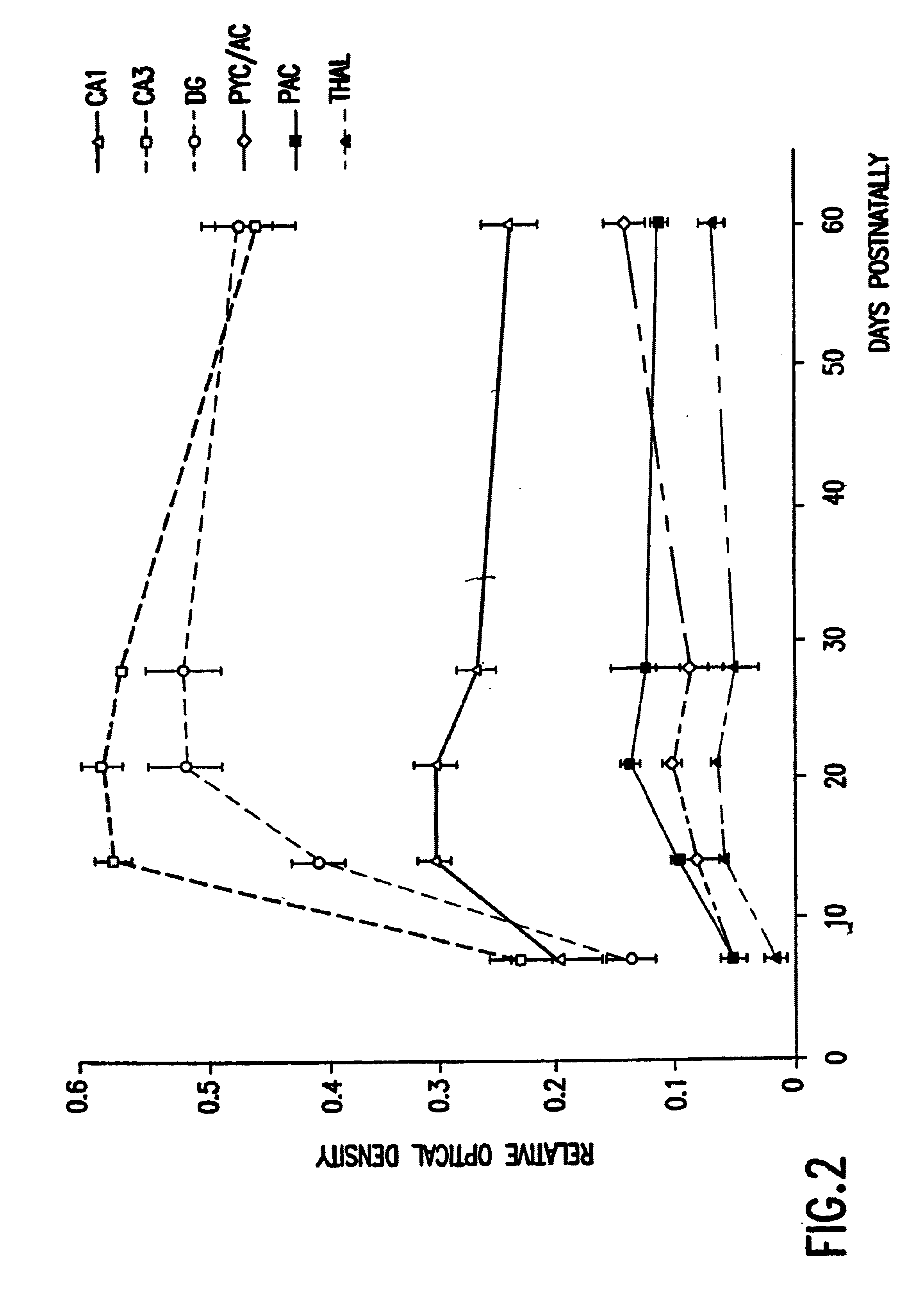
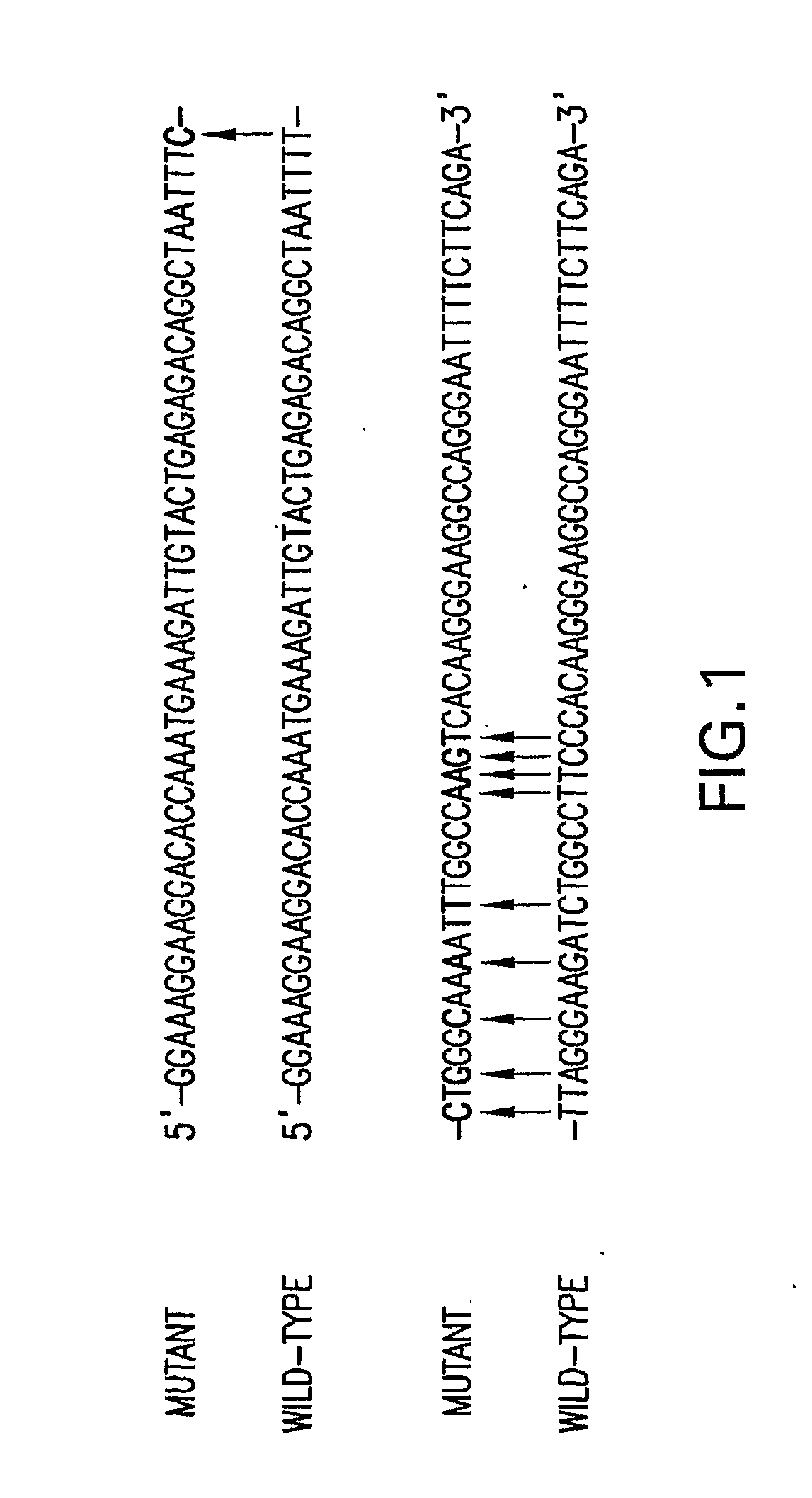
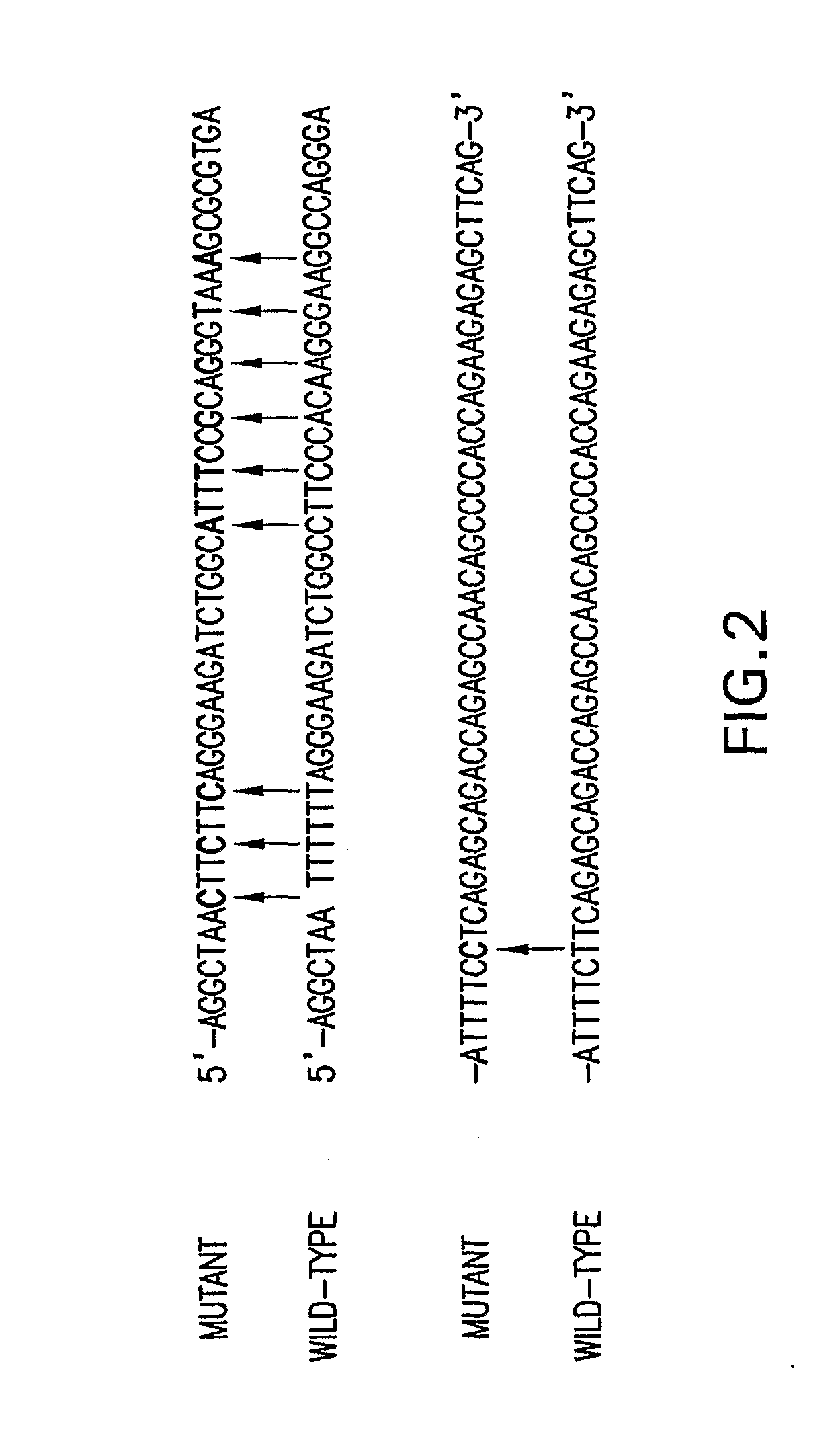
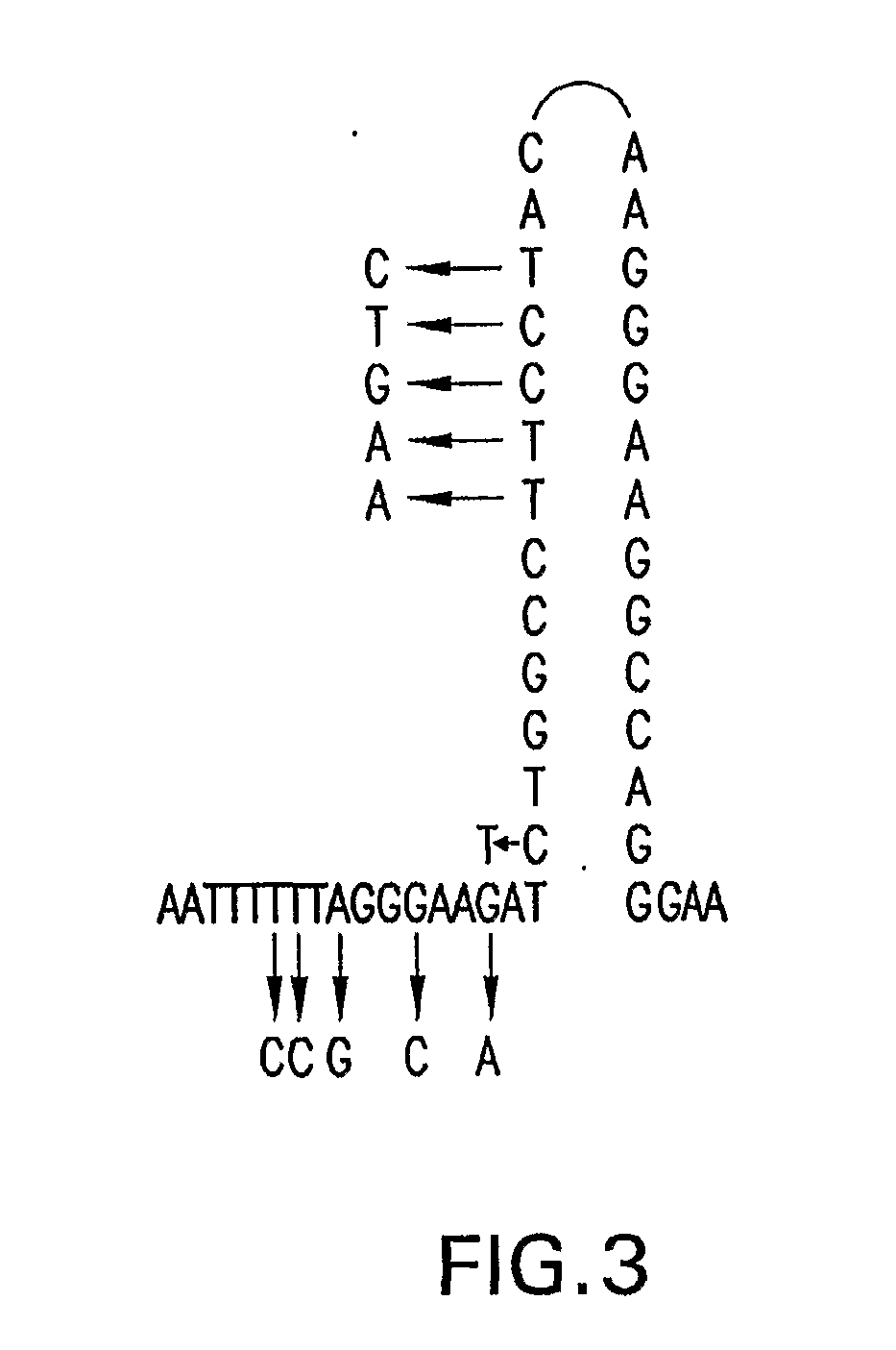
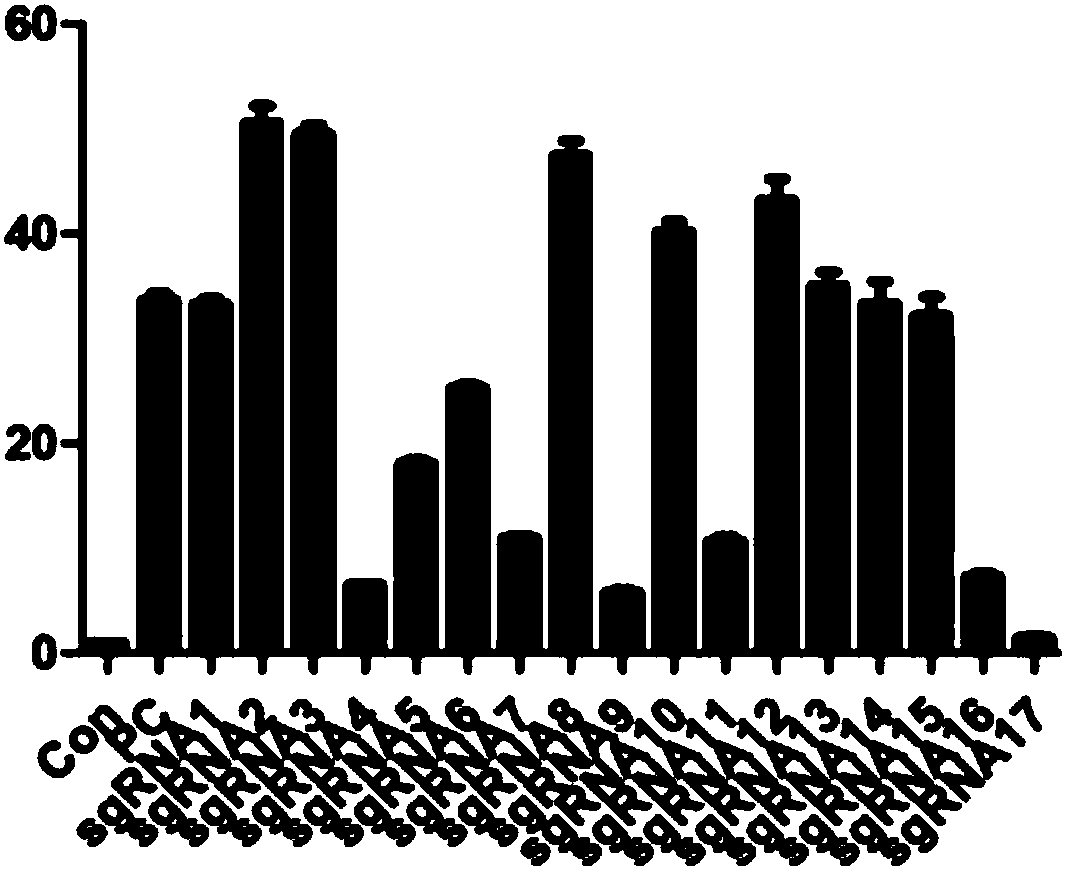
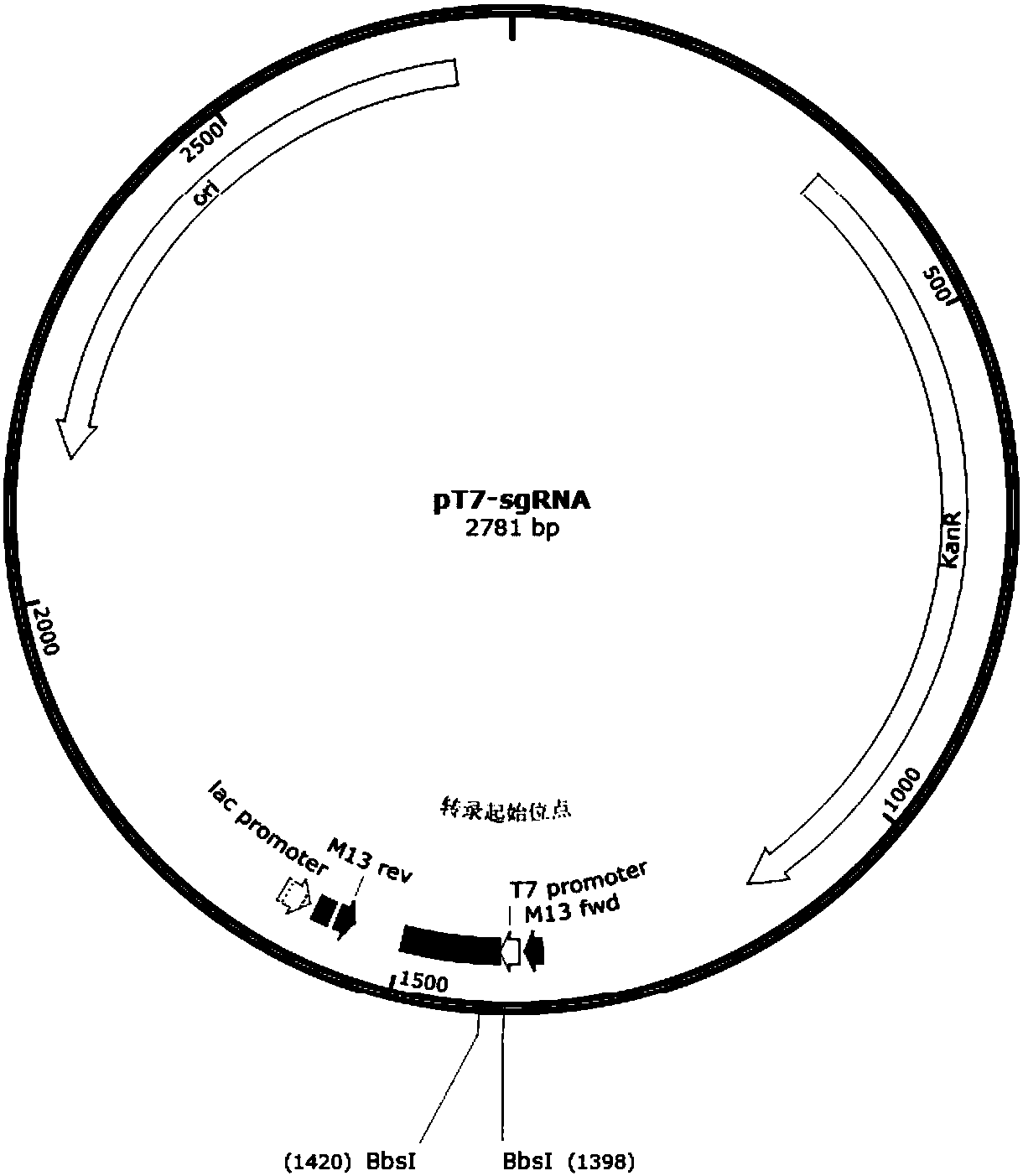
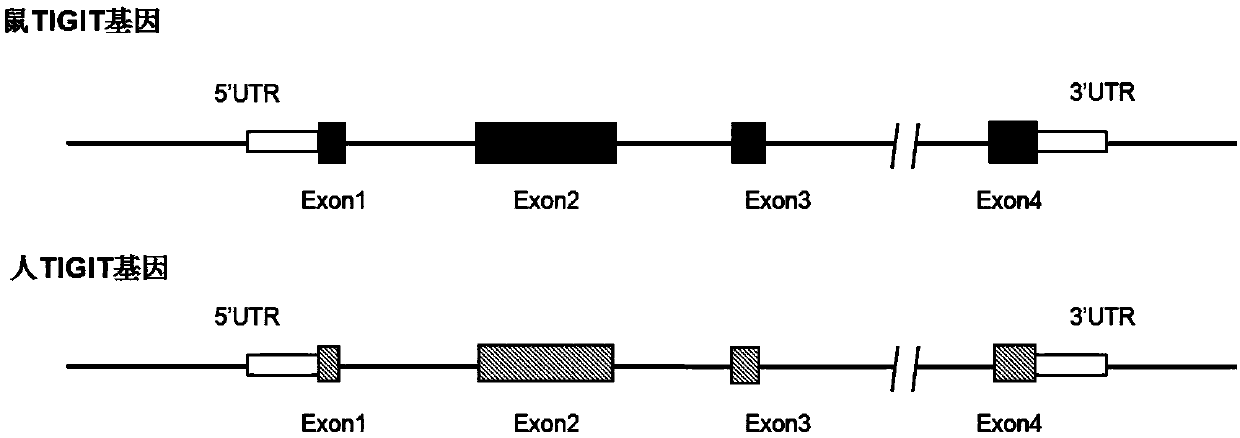
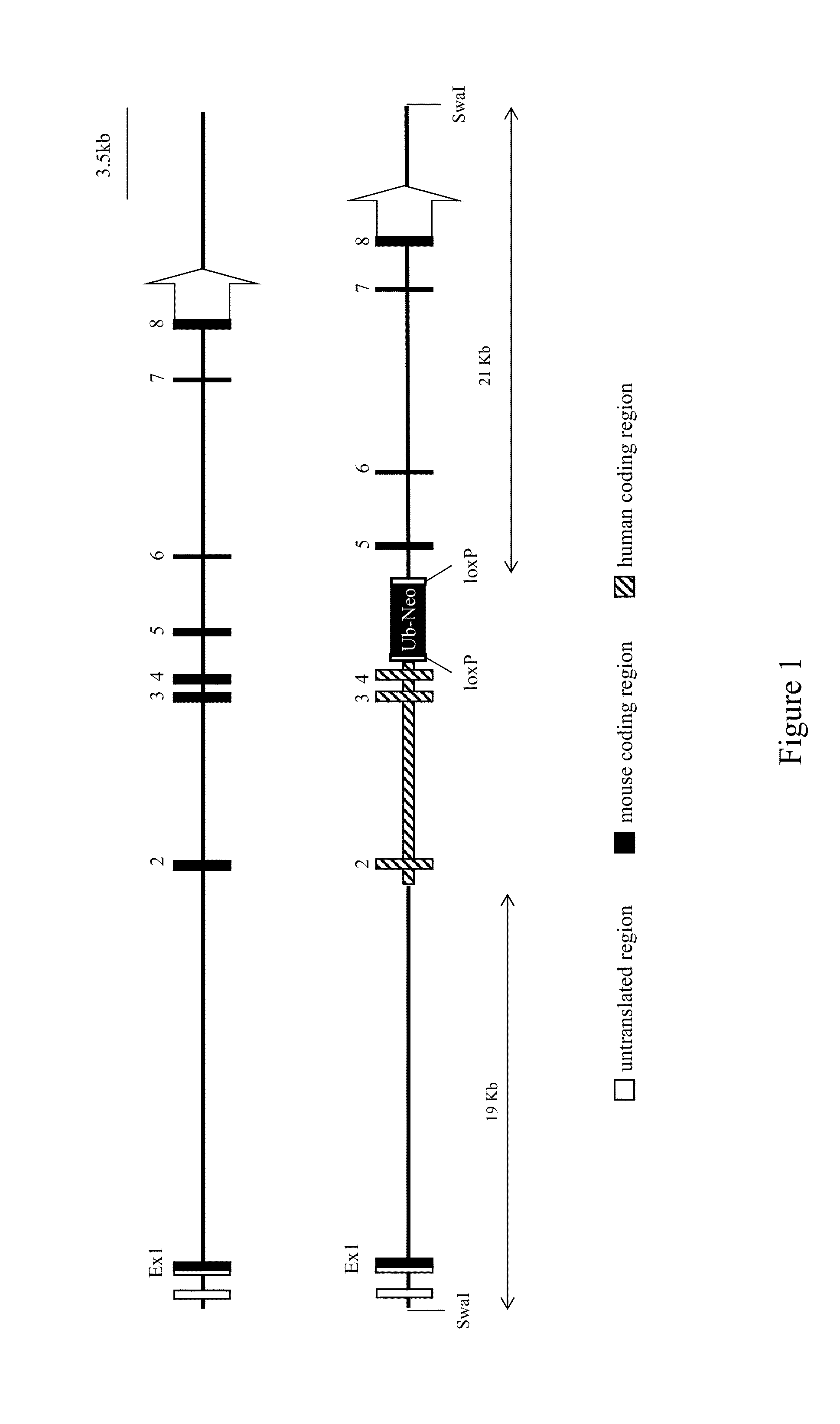
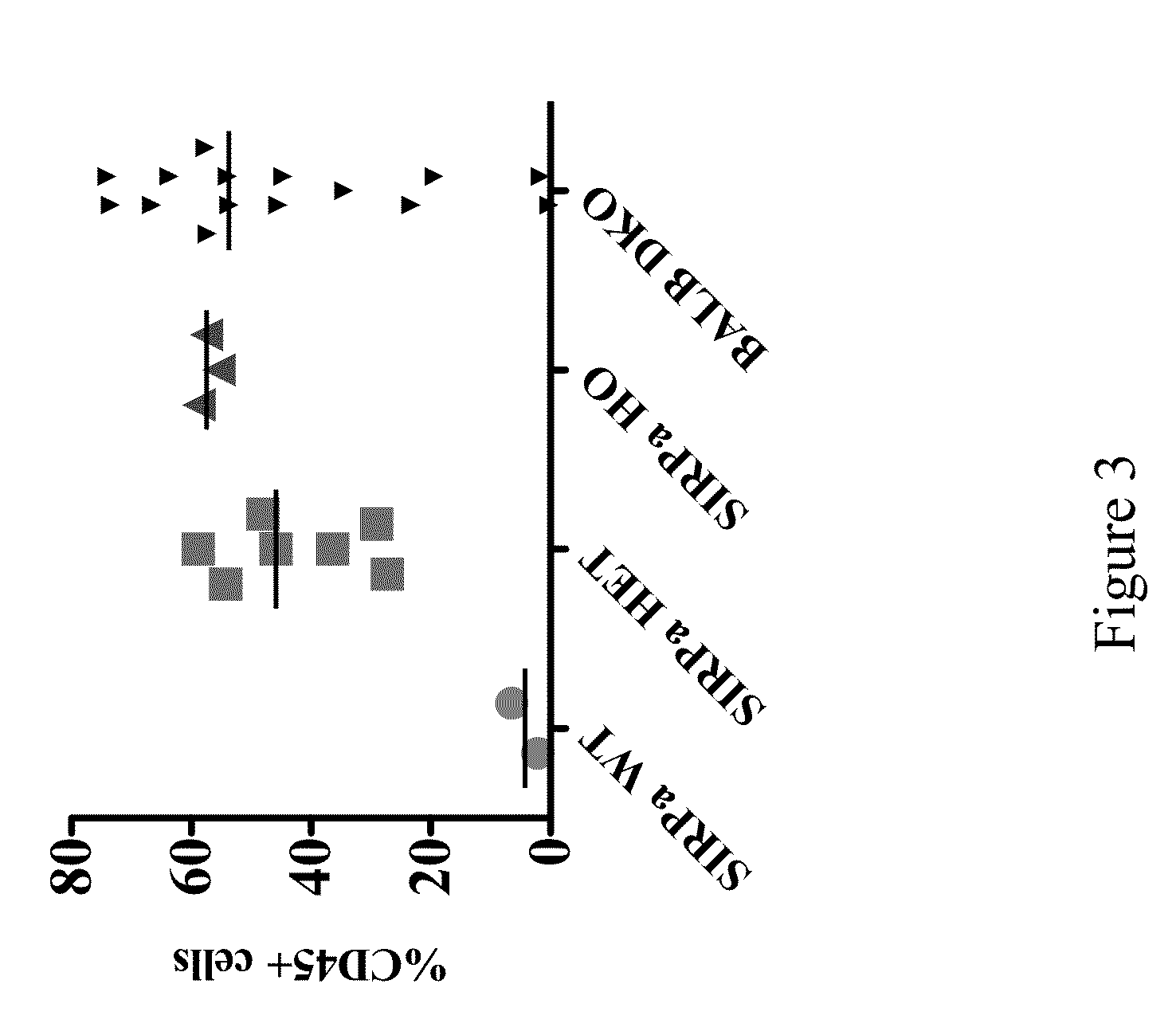Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
213 results about "Genetically modified mouse" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genetically modified mouse (Mus musculus) is a mouse that has had its genome altered through the use of genetic engineering techniques. Genetically modified mice are commonly used for research or as animal models of human diseases, and are also used for research on genes.
