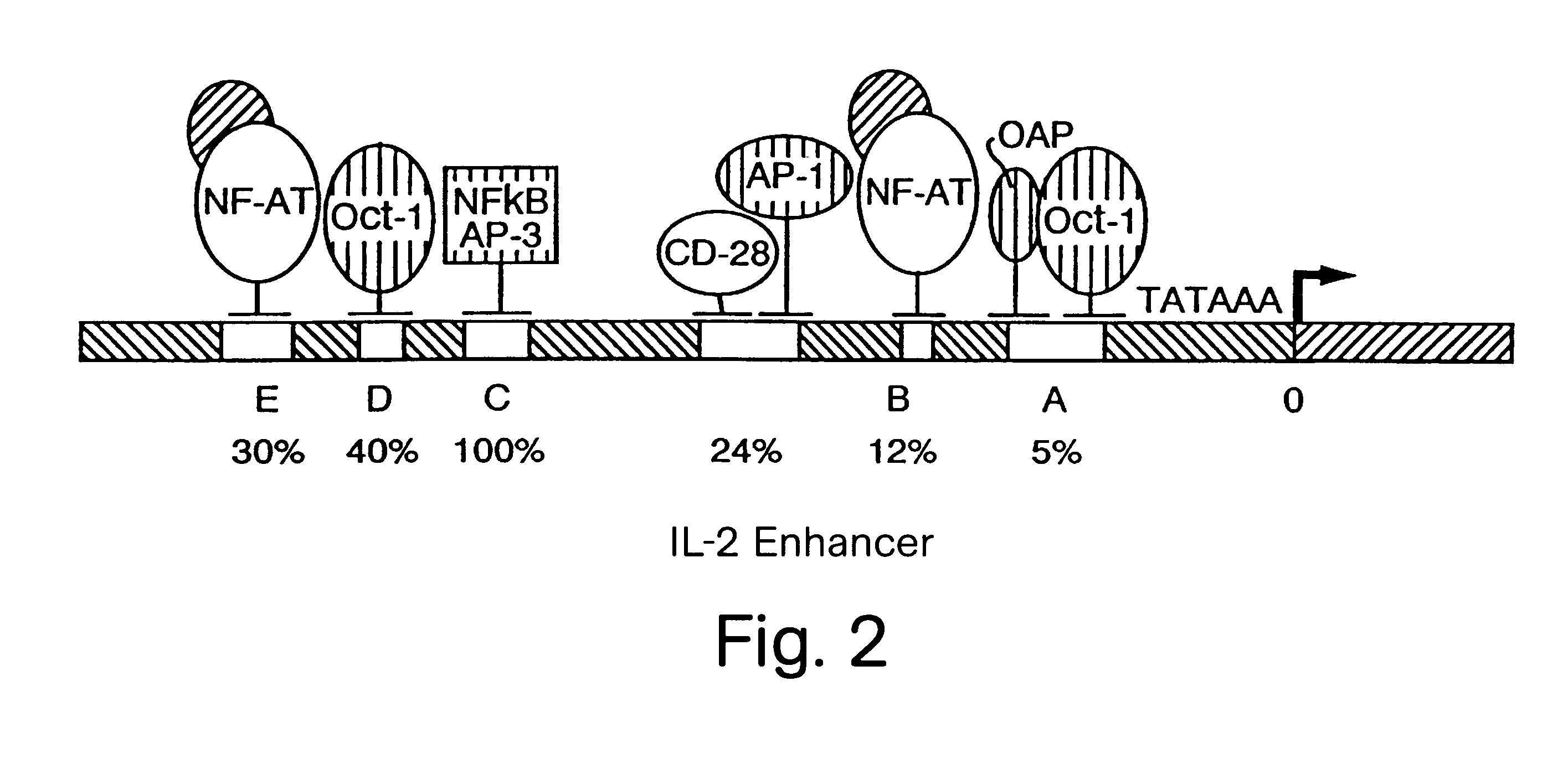
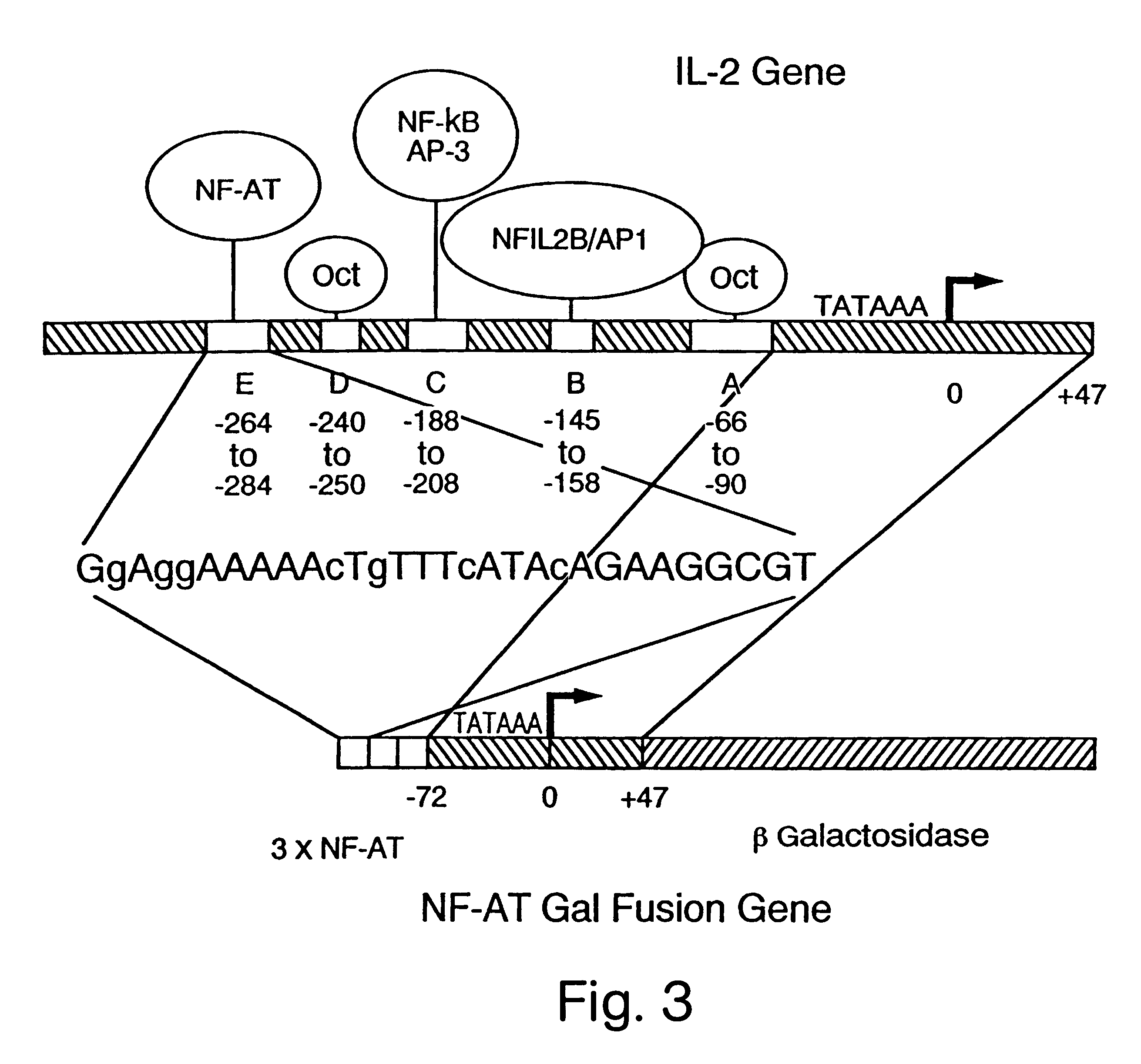
NF-AT polypeptides and polynucleotides and screening methods for immunosuppressive agents
InactiveUS6352830B1Inhibiting transcriptionInhibition of translationCompound screeningPeptide librariesHybridization probeNucleotide
The invention provides novel polypeptides which are associated with the transcription complex NF-AT, polynucleotides encoding such polypeptides, antibodies which are reactive with such polypeptides, polynucleotide hybridization probes and PCR amplification probes for detecting polynucleotides which encode such polypeptides, transgenes which encode such polypeptides, homologous targeting constructs that encode such polypeptides and / or homologously integrate in or near endogenous genes encoding such polypeptides, nonhuman transgenic animals which comprise functionally disrupted endogenous genes that normally encode such polypeptides, and transgenic nonhuman animals which comprise transgenes encoding such polypeptides. The invention also provides methods for detecting T cells (including activated T cells) in a cellular sample, methods for treating hyperactive or hypoactive T cell conditions, methods for screening for immunomodulatory