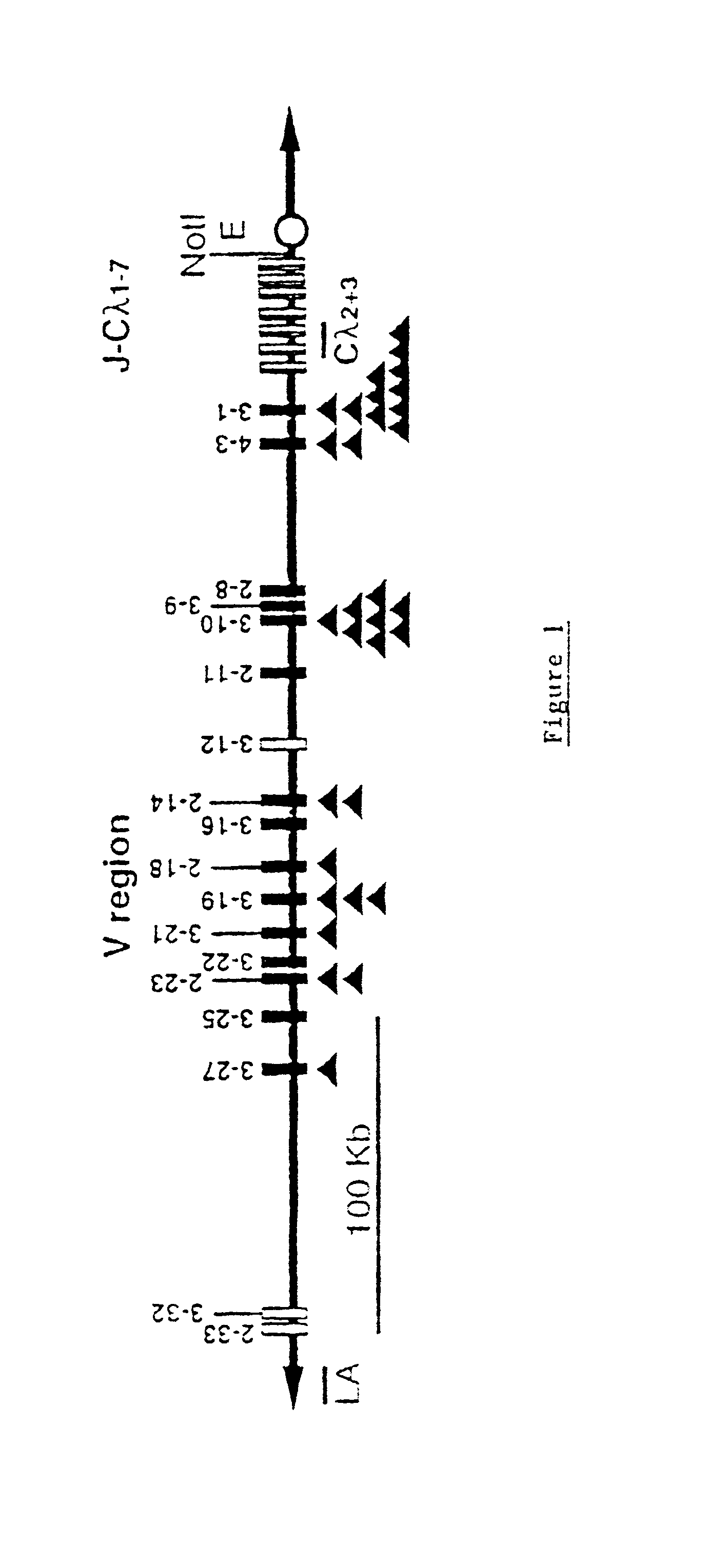
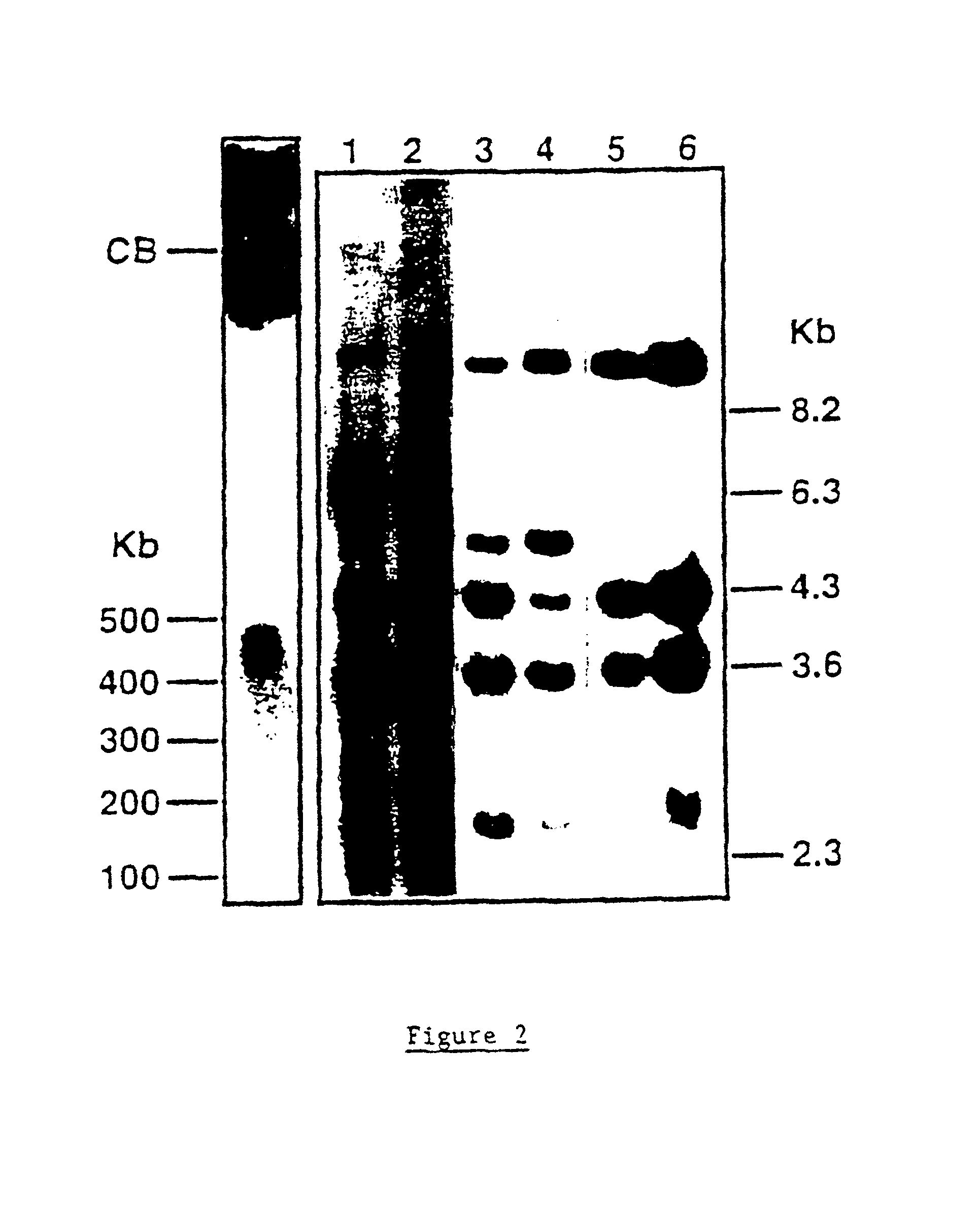
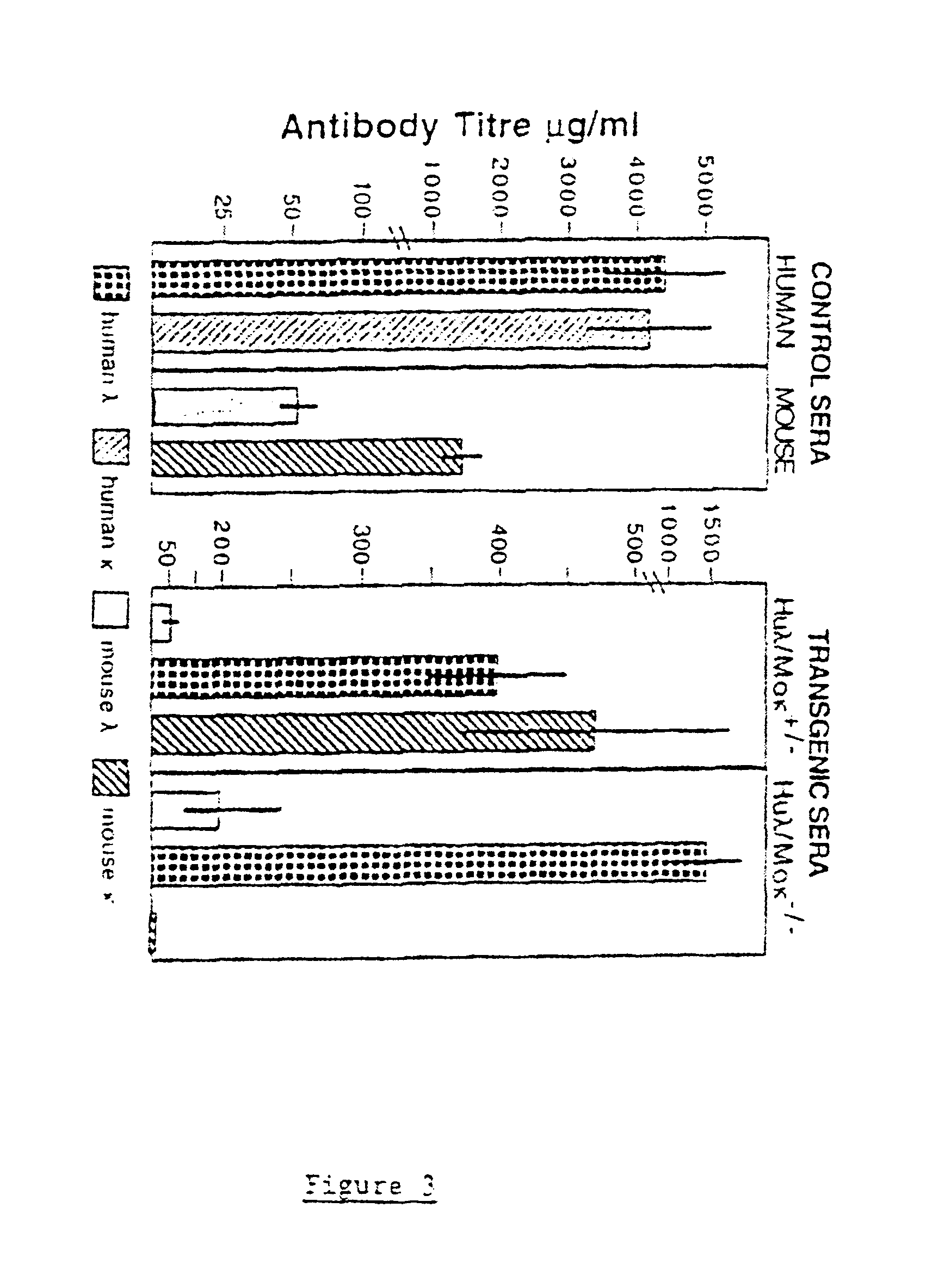
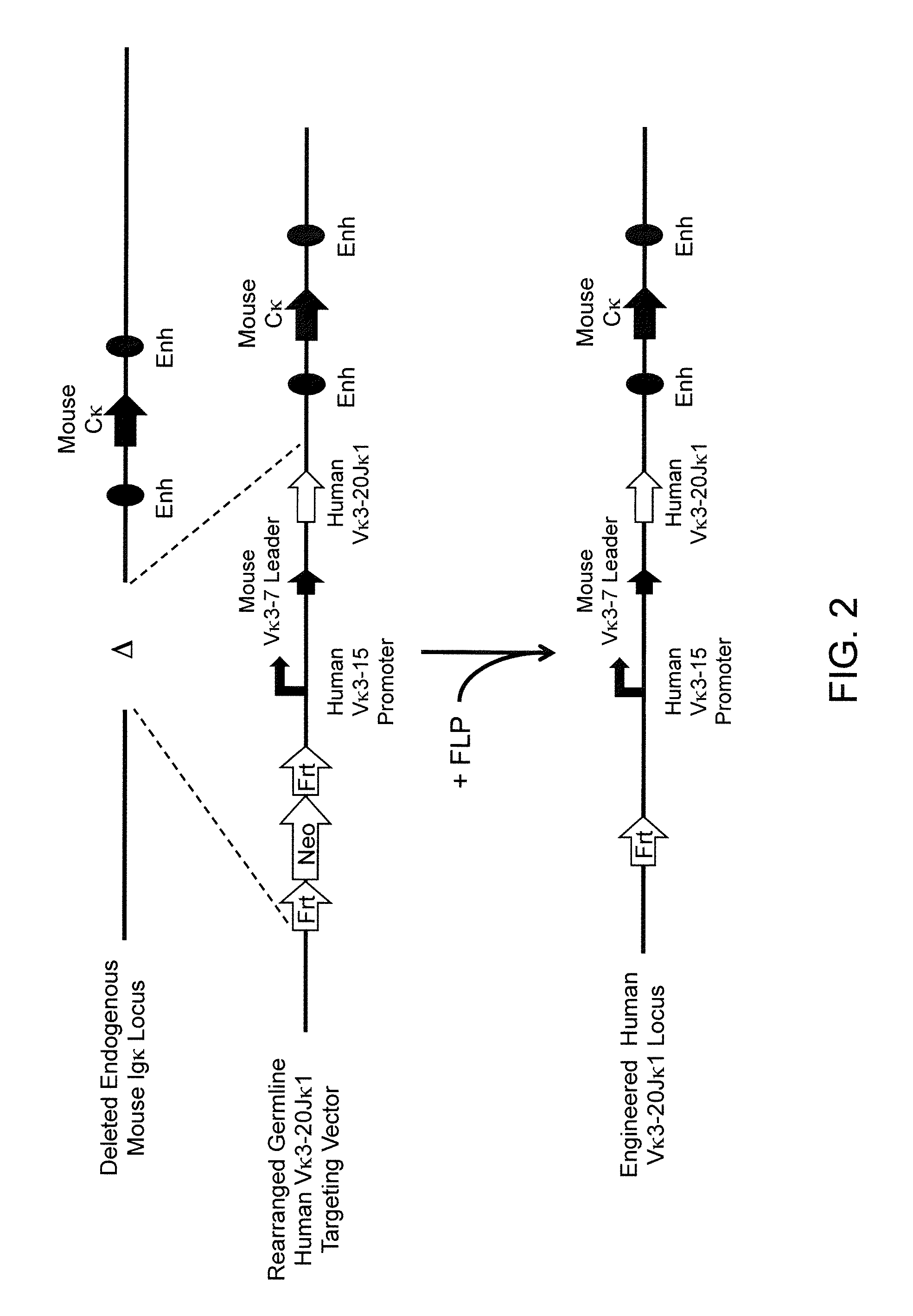
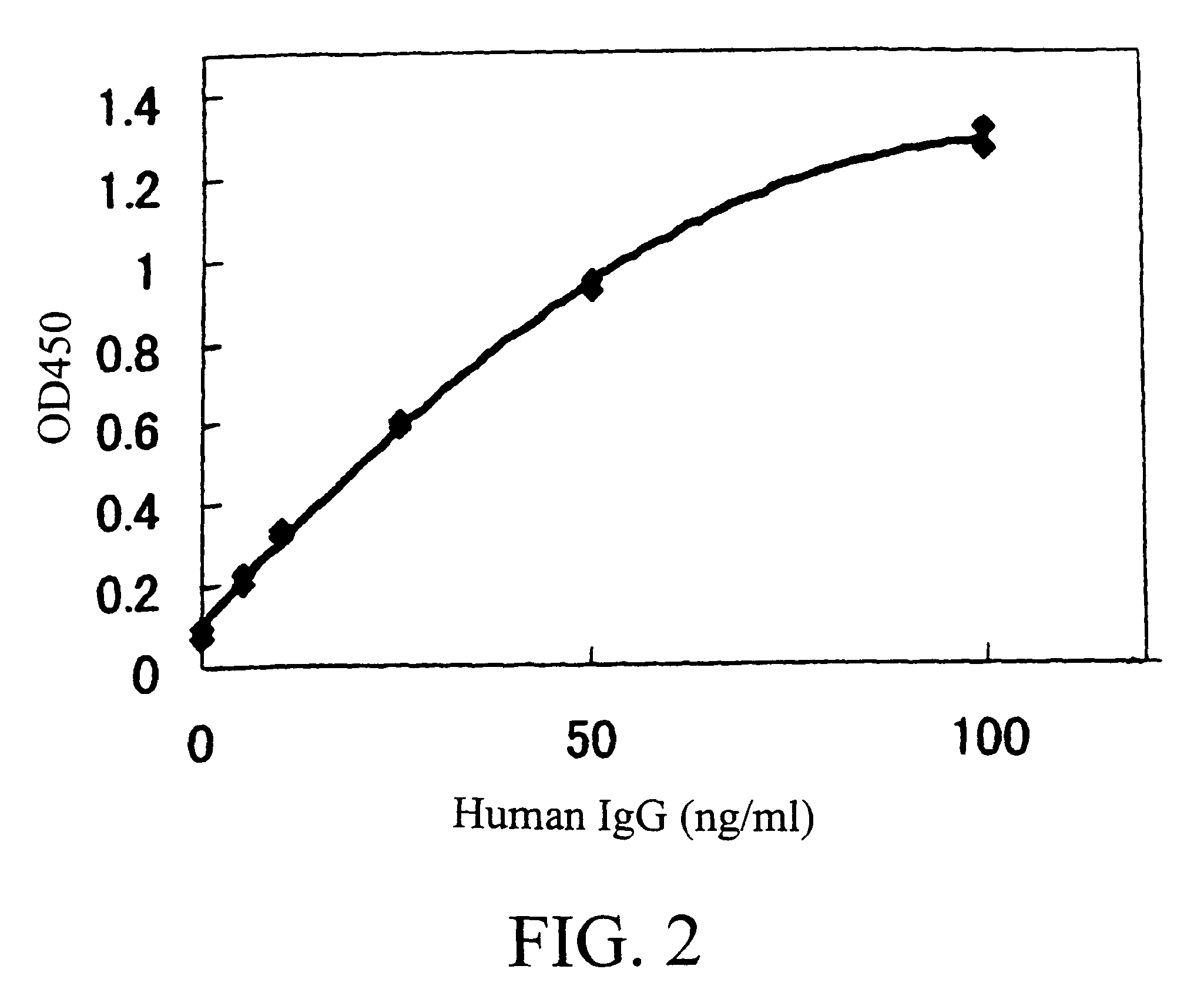
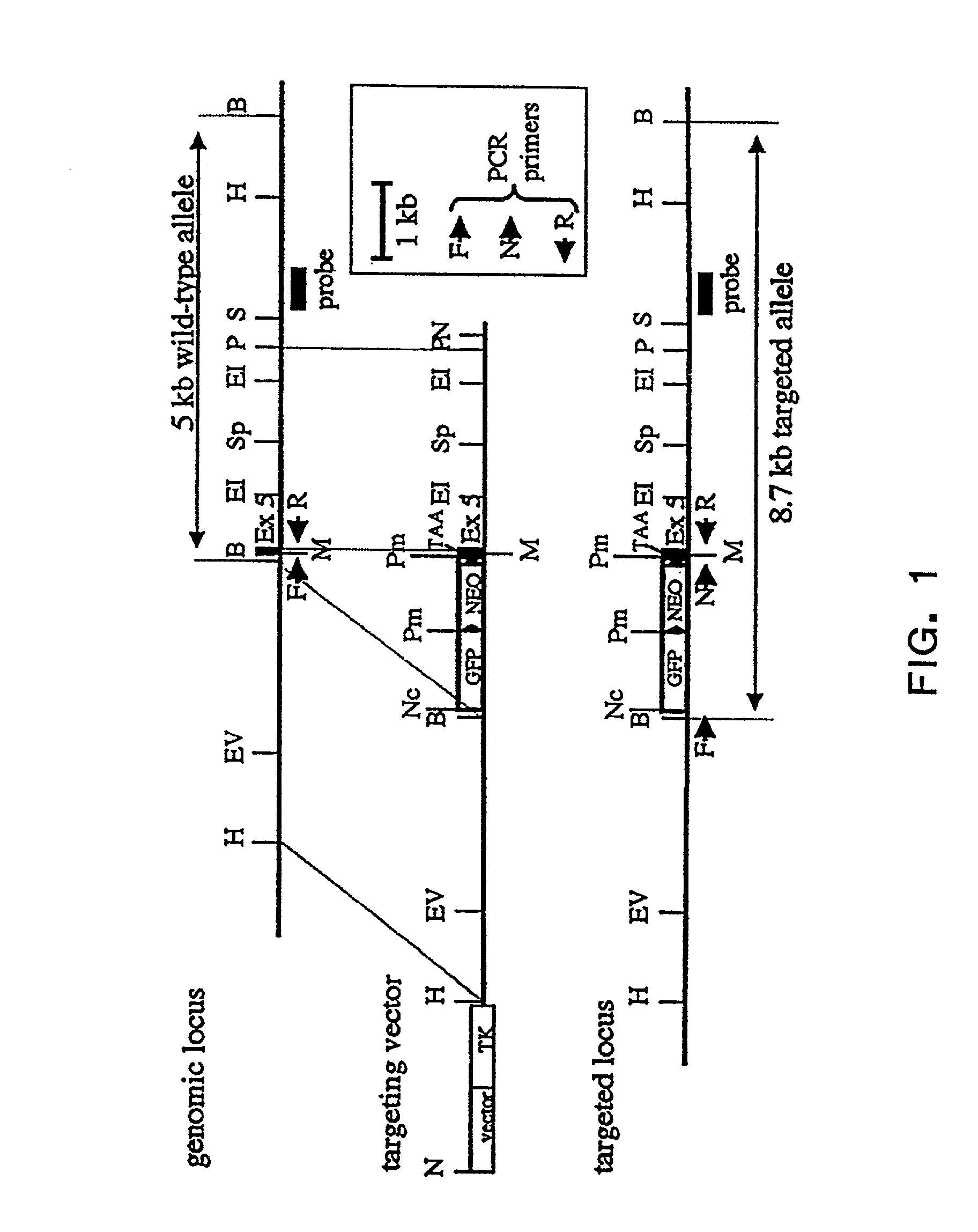
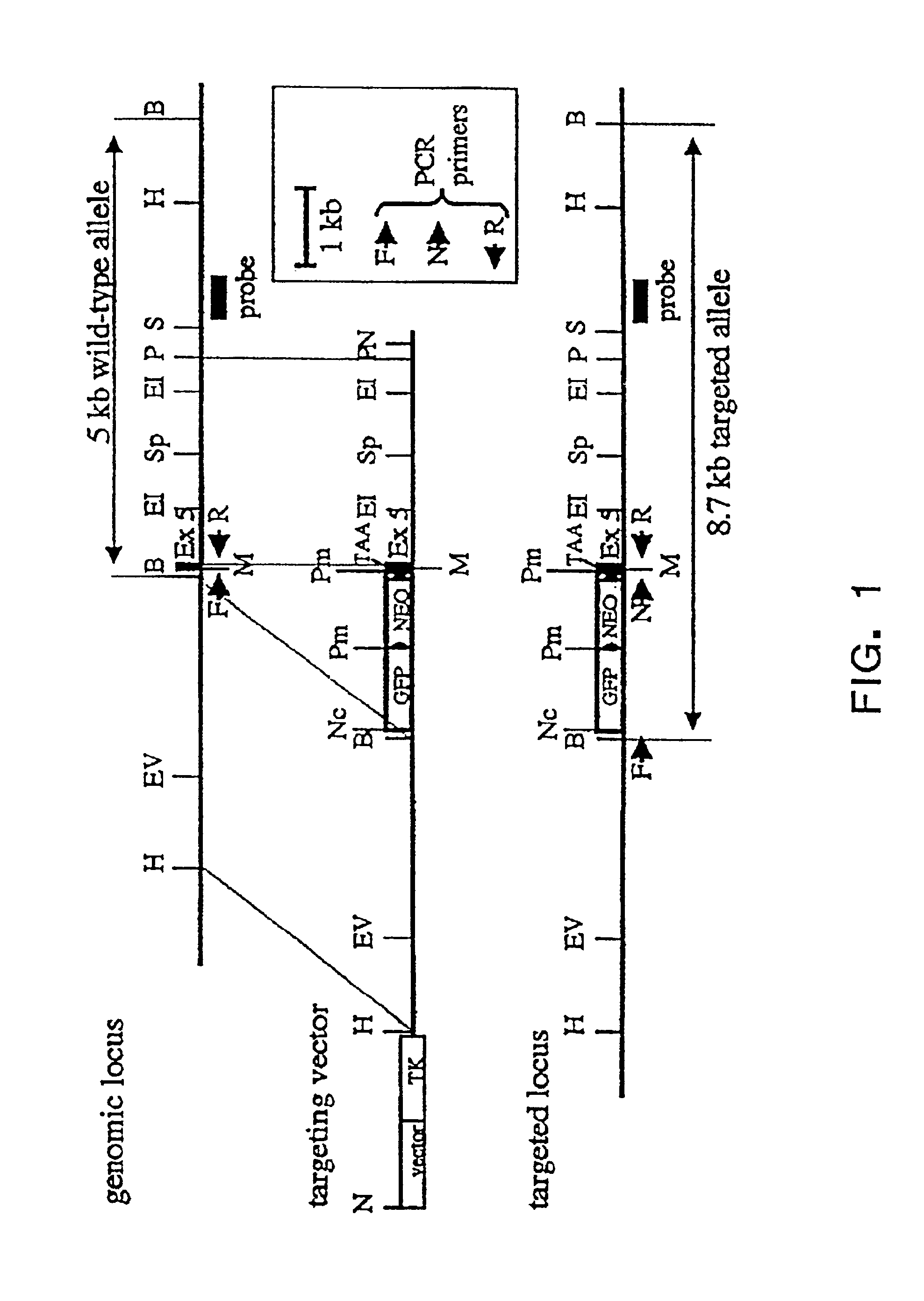
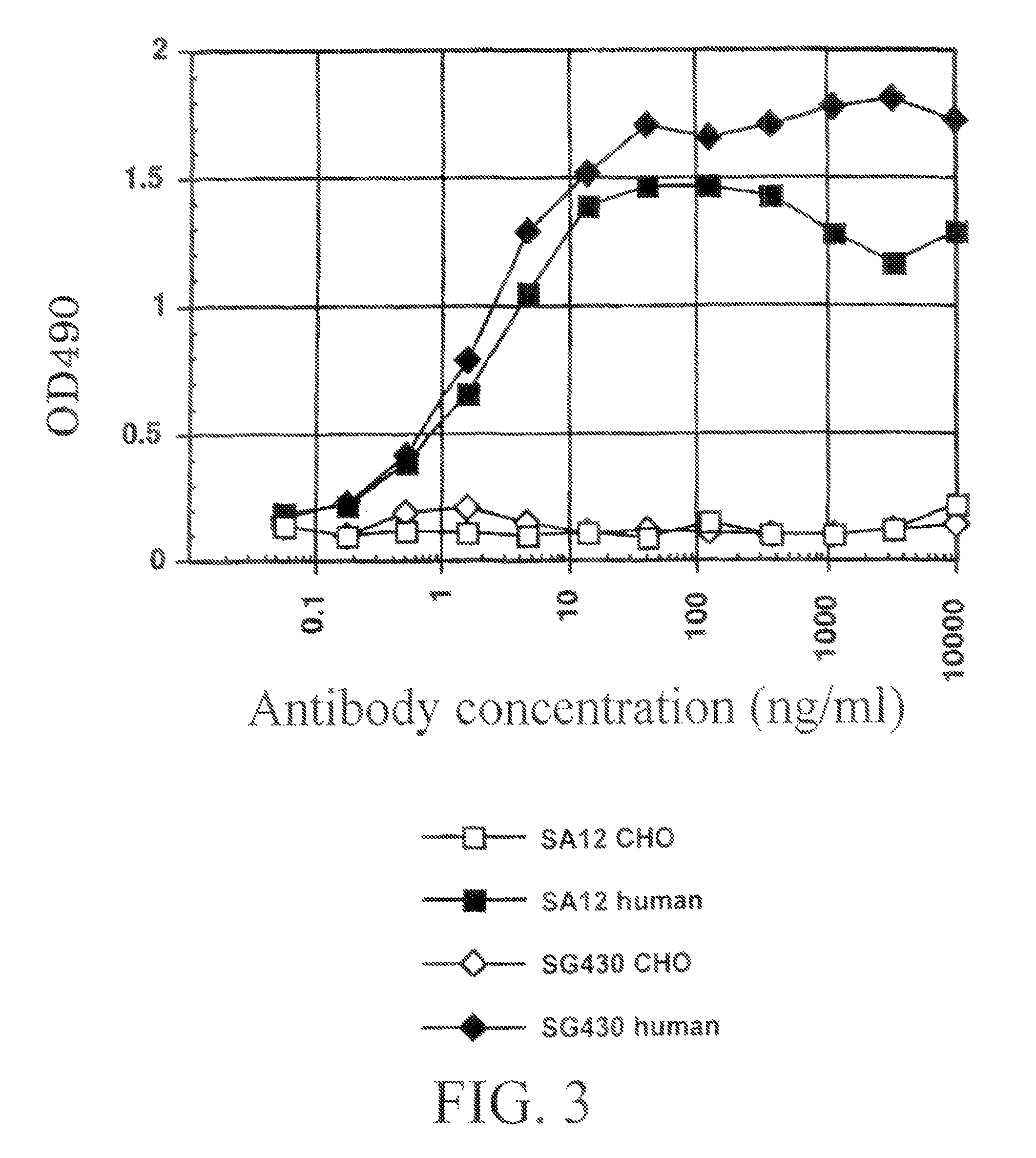
Murine expression of a human IgA lambda locus
InactiveUS6998514B2High expressionTissue cultureImmunoglobulinsImmunoglobulin light chainMonoclonal antibody
In humans, approximately 60% of expressed immunoglobulin light chains are of the Kappa type and 40% of the Lambda type. In mice, there is almost no expression from the Lambda locus and over 95% of light chains are of Kappa type. The present invention discloses, among other things, transgenic mice carrying most of the human Ig Lambda light chain locus in their genome. The resulting mice express light chains with Kappa / Lambda ratio similar to the human ratio. Breeding of HuIg Lamda mice to Kappa-deficient mice also is described, as well as the generation of human monoclonal antibodies from transgenic mice with human Ig Lambda locus.
Owner:BABRAHAM INST
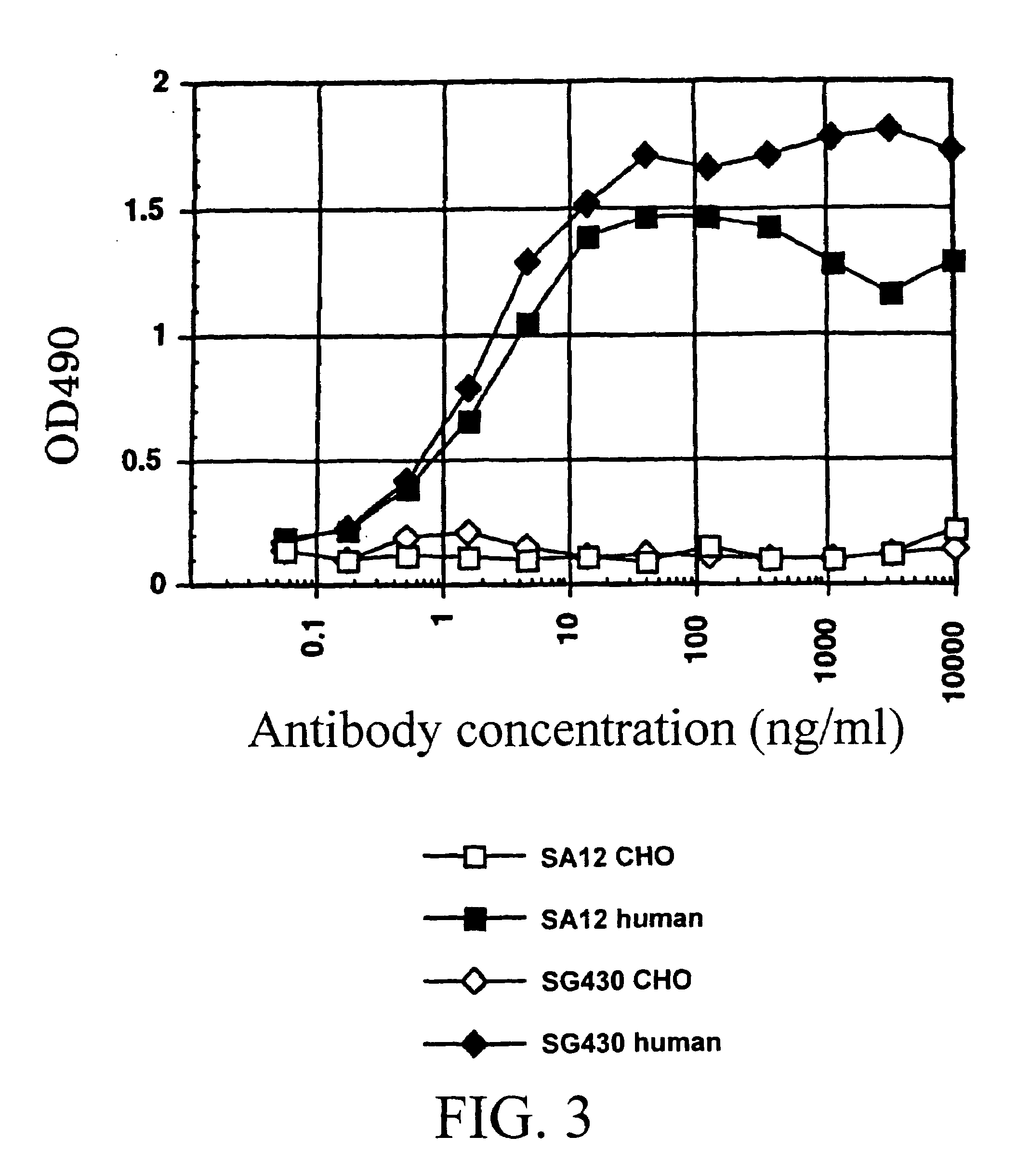
Methods For Making Fully Human Bispecific Antibodies Using A Common Light Chain
InactiveUS20130045492A1Reduce in quantitySimple methodAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeProtein insertion
A genetically modified mouse is provided, wherein the mouse expresses an immunoglobulin light chain repertoire characterized by a limited number of light chain variable domains. Mice are provided that express just one or a few immunoglobulin light chain variable domains from a limited repertoire in their germline. Methods for making bispecific antibodies having universal light chains using mice as described herein, including human light chain variable regions, are provided. Methods for making human variable regions suitable for use in multispecific binding proteins, e.g., bispecific antibodies, and host cells are provided. Bispecific antibodies capable of binding first and second antigens are provided, wherein the first and second antigens are separate epitopes of a single protein or separate epitopes on two different proteins are provided.
Owner:REGENERON PHARM INC
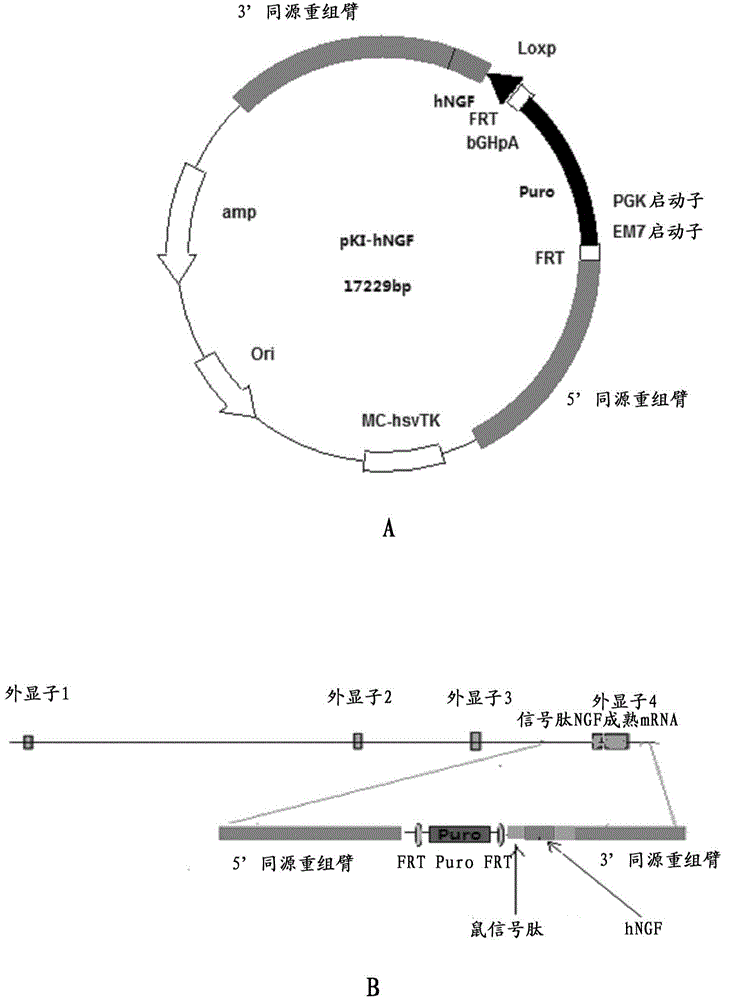
Preparation method for transgenic mice capable of producing human nerve growth factor
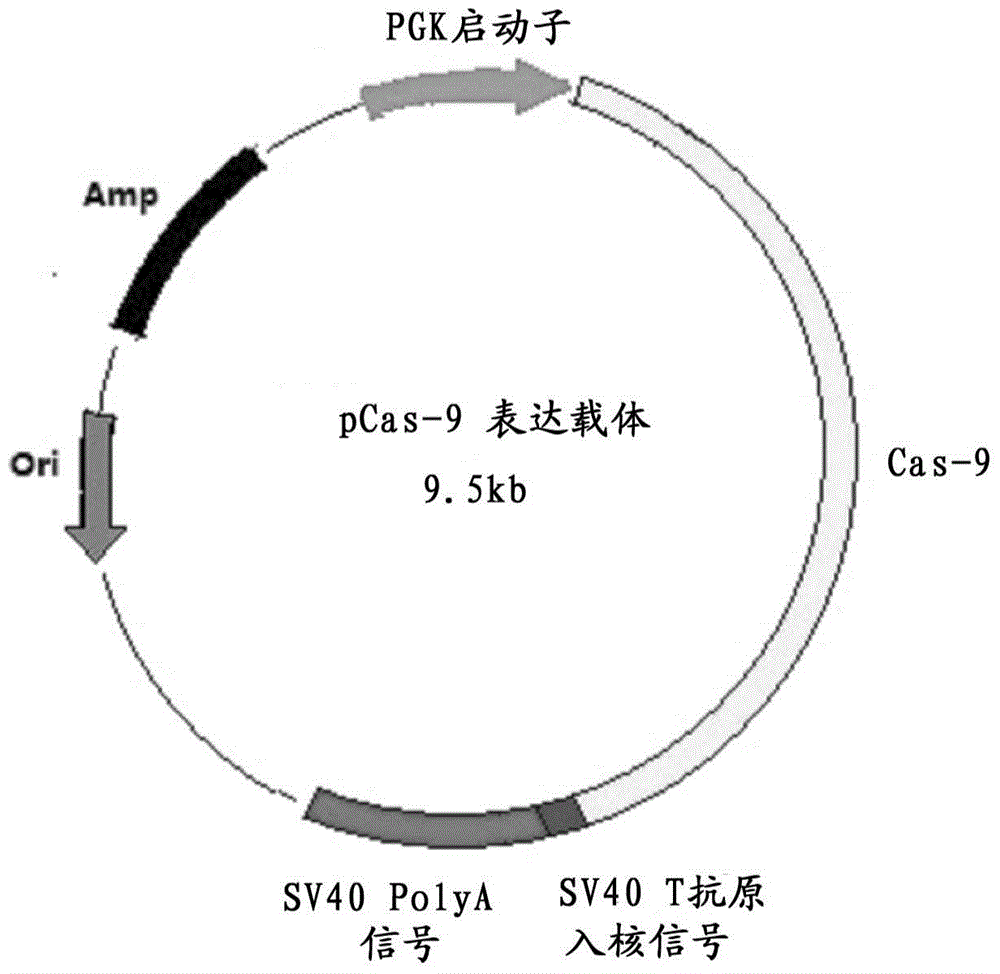
ActiveCN104561095AEasy to operateIncrease success rateAnimals/human peptidesVector-based foreign material introductionPlasmid dnaGenetically modified mouse
The invention discloses a method for producing transgenic mice through a homologous recombination technology. The method comprises the following steps: replacing mouse NGF genes on mouse chromosomes by human NGF genes through a Cas-9 / CRISPR gene knock-in technology, and obtaining the genes with homozygous human NGF genes through breeding and knocking the genes in mice, thus obtaining filial generation mice with salivary glands capable of secreting human NGF. Compared with the conventional targeting technology utilizing positive and negative double screening homologous recombination genes, the method disclosed by the invention is simple to operate, capable of being realized only by transfecting mouse embryonic stem cells by three plasmid DNAs or carrying out pronucleus injection on the zygotes of mice, and high in success rate which is up to 2-5% and remarkably higher than the mouse embryonic stem cell positive rate of 0.1% of the common gene targeting technology.
Owner:深圳市国创纳米抗体技术有限公司
G protein coupled receptors and uses thereof
InactiveUS20060134109A1Peptide/protein ingredientsMuscular disorderAgonistGenetically modified mouse
Owner:OMEROS CORP
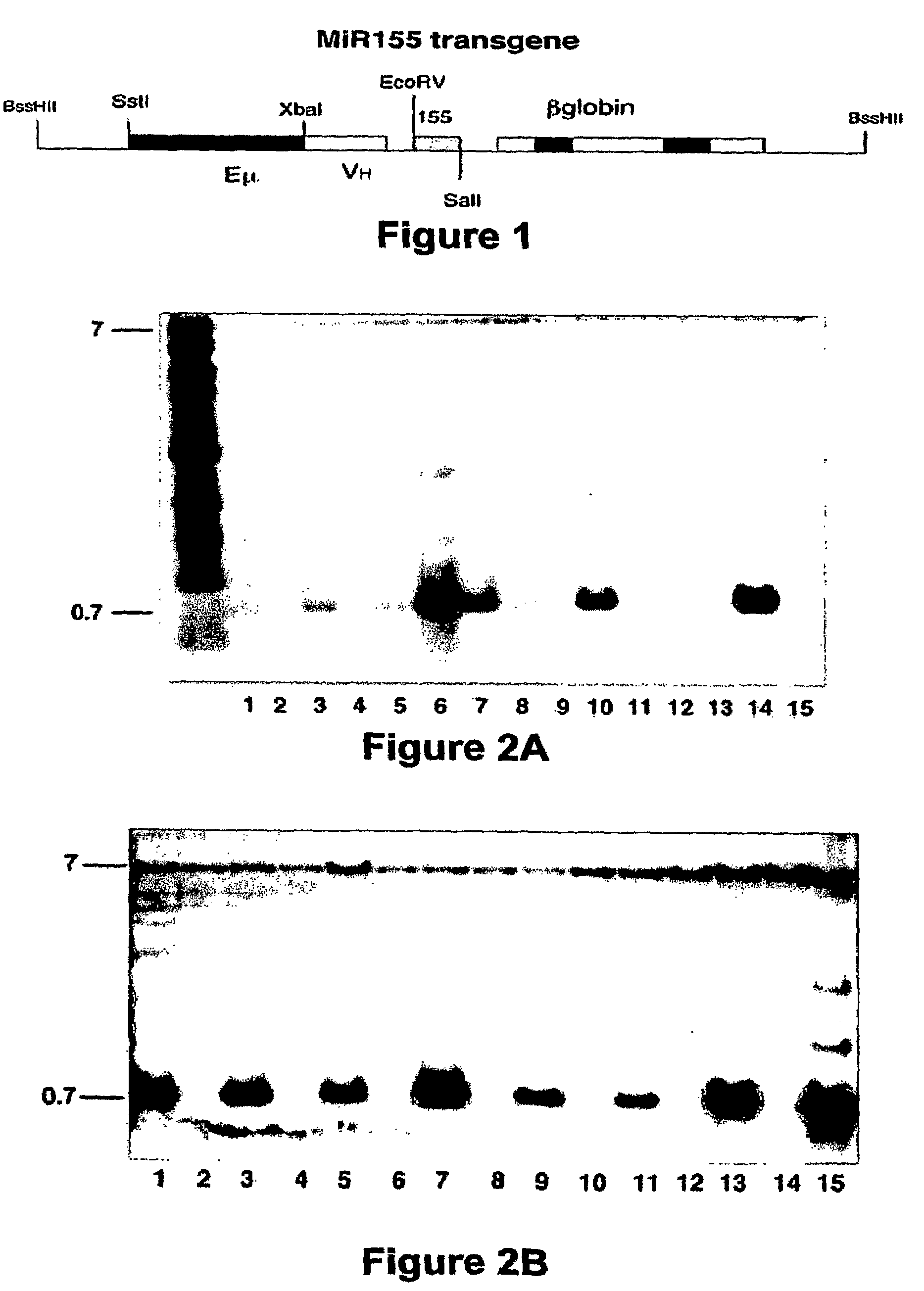
Transgenic mouse model of B cell malignancy
A transgenic non-human animal, such as a mouse, has a genome that include a nucleic acid construct having at least one transcriptional regulatory sequence capable of directing expression in B cells of the animal, wherein the transcriptional regulatory sequence is operably linked to a nucleic acid encoding a miR155 gene product. A method of testing the therapeutic efficacy of an agent in treating or preventing a lymphoproliferative condition includes assessing the effect(s) of the agent on a transgenic non-human animal.
Owner:THE OHIO STATE UNIV RES FOUND
Methods of treating an inflammatory disorder and prohibiting proliferation, cytokine production, and signal transduction with antibody against costimulatory signal transduction molecule AILIM
InactiveUS7166283B2Increase valueEliminate the effects ofOrganic active ingredientsPeptide/protein ingredientsFhit geneGenetically modified mouse
Immunization of human antibody-producing transgenic mice, which have been created using genetic engineering techniques, with AILIM molecule as an antigen resulted in various human monoclonal antibodies capable of binding to AILIM and capable of controlling a variety of biological reactions (for example, cell proliferation, cytokine production, immune cytolysis, cell death, induction of ADCC, etc.) associated with AILIM-mediated costimulatory signal (secondary signal) transduction. Furthermore, it has been revealed that the human monoclonal antibody is effective to treat and prevent various diseases associated with AILIM-mediated costimulatory signal transduction, being capable of inhibiting the onset and / or advancement of the diseases.