agents, methods for diagnostic staging of lymphocyte differentiation, methods for producing NF-AT proteins for use as research or diagnostic reagents, methods for producing antibodies reactive with the novel polypeptides, and methods for producing transgenic nonhuman animals. Also included are methods and agents for activation of NF-AT dependent transcription, including agents which interfere with the production, modification of nuclear or cytoplasmic subunits, or the nuclear import of the cytoplasmic subunits. In particular, screening tests for novel immunosuppressants are provided based upon the ability of NF-AT to activate transcription.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Animal-wearable device with warning
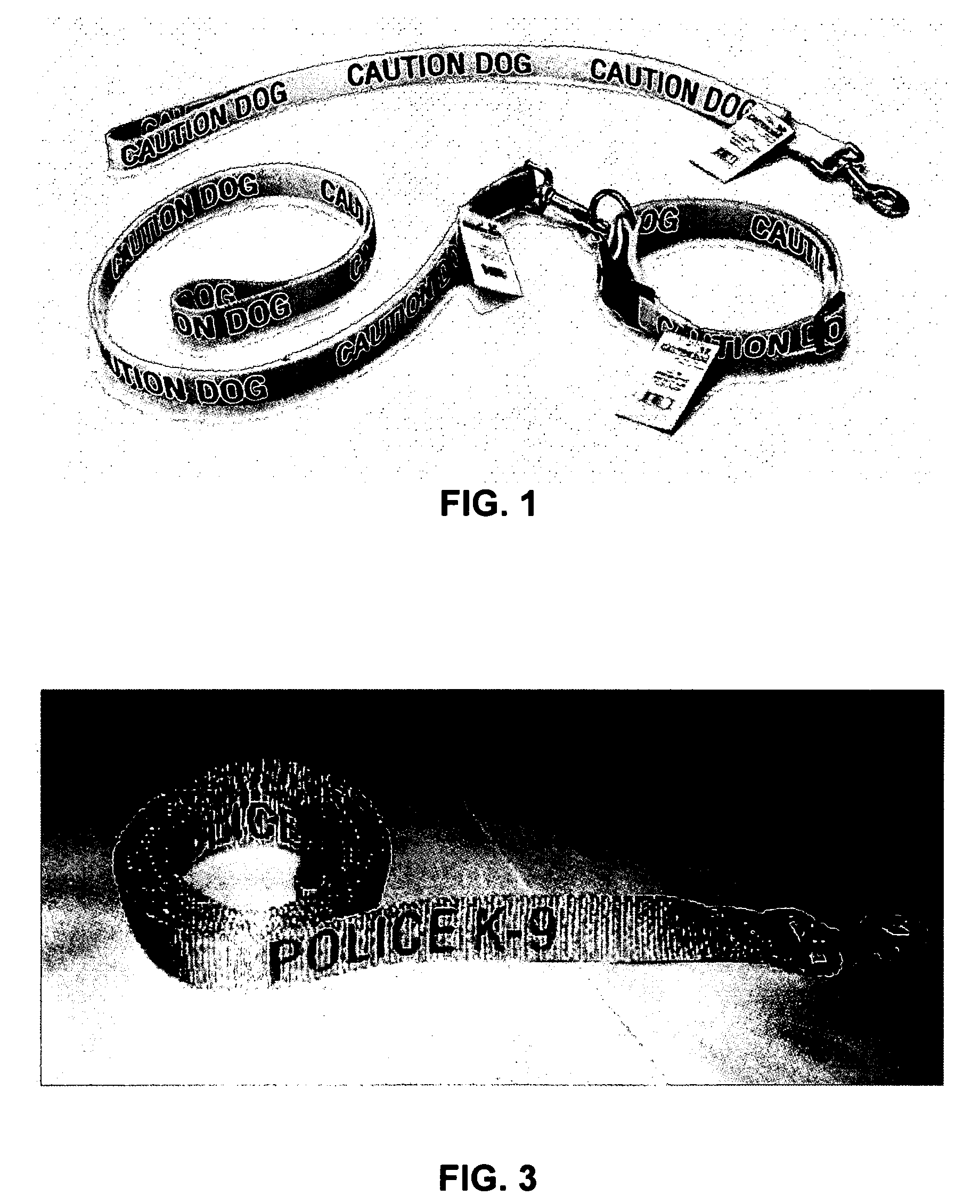
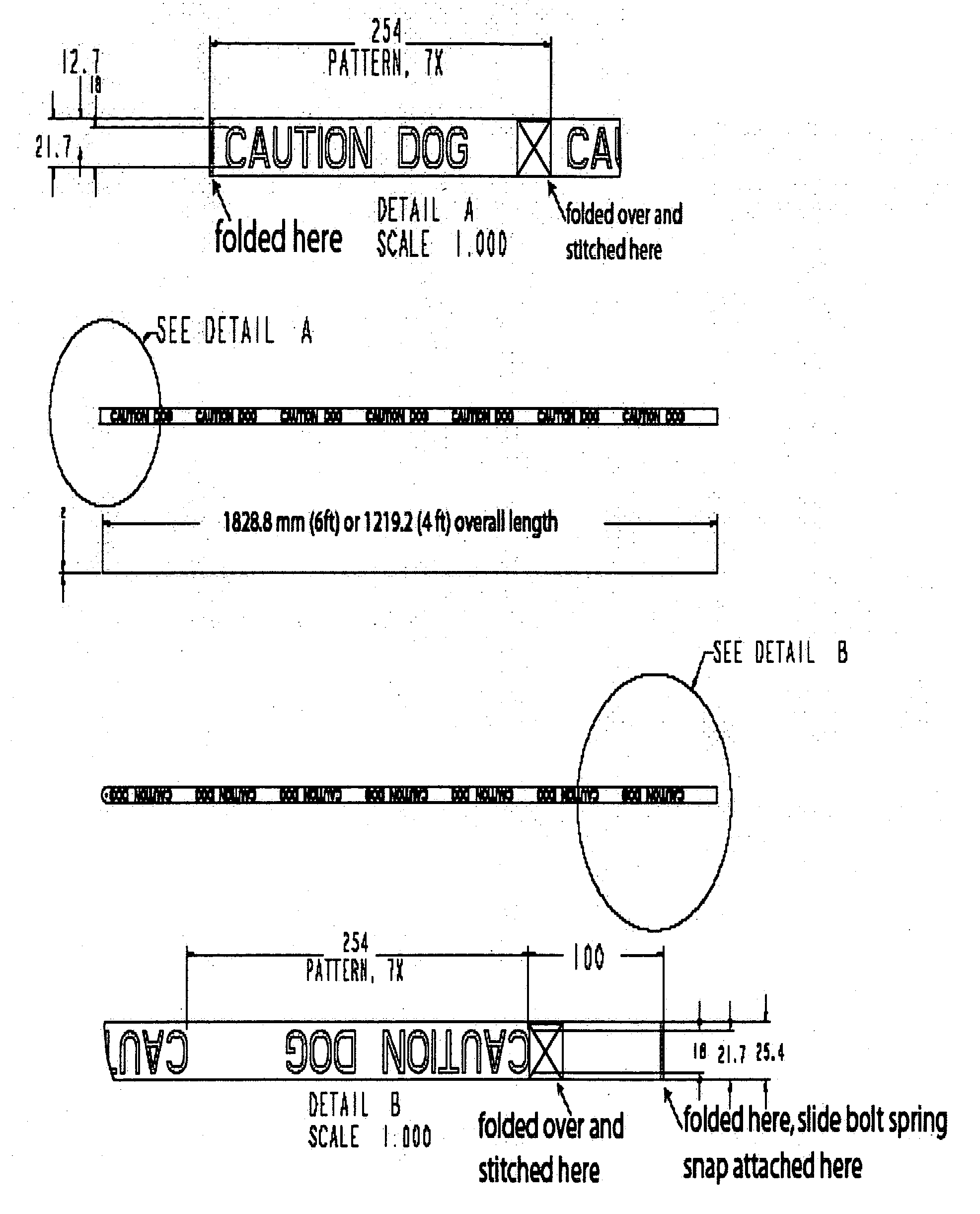
Provided is a device that includes an item of apparel for a nonhuman animal and a means for communicating a warning effective at a distance from the animal to discourage an individual from approaching the animal. Optionally, the device of claim excludes any indication that the animal is a service animal. Also provided are a kit of the device packaged with instructions for using of the device and a method for providing a warning.
Owner:AEBI CAROLINE ESTHER
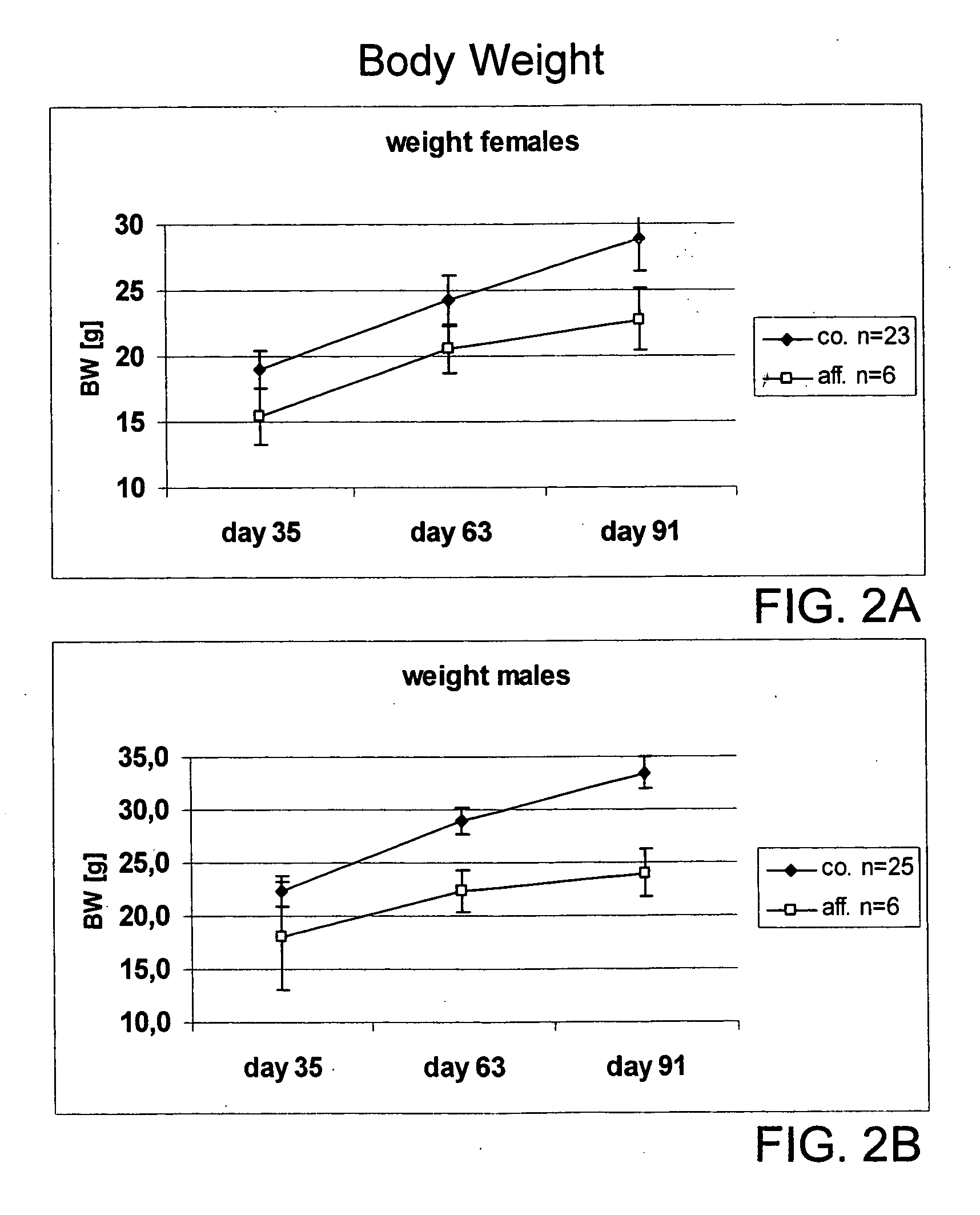
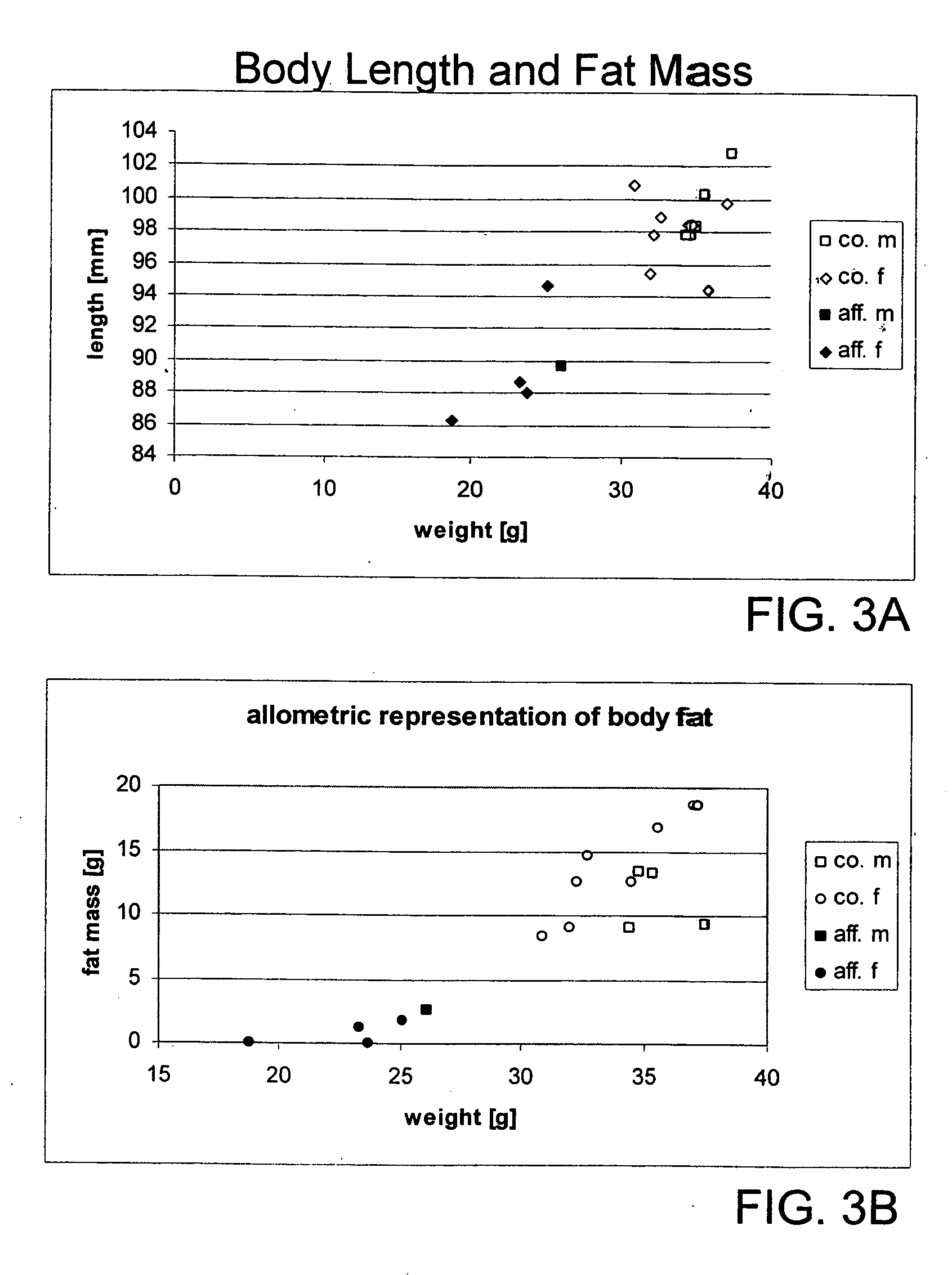
Spinster-like protein genes, expression products, non-human animal model: uses in human metabolic disorders
The present invention relates to a non-human vertebrate animal model displaying an alteration in fat metabolism or in the sensitivity towards leptin or insulin, which model bears a mutation in the gene encoding the spinster like 1 protein (Spinl1). The invention also relates to mutant Spinl1 proteins and nucleic acid sequences encoding these proteins. Furthermore, the invention relates to the use of the non-human vertebrate animal model for the identification of diagnostic markers, or as a model for studying the molecular and physiological mechanisms associated with an alteration in fat metabolism or an alteration in the sensitivity towards leptin or insulin, or for the identification and testing of agents useful in the prevention, amelioration, or treatment of the above conditions. Agents, pharmaceutical compositions, and methods for treating the above conditions are likewise described, as are methods for identifying said agents.