Owner:JAPAN TOBACCO INC
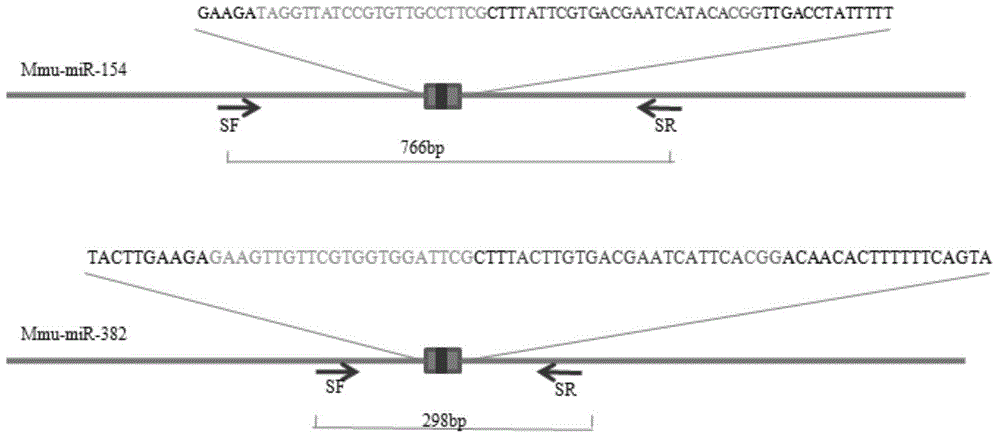
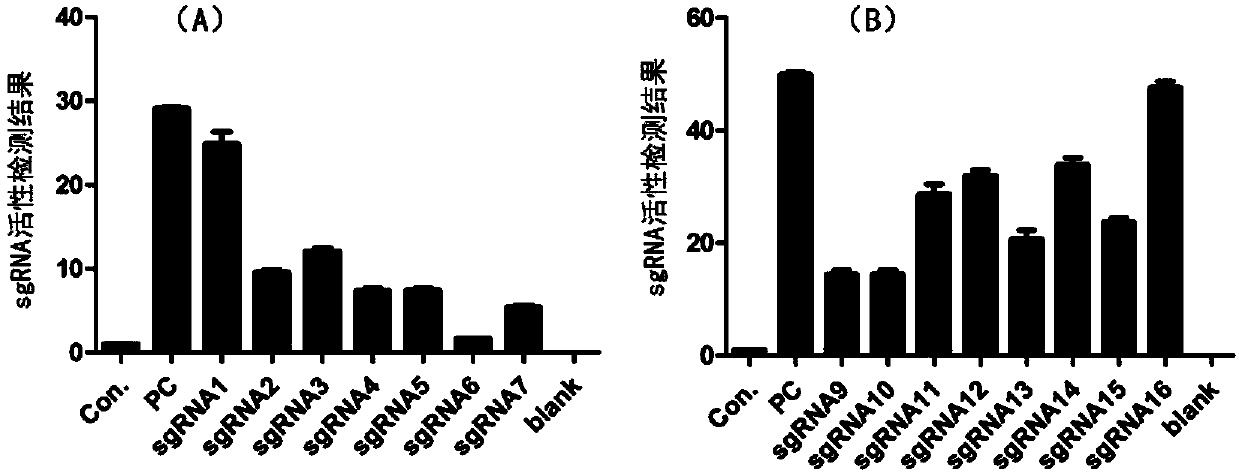
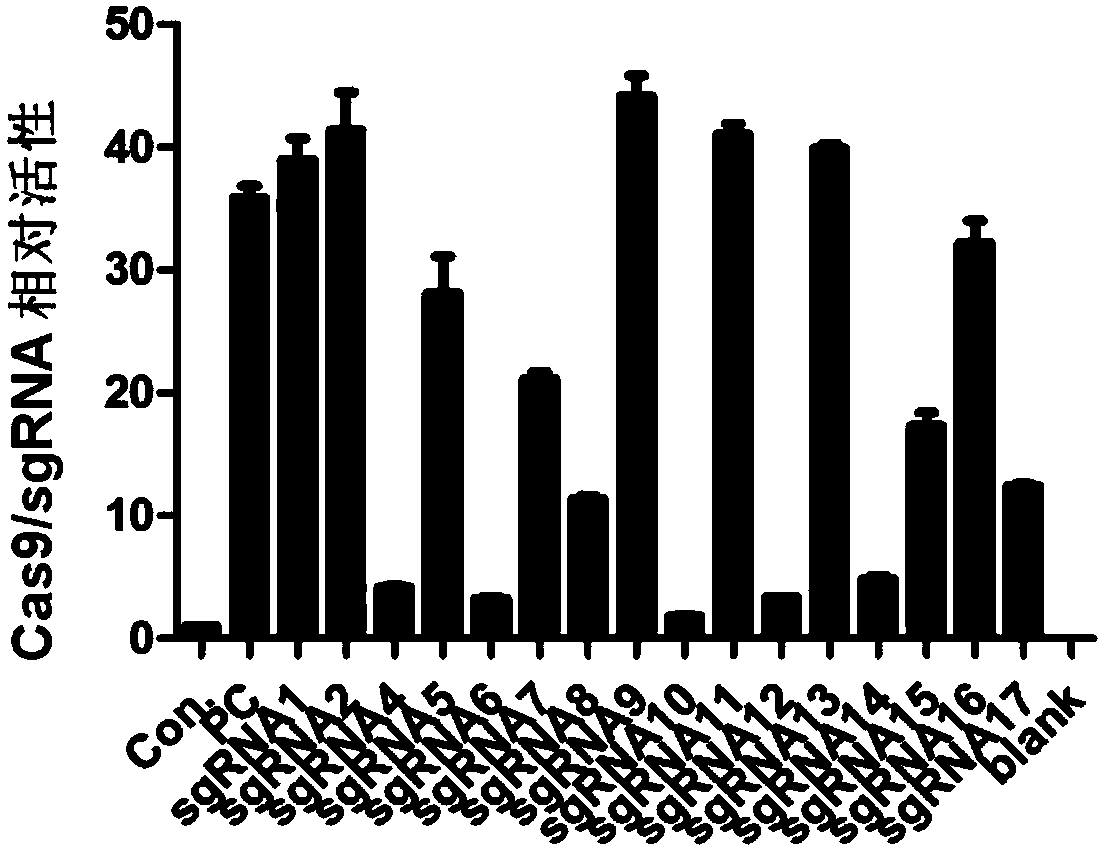
Method for knocking out microRNA gene family by utilizing CRISPR-Cas9 specificity
InactiveCN104651398AShort cycleReduce investmentVector-based foreign material introductionAnimal husbandryF1 generationMicroRNA Gene
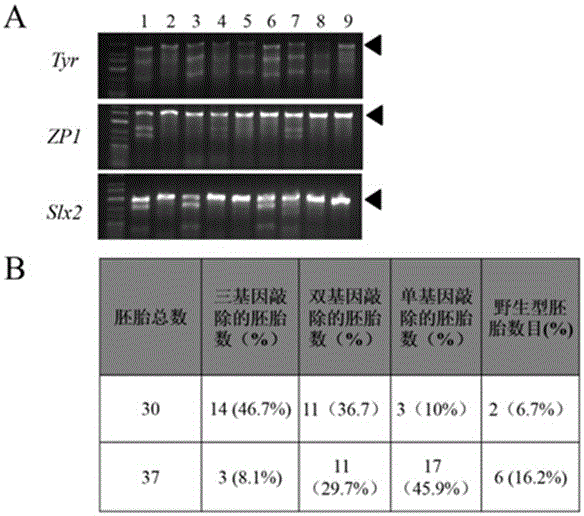
The invention discloses a method for knocking out a microRNA gene family by utilizing CRISPR-Cas9 specificity, wherein a target gene is knocked out by mainly adopting a CRISPR / Cas9 system. The microRNA family is knocked out by utilizing the specificity of the CRISPR / Cas9 system for the first time; the disadvantage that only one gene can be knocked out for one time in traditional transgenosis can be overcome by utilizing the novel method; the time for establishing a model organism is reduced to three weeks; furthermore, the construction step is simple; an expensive molecular reagent is also reduced; the period for constructing a genetically modified mouse is greatly shortened to four months; multiple genes constructed for one time can be knocked out simultaneously; the fund investment is obviously reduced; F1 generations of mice can be obtained only in need of 50000 Yuan; the gene modification efficiency can be above 90%; the unreliability of the traditional technology is reduced; the operation technology is simple; and a series of complex steps including constructing a targeting vector, screening ES (Embryonic Stem) cells, selectively breeding chimeric mice and the like are unnecessary.
Owner:HANGZHOU NORMAL UNIVERSITY
NKX-2.2 and NKX-6.1 transgenic mouse models for diabetes, depression, and obesity
InactiveUS6127598ASuitable for useEasy to detectBiocidePeptide/protein ingredientsInsulin producing cellObesity
The present invention features mouse models for Nkx-2.2 gene function and for Nkx-6.1 gene function, wherein the transgenic mouse is characterized by having a defect in Nkx-2.2 gene function or a defect in Nkx-6.1 gene function (where, because Nkx-2.2 acts upstream of Nkx-6.1, a defect in Nkx-2.2 gene function affects Nkx-6.1 gene function) and by having a decreased number of insulin-producing cells relative to a normal mouse. Where the transgenic mouse contains a defect in Nkx-2.2 gene function, the mouse is further characterized by a decreased number of serotonin-producing cells relative to a normal mouse. The transgenic mice may be either homozygous or heterozygous for the Nkx-2.2 or Nkx-6.1 defect.
Owner:RGT UNIV OF CALIFORNIA
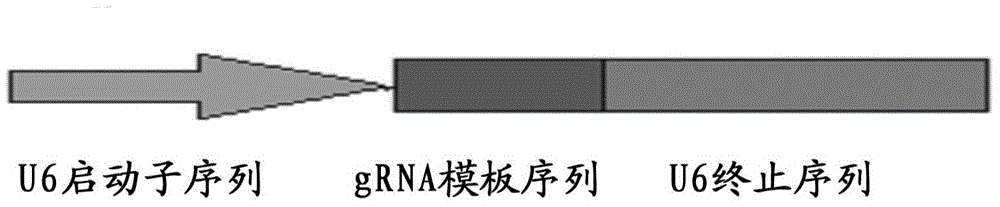
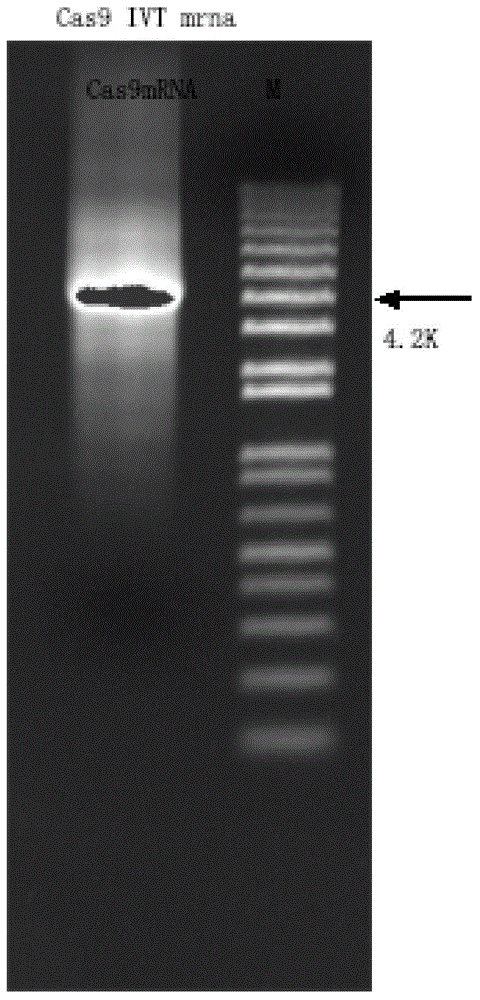
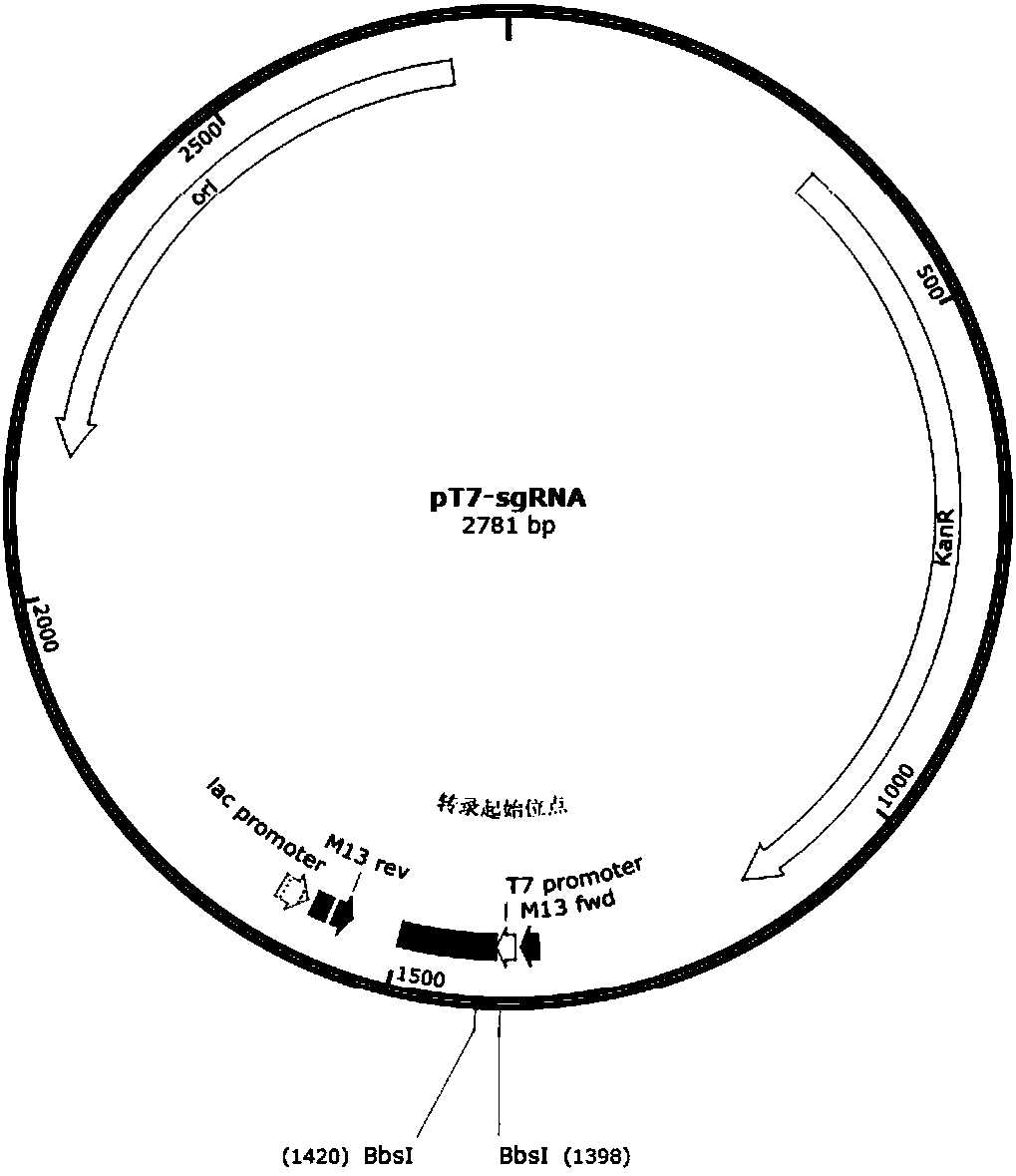
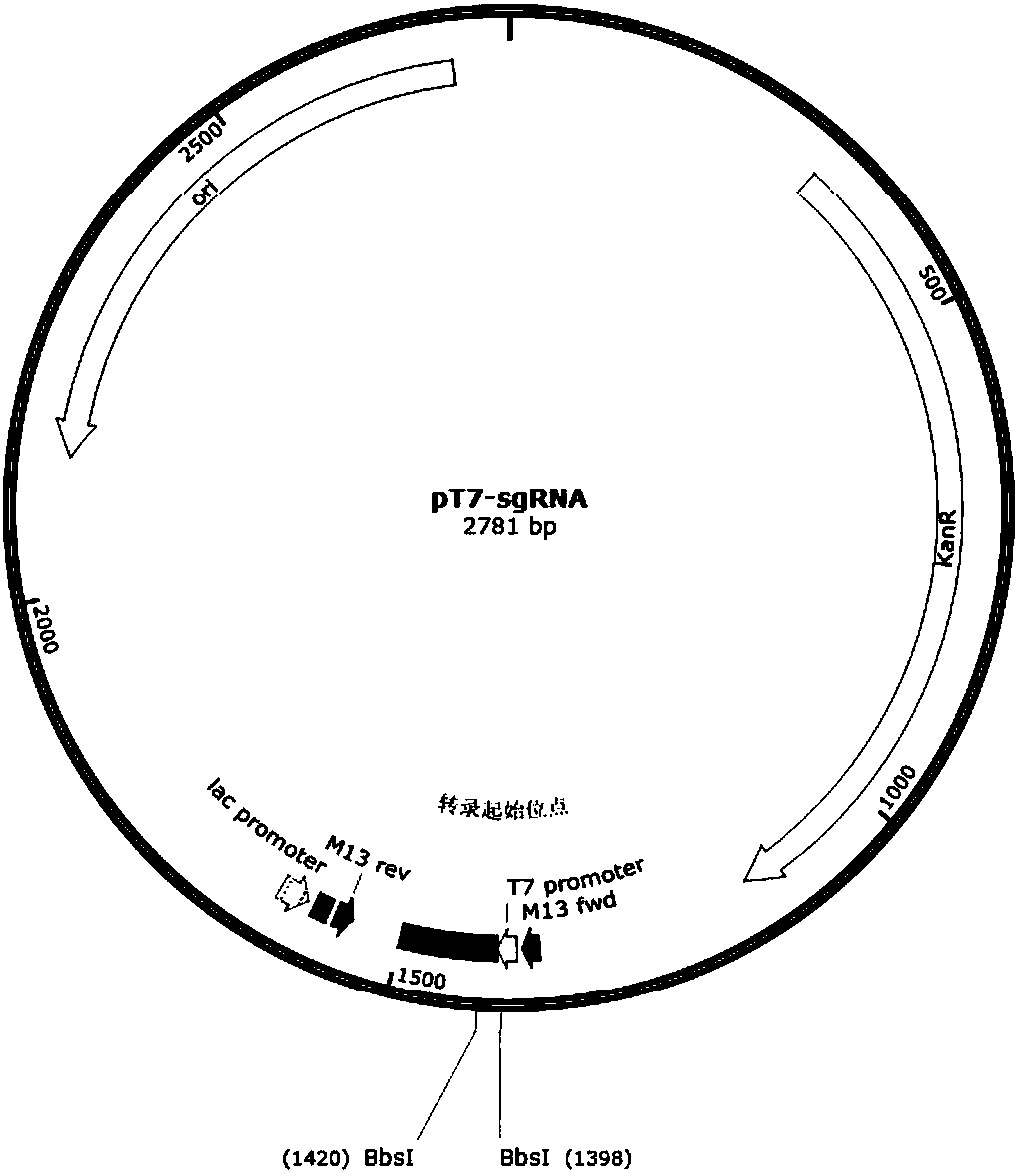
Preparation method of staphylococcus aureus CRISPR/Cas9 system and application of system in constructing mouse model
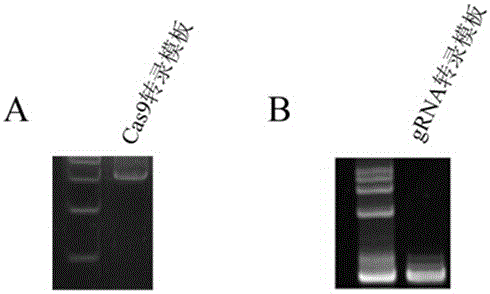
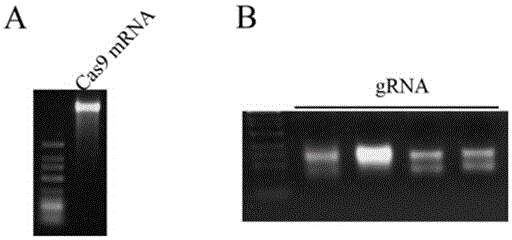
The invention discloses a preparation method of a staphylococcus aureus CRISPR / Cas9 system and an application of the system in constructing a genetically modified mouse model. The staphylococcus aureus CRISPR / Cas9 system is composed of two components, namely Cas9 mRNA and gRNA, wherein a preparation method of the Cas9 mRNA is achieved by adding a T7 promoter to the upstream region of original Cas9 coding DNA, and a preparation method of the gRNA is achieved by adding the T7 promoter to the upstream region of an original gRNA coding sequence. The staphylococcus aureus Cas9 mRNA and gRNA, which are injected to mouse fertilized embryos through micro-injection, can achieve gene editing and modification of various types, such as single-gene knockout, multi-gene knockout and / or gene knock-in and the like, on the mouse fertilized embryos; therefore, the CRISPR / Cas9 system has a good application prospect in the aspects of fertilized embryo gene editing and modification of such animals as mouse and the like as well as construction of animal models.