Owner:INGENIUM PHARMACEUTICALS AG
Bone xenografts
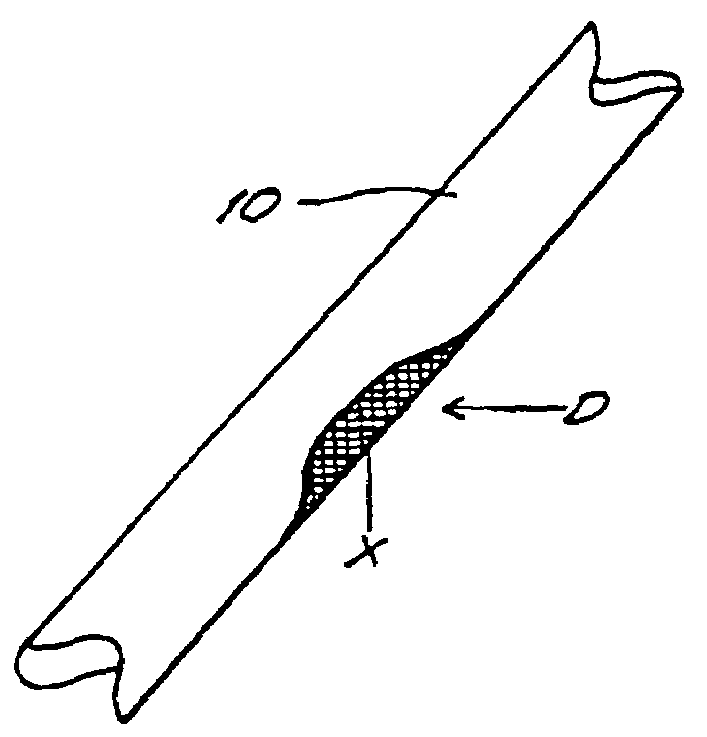
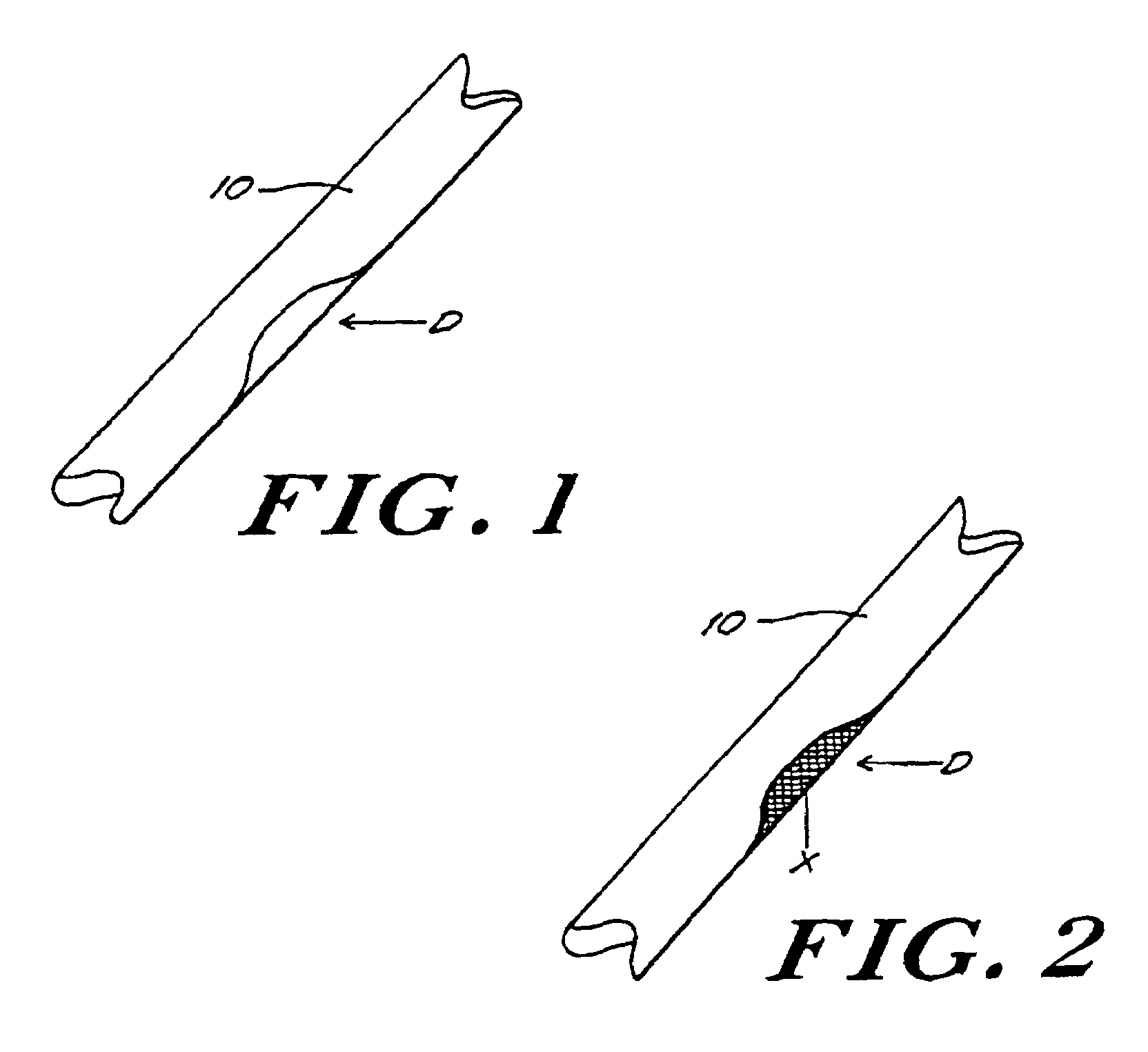
The invention provides an article of manufacture comprising a substantially non-immunogenic bone xenograft for implantation into humans. The invention further provides a method for preparing a bone xenograft by removing at least a portion of a bone from a non-human animal to provide a xenograft (X); washing the xenograft in saline and alcohol; subjecting the xenograft to a cellular disruption treatment; and treating the xenograft with a glycosidase to remove surface carbohydrate moieties. The invention also provides an article of manufacture produced by the above identified method of invention. The invention further provides a bone xenograft for implantation into a human including a portion (10) of a bone from a nonhuman animal, wherein the portion has substantially no surface