Owner:GUANGZHOU MAGIGEN BIOTECH
Muir-torre-like syndrome in Fhit deficient mice
The invention provides nonhuman transgenic animals with a disrupted FHIT gene. The invention further provides transgenic mice in which one or both Fhit alleles have been inactivated. Preferably, the Fhit-deficient mice develop multiple tumors of both visceral and sebaceous origin, similar to those of Muir-Torre familial cancer syndrome. The present invention further relates to the generation of these transgenic mice and their use as model systems to study the effects of carcinogenic agents in promoting clonal expansion of neoplastic cells in cancers, preferably gastrointestinal cancers of which Muir-Torre syndrome is a subset. The invention further relates to testing therapeutic agents for their efficacy in the prevention and treatment of cancer, preferably gastrointestinal cancer.
Owner:THOMAS JEFFERSON UNIV
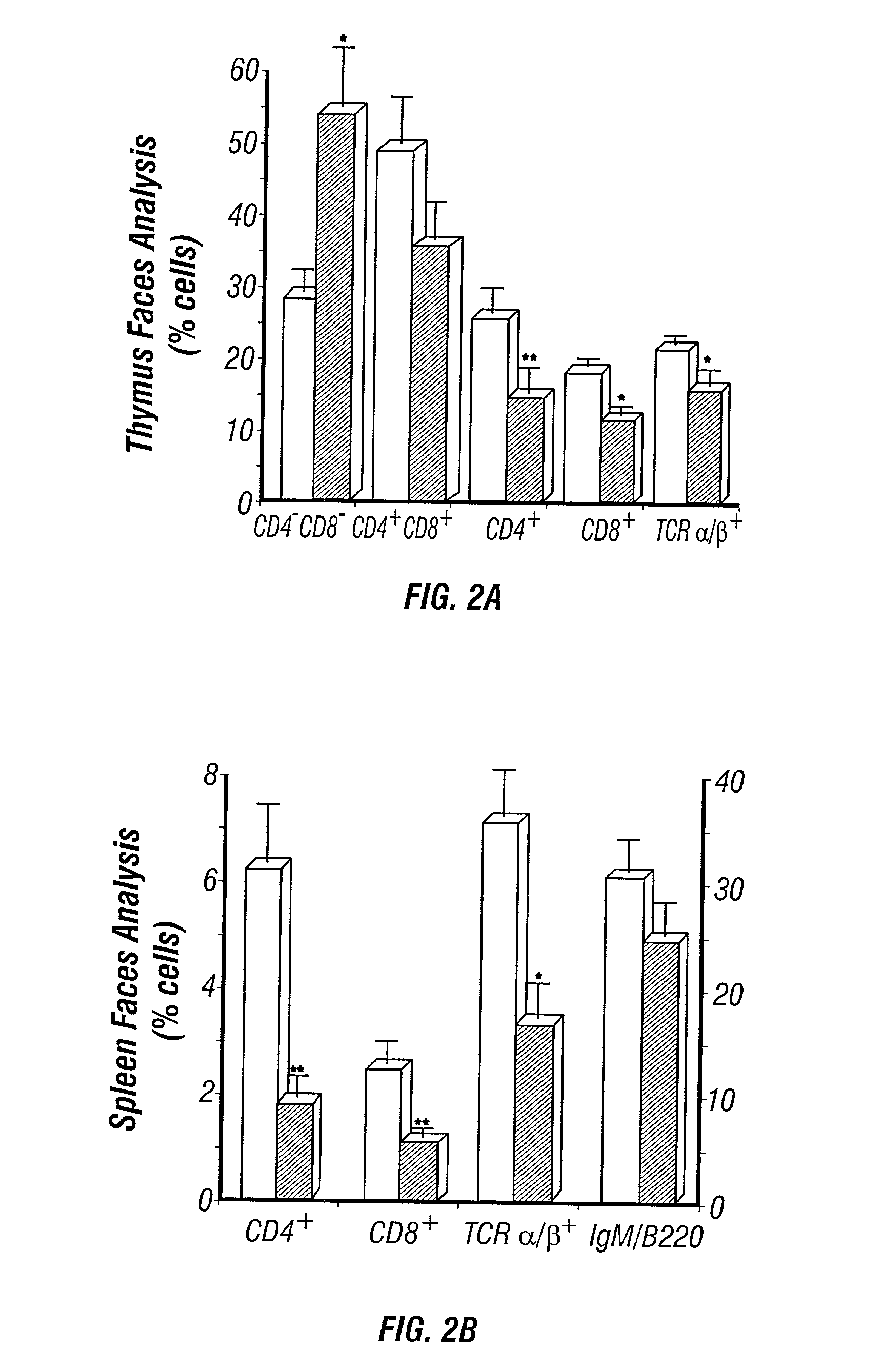
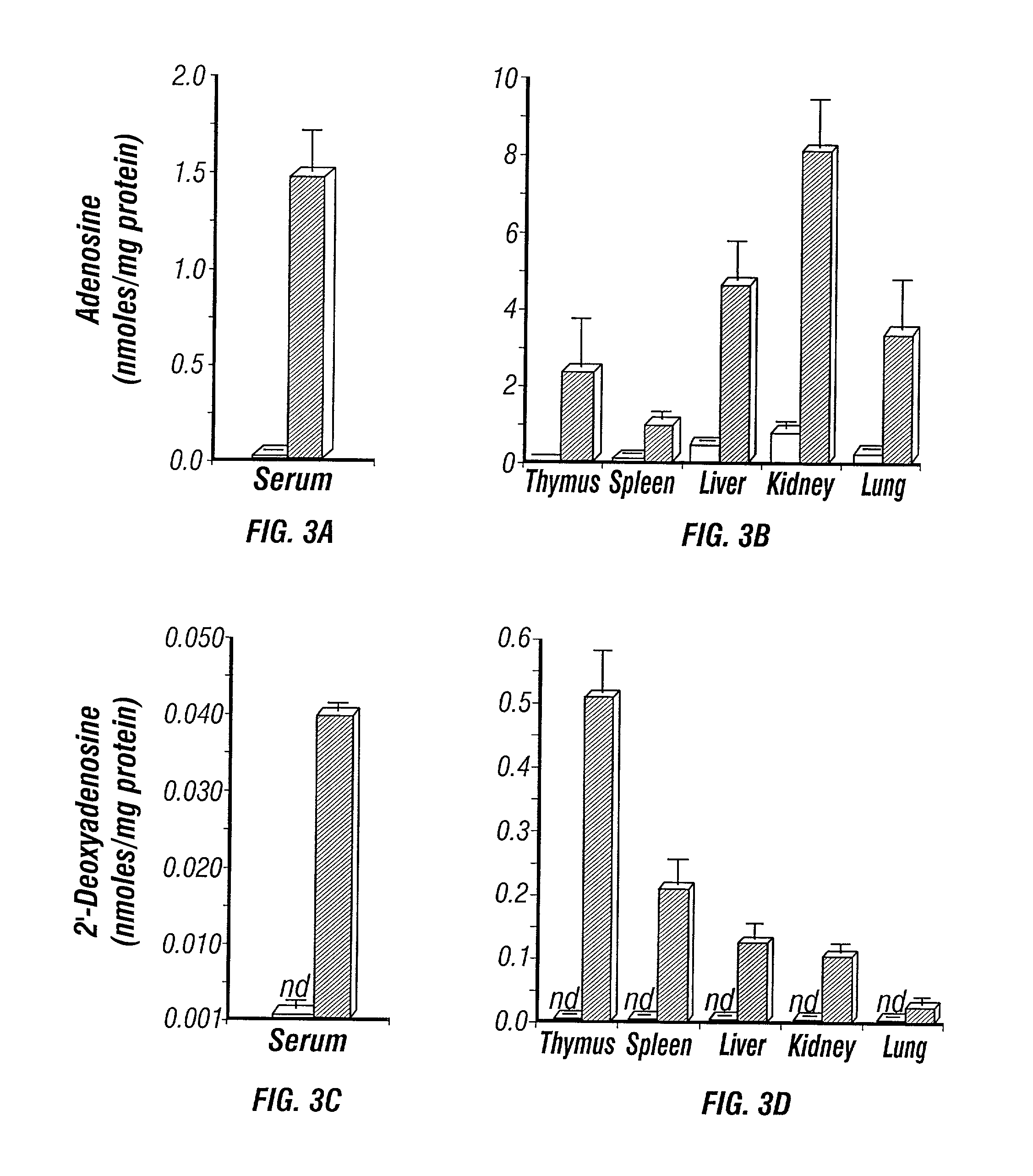
Adenosine deaminase deficient transgenic mice and methods for the use thereof
InactiveUS6207876B1Determining effectGuaranteed flatnessVectorsTissue cultureMedicineDeficient mouse
The present invention relates to the production of adenosine deaminase (ADA) deficient mice and the use of such mice as an animal model for dysfunctions associated with elevated adenosine levels. Also, provided by the present invention are methods of treating dysfunctions associated with elevated adenosine levels and methods of screening compounds for pharmaceutical activity in the treatment of dysfunctions associated with elevated adenosine levels.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Muir-torre-like syndrome in Fhit deficient mice
The invention provides nonhuman transgenic animals with a disrupted FHIT gene. The invention further provides transgenic mice in which one or both Fhit alleles have been inactivated. Preferably, the Fhit-deficient mice develop multiple tumors of both visceral and sebaceous origin, similar to those of Muir-Torre familial cancer syndrome. The present invention further relates to the generation of these transgenic mice and their use as model systems to study the effects of carcinogenic agents in promoting clonal expansion of neoplastic cells in cancers, preferably gastrointestinal cancers of which Muir-Torre syndrome is a subset. The invention further relates to testing therapeutic agents for their efficacy in the prevention and treatment of cancer, preferably gastrointestinal cancer.
Owner:THOMAS JEFFERSON UNIV
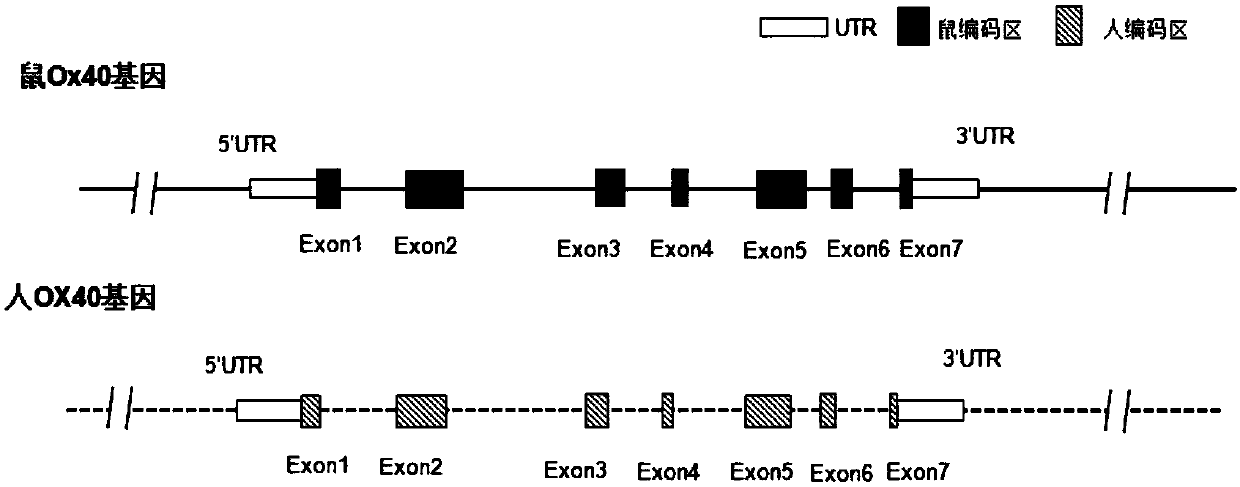
Preparation method and application of humanized gene modification animal model
ActiveCN107815465AReduce development riskSpeed up the R&D processNGF/TNF-superfamilyStable introduction of DNAGene ModificationGenetically modified mouse
The invention relates to a humanized gene genetically modified non-human animal, particularly a genetically modified rodent, especially a genetically modified mouse, and in particular relates to a construction method of a humanized OX-40 gene animal model and application of the model in the biomedicine field.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD +1
Methods of treating systemic lupus erythematosus with an antibody against costimulatory signal transduction molecule ailim
InactiveUS7988965B2Increase valueEliminate the effects ofOrganic active ingredientsPeptide/protein ingredientsDiseaseImmune cytolysis
Immunization of human antibody-producing transgenic mice, which have been created using genetic engineering techniques, with AILIM molecule as an antigen resulted in various human monoclonal antibodies capable of binding to AILIM and capable of controlling a variety of biological reactions (for example, cell proliferation, cytokine production, immune cytolysis, cell death, induction of ADCC, etc.) associated with AILIM-mediated costimulatory signal (secondary signal) transduction. Furthermore, it has been revealed that the human monoclonal antibody is effective to treat and prevent various diseases associated with AILIM-mediated costimulatory signal transduction, being capable of inhibiting the onset and / or advancement of the diseases.