carbohydrate moieties which are susceptible to glycosidase digestion. Each xenograft of the invention has substantially the same mechanical properties as a corresponding native bone.
Owner:APERION BIOLOGICS
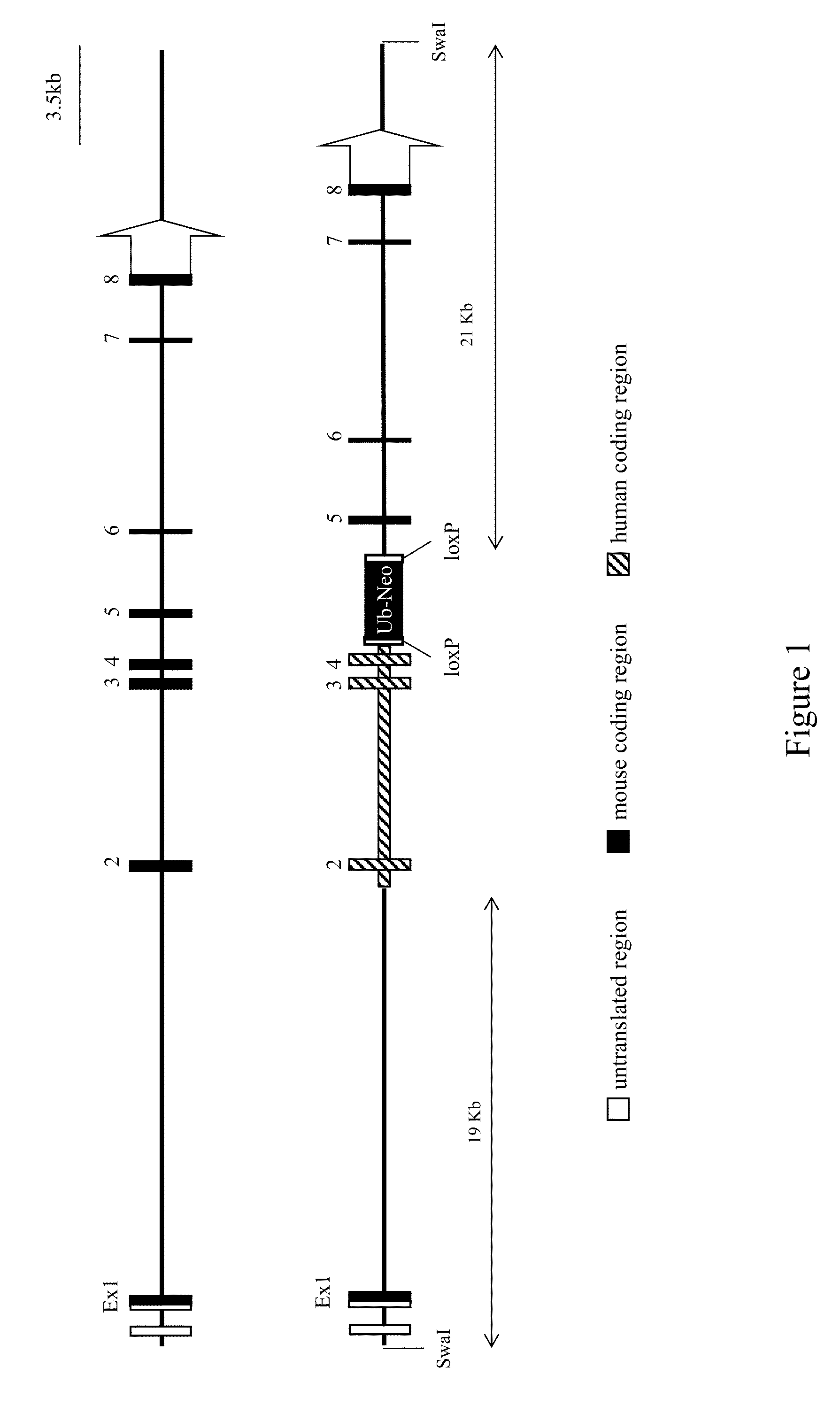
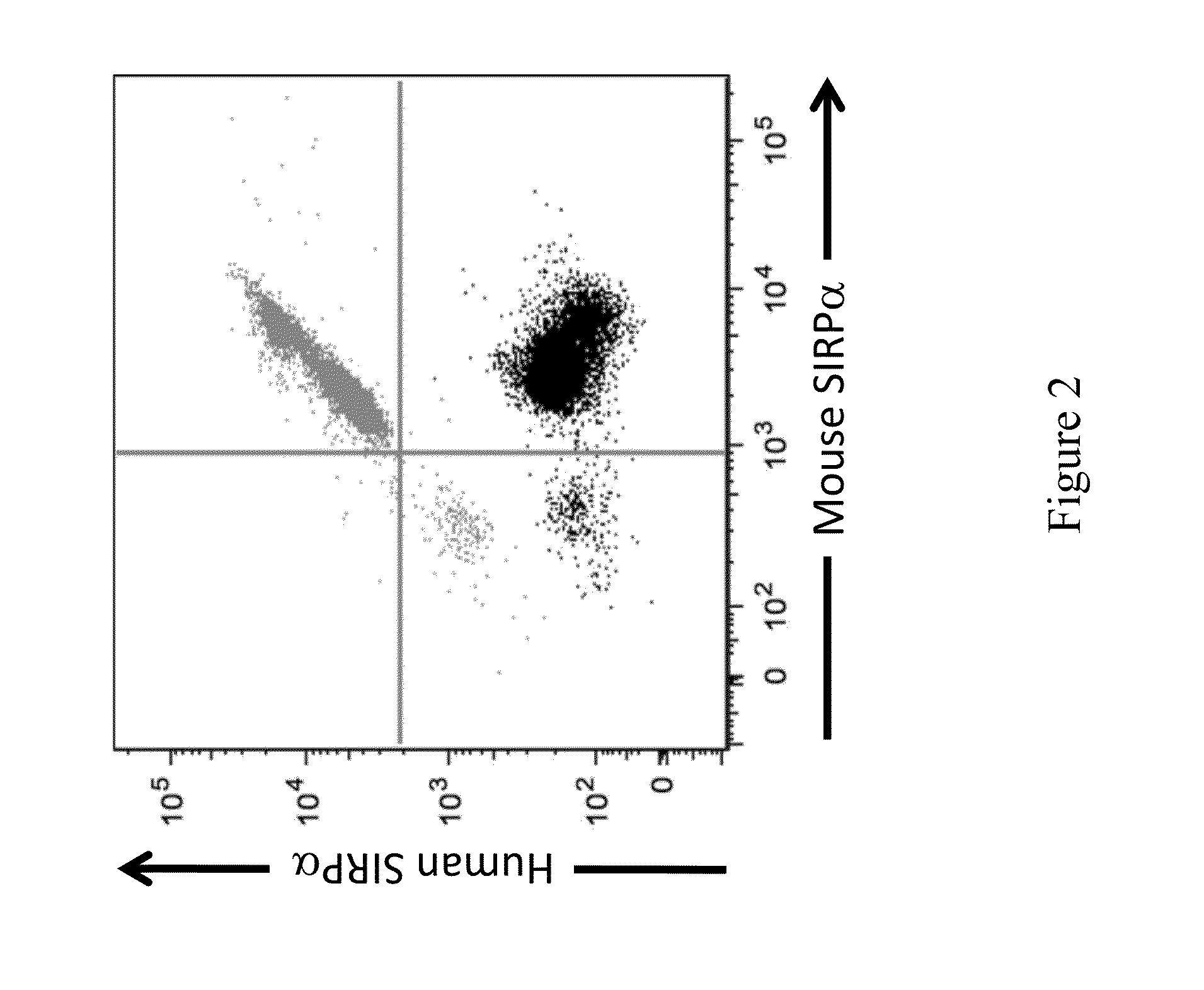
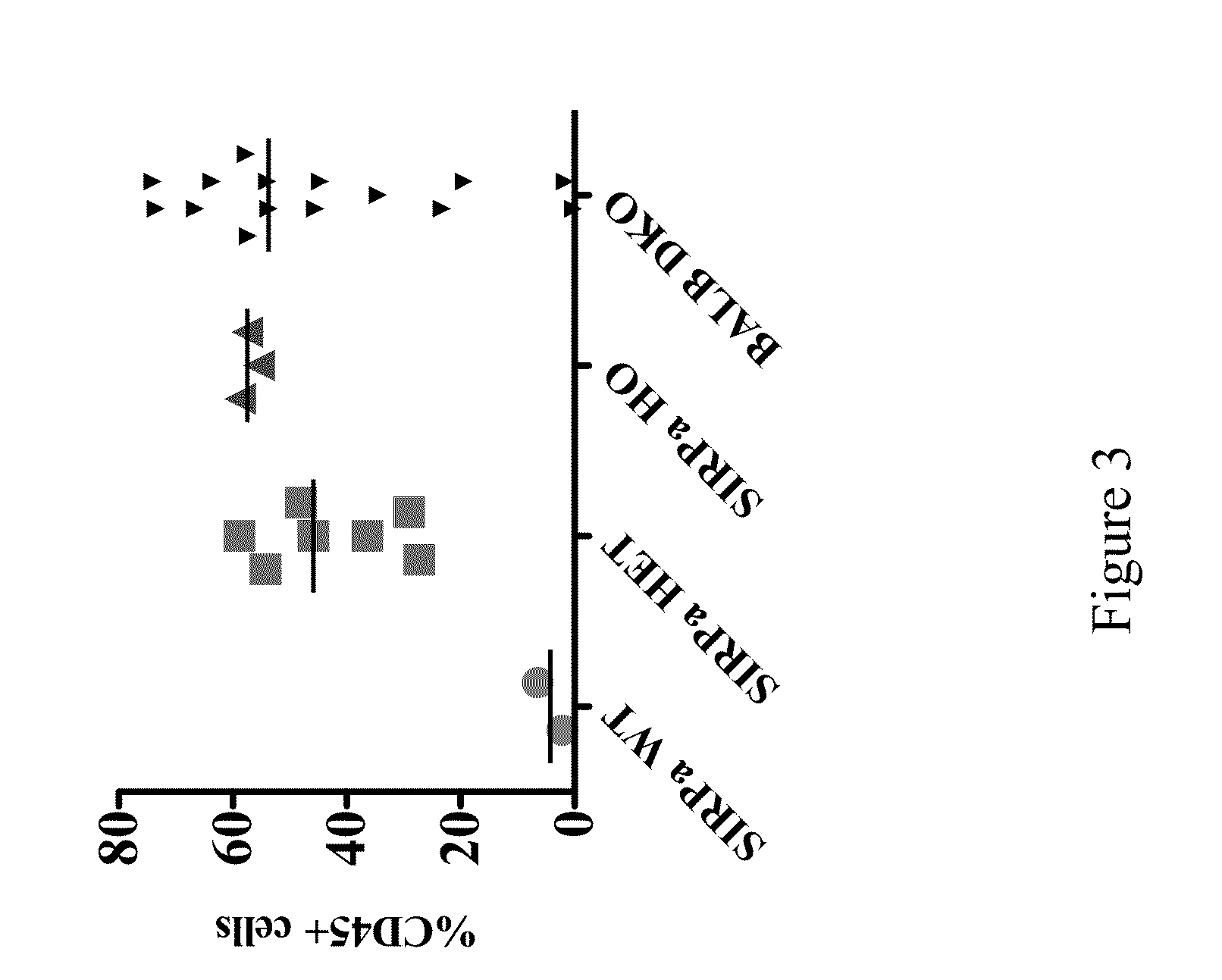
Non-human animals having a humanized signal-regulatory protein gene
ActiveUS20150089678A1Increased susceptibilityIncrease and decrease in food consumptionCompounds screening/testingCompound screeningHuman animalGenetically modified mouse
Genetically modified non-human animals and methods and compositions for making and using the same are provided, wherein the genetic modification comprises a humanization of an endogenous signal-regulatory protein gene, in particular a humanization of a SIRPα gene. Genetically modified mice are described, including mice that express a human or humanized SIRPα protein from an endogenous SIRPα locus.
Owner:REGENERON PHARM INC
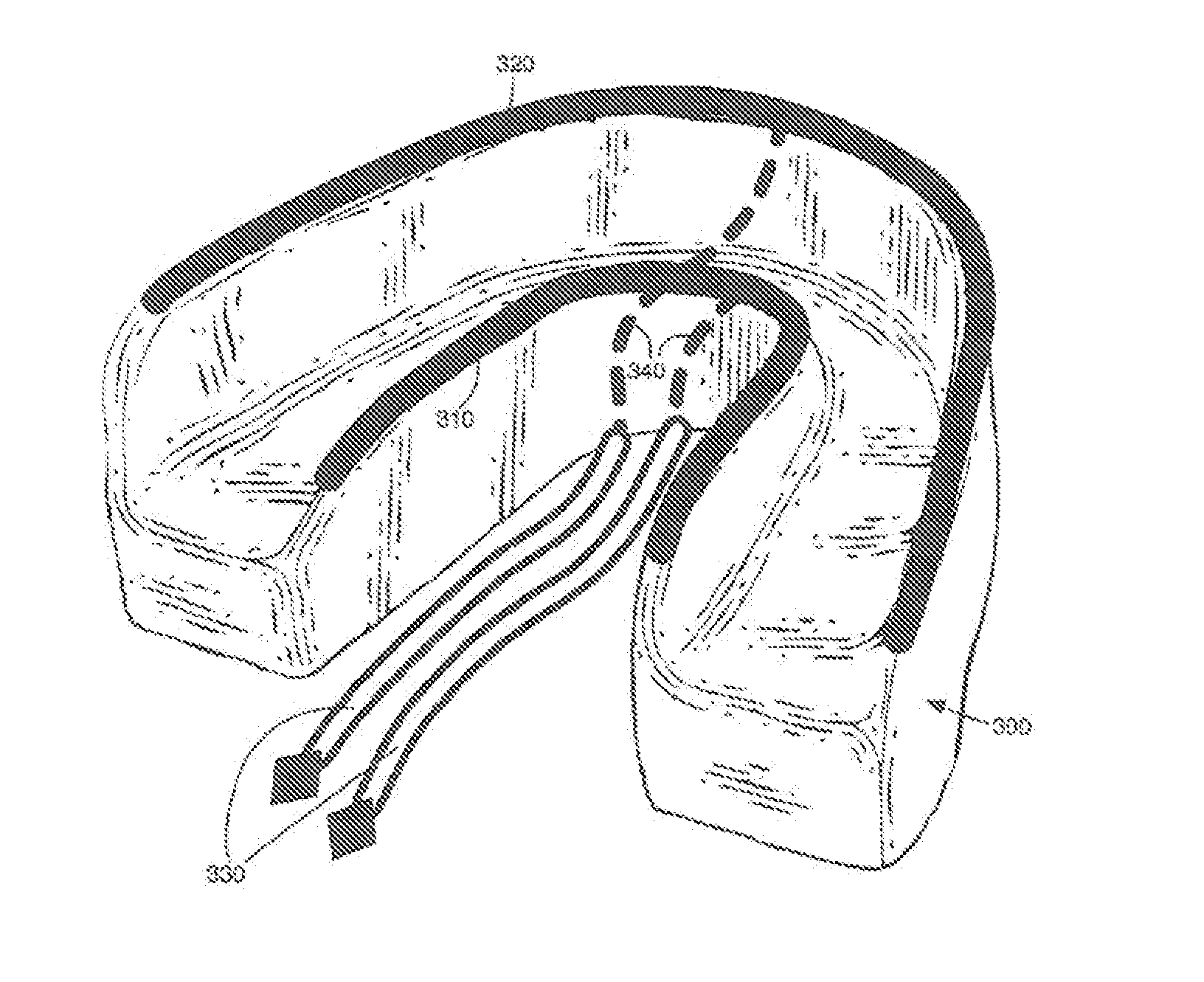
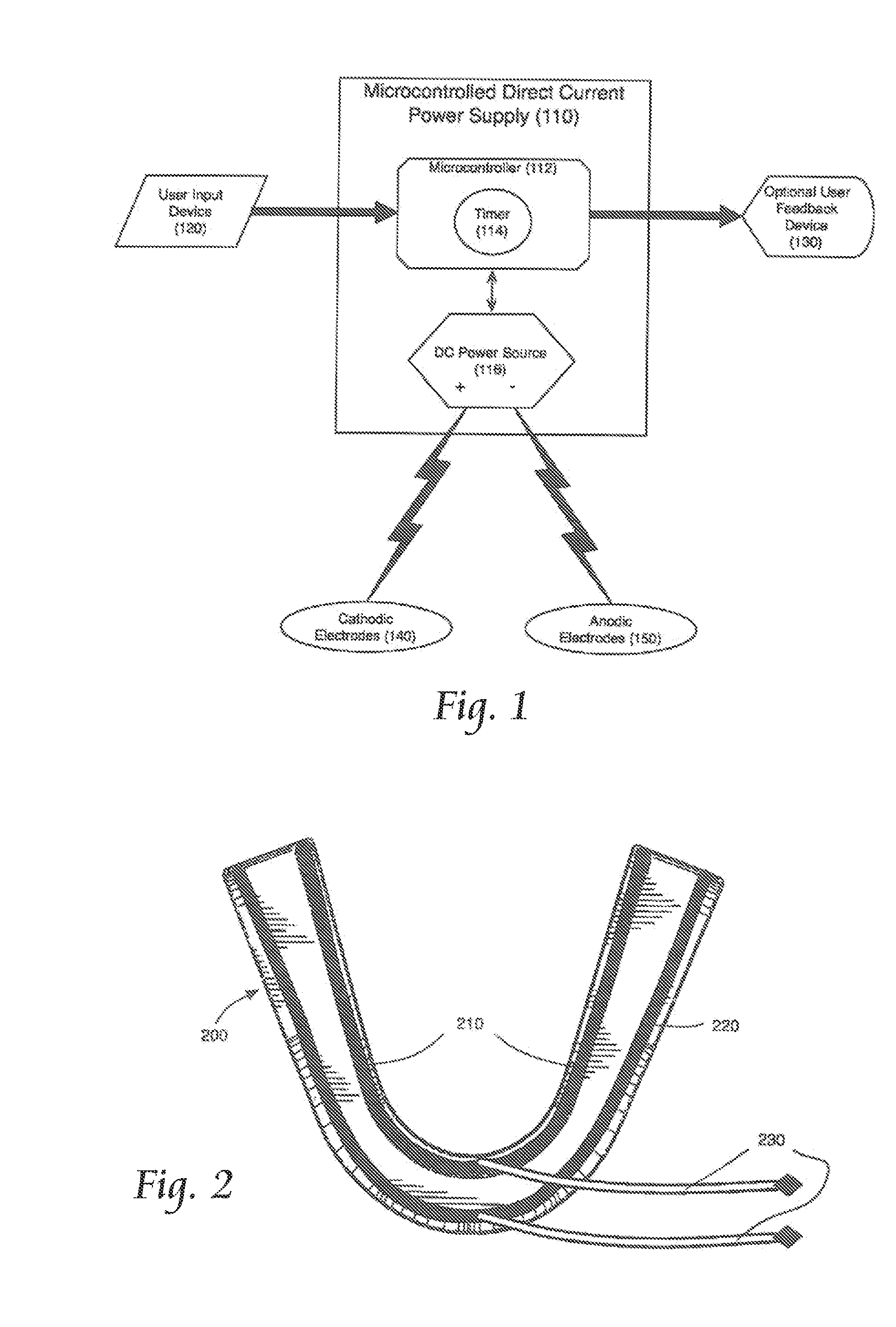
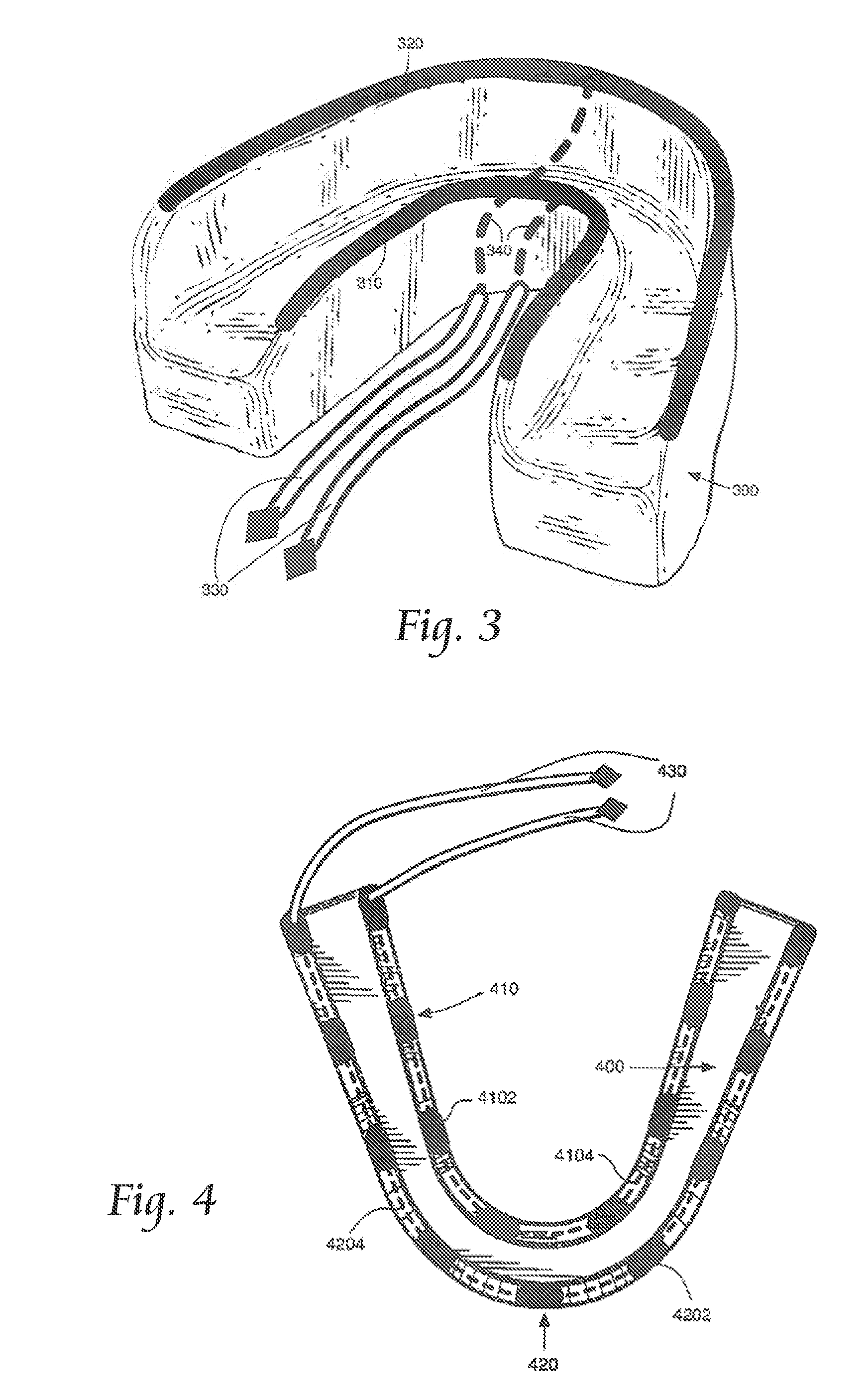
Concurrent Treatment of Oral and Systemic Maladies in Animals Using Electrical Current
ActiveUS20140093832A1Reducing oral biofilmsIncrease blood flowHead electrodesDentistryOral Cavity DisorderGingival tissue
A method and apparatus for the concurrent treatment of multiple oral diseases and defects while promoting general oral hygiene utilizing electricity are provided for non-human animals. Electrodes are used to deliver an electrical current to the gingival tissues of a mouth in order to achieve a number of therapeutic, prophylactic, and regenerative benefits. These benefits include killing oral microbes, increasing oral vasodilation, reducing oral biofilm, improving oral blood circulation, reversing oral bone resorption, promoting oral osteogenesis, treating gum recession, and fostering gingival regeneration. Other benefits include the treatment of gingivitis, periodontitis, and oral malodor, and other systemic diseases correlated with oral pathogens.
Owner:BIOLECTRICS
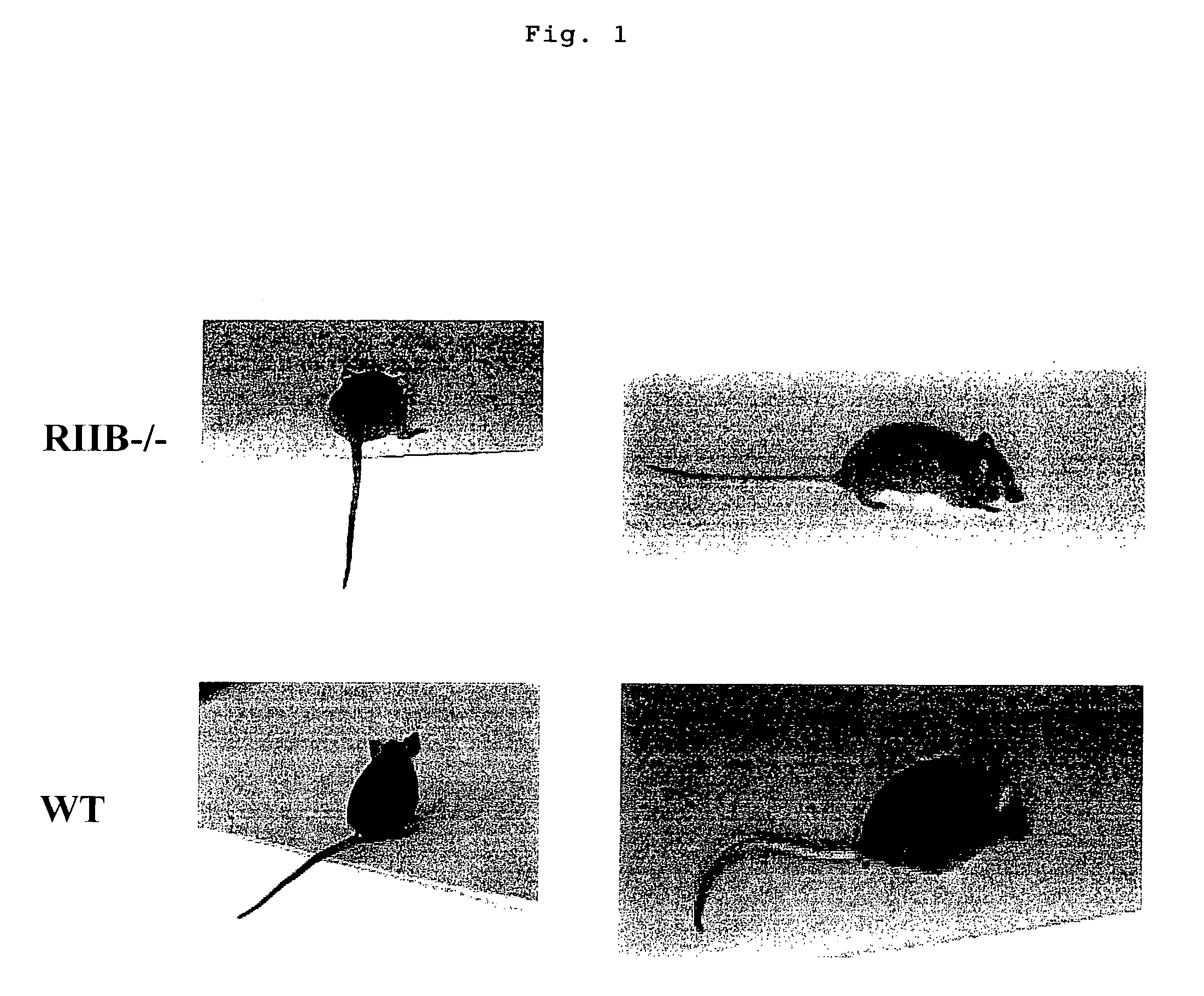
Nonhuman model animal suffering from Guillain-Barré syndrome and/or fisher syndrome
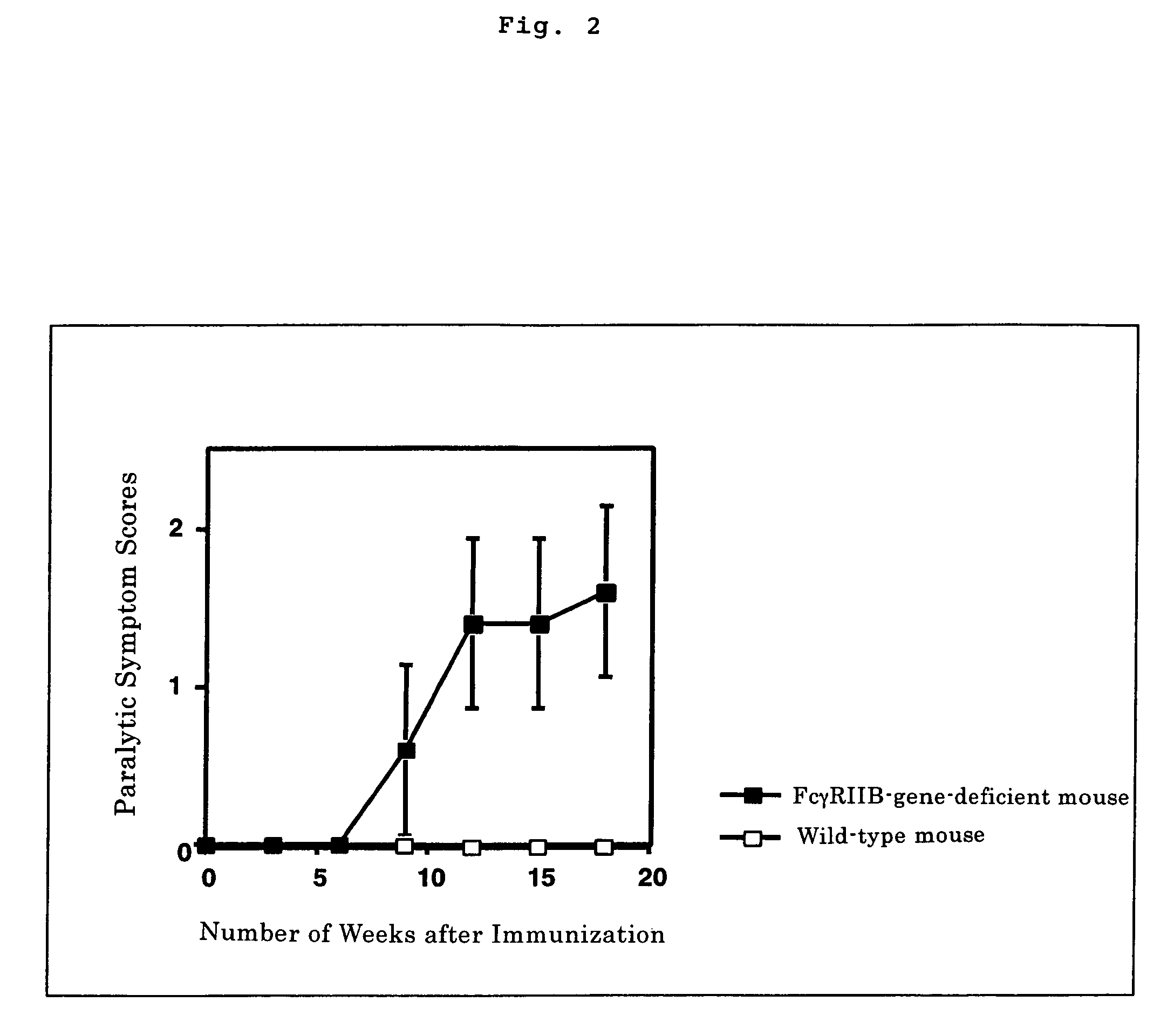
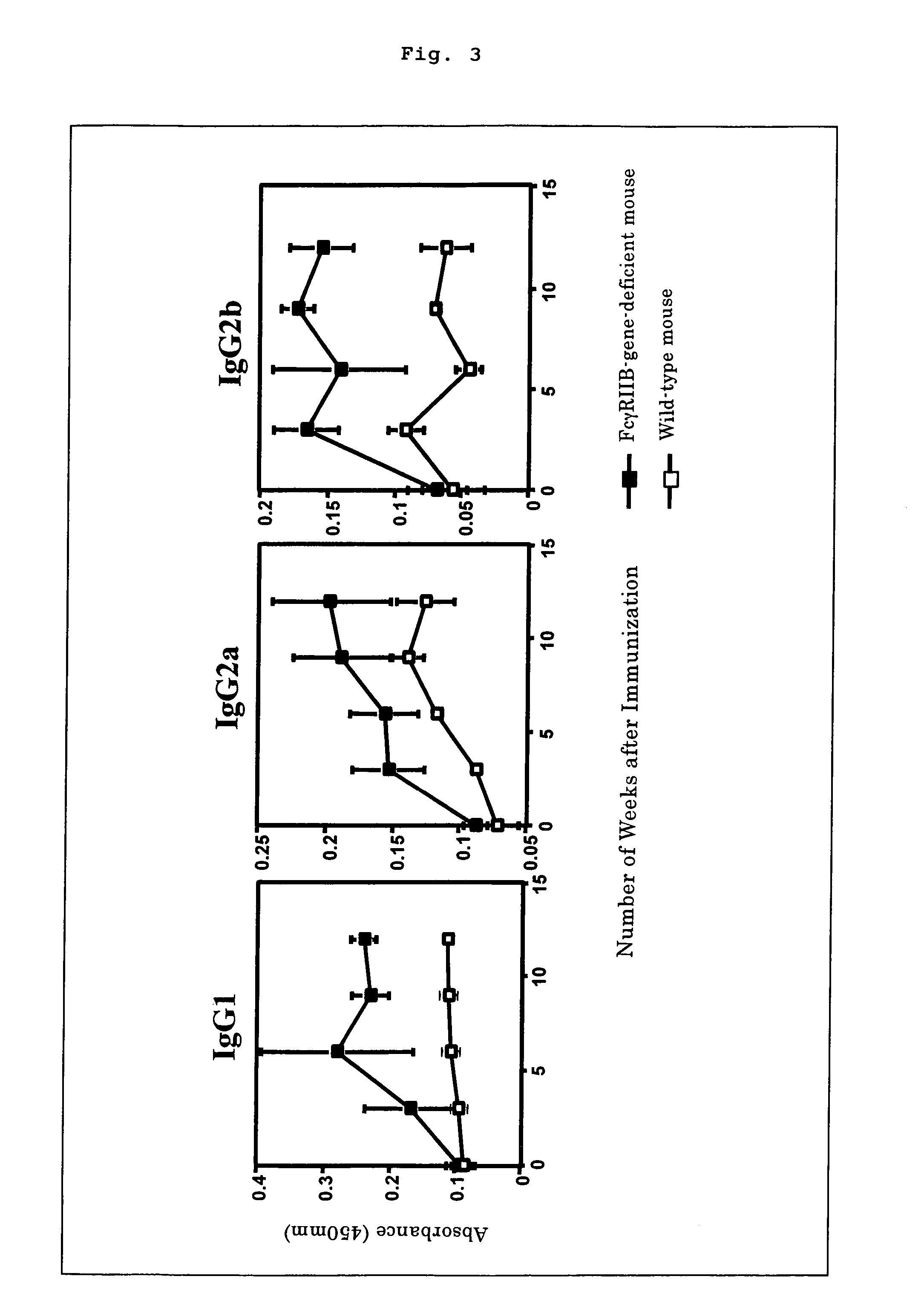
The present invention intends to provide a non-human animal model of Guillain-Barré syndrome, which can be obtained by immunizing FcγRIIB-gene-deficient non-human animal with ganglioside GQ1b, and a screening method of a therapeutic agent for Guillain-Barré syndrome using the non-human animal model. A mouse model of Guillain-Barré syndrome is generated by immunizing FcγRIIB-gene-deficient mice with gangliosides GM1, GM2, GD1a, and GQ1b together with Freund's adjuvant every three weeks four times in total.
Owner:JAPAN SCI & TECH CORP
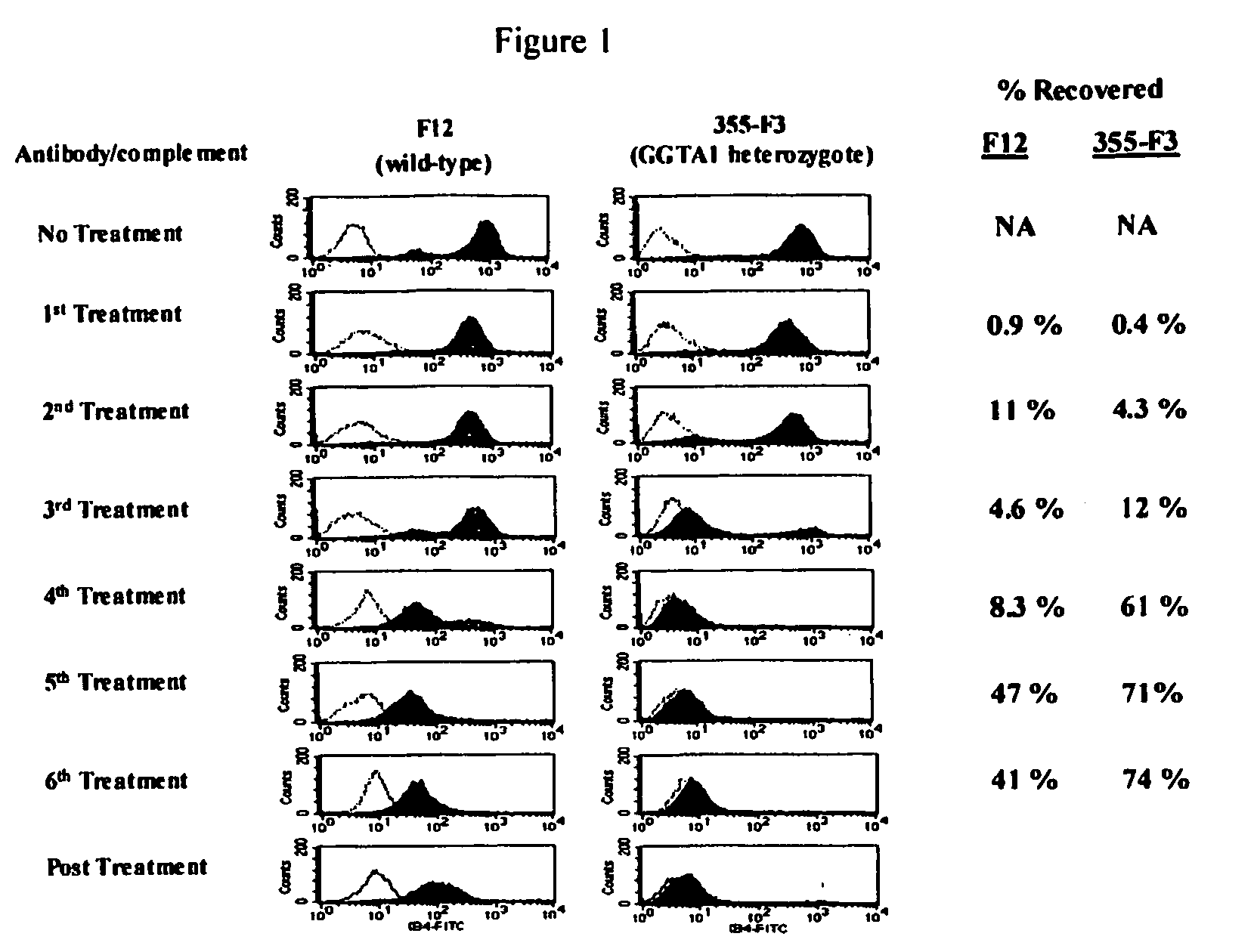
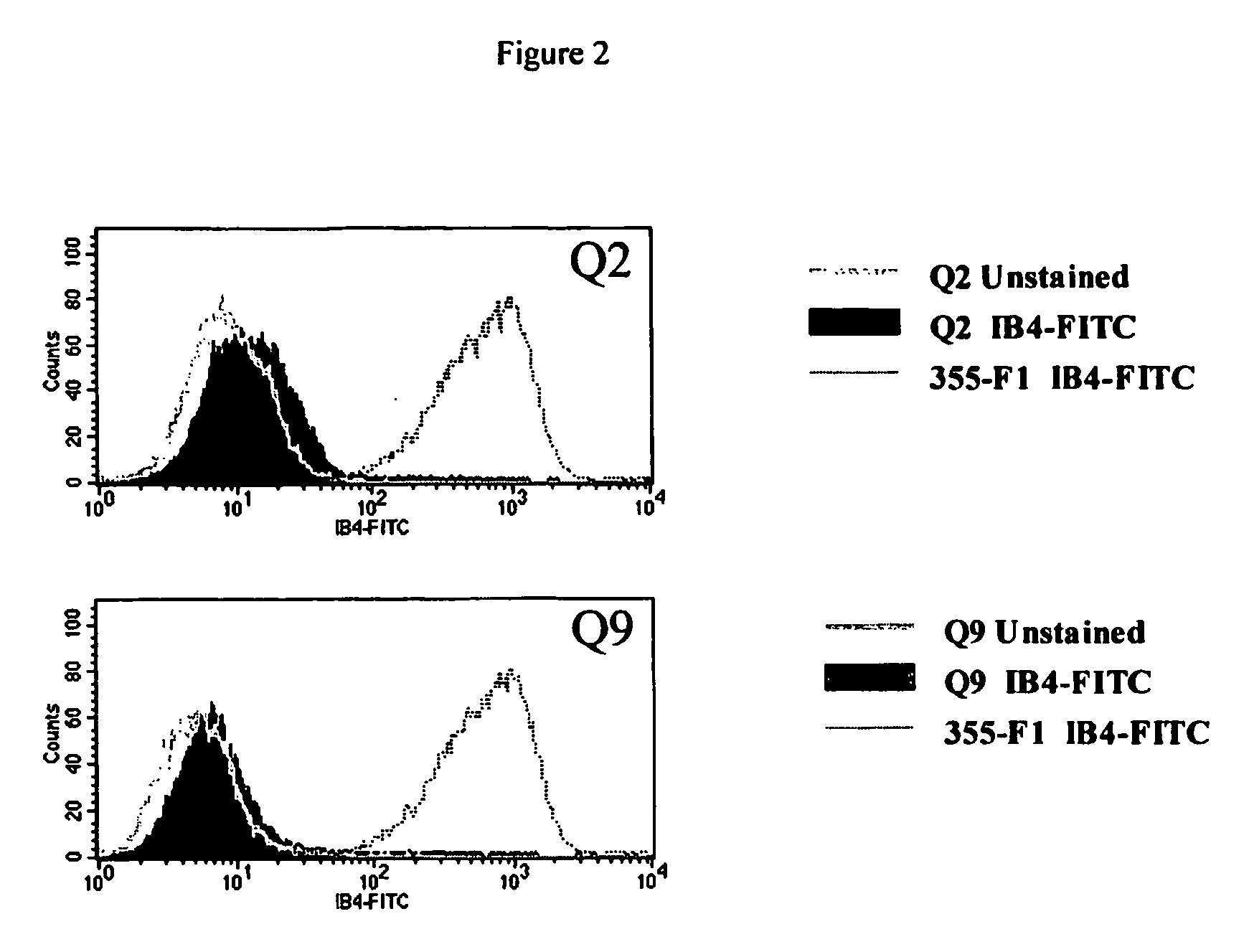
Alpha(1,3)-galactosyltransferase null cells, methods of selecting and alpha(1,3)-galactosyl transferase null swine produced therefrom
ActiveUS20060242722A1New breed animal cellsMicrobiological testing/measurementHuman animalCell selection
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides a method of selecting GGTA 1 null cells, a viable GGTA 1 null swine, methods for making such swine, and methods of using cells, tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com