Owner:JAPAN TOBACCO INC
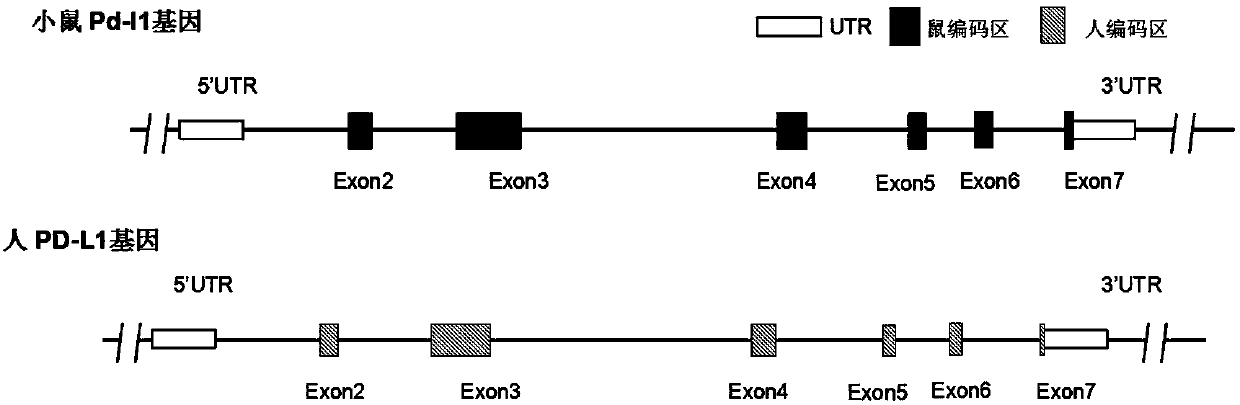
Preparation method and application of humanized gene modification animal model
ActiveCN107815466AReduce development riskSpeed up the R&D processCell receptors/surface-antigens/surface-determinantsSugar derivativesPD-L1Gene Modification
The invention relates to a humanized gene genetically modified non-human animal, particularly a genetically modified rodent, especially a genetically modified mouse, and in particular relates to a construction method of a humanized PD-L1 gene animal model and application of the model in the biomedicine field.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
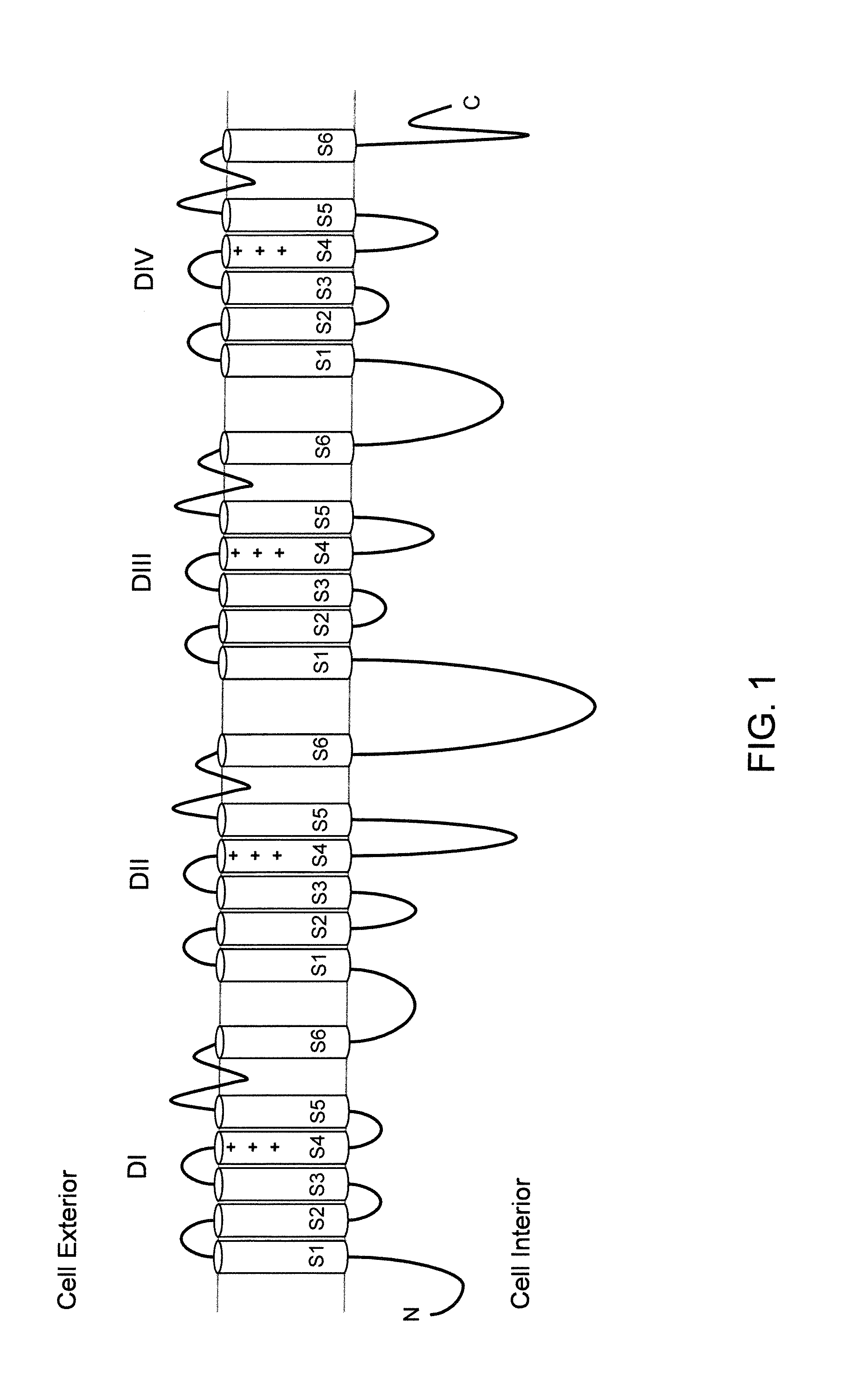
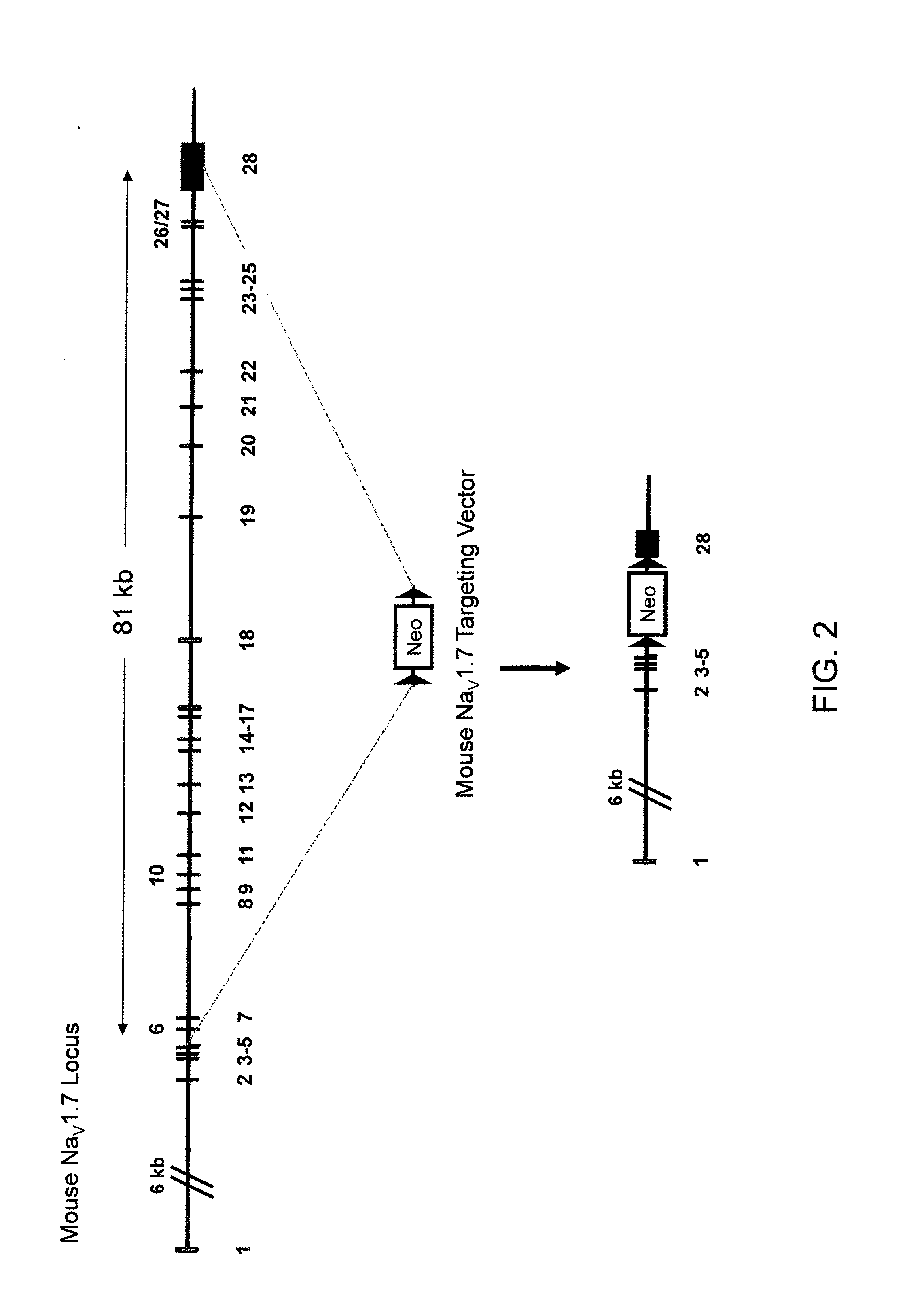
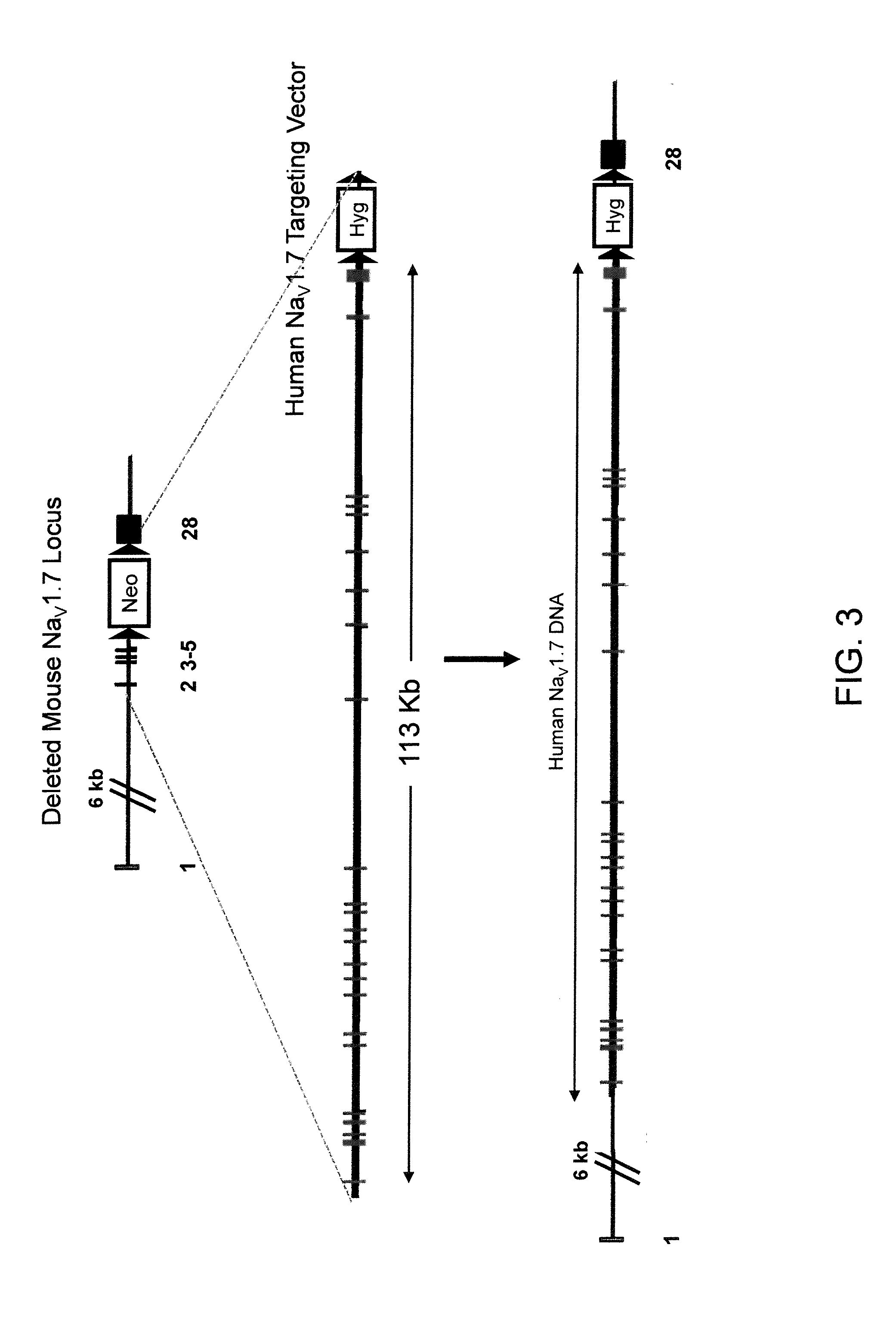
Mice Expressing Human Voltage-Gated Sodium Channels
ActiveUS20110307966A1High activityReduced activityAnimal cellsFermentationExcitable cellGenetically modified mouse
Genetically modified non-human animals and methods and compositions for making and using them are provided, wherein the genetic modification comprises a humanization of an extracellular loop of an endogenous NaV channel gene, in particular a humanization of the one or more extracellular pore loops of a NaV1.7 channel protein. Genetically modified non-human animals are also provided, wherein the genetic modification comprises replacement of an endogenous NaV channel gene, in particular a replacement of the endogenous NaV1.7 gene with a human NaV1.7 gene, and wherein the genetically modified non-human animals are capable of generating action potentials and communicating through the excitable cells of the genetically modified non-human animals via the expressed human or humanized NaV1.7 protein the surface of the excitable cells. Genetically modified mice are described, including mice that express the human or humanized NaV1.7 gene from the endogenous NaV1.7 locus, and wherein the mice comprise functional β-subunits.
Owner:REGENERON PHARM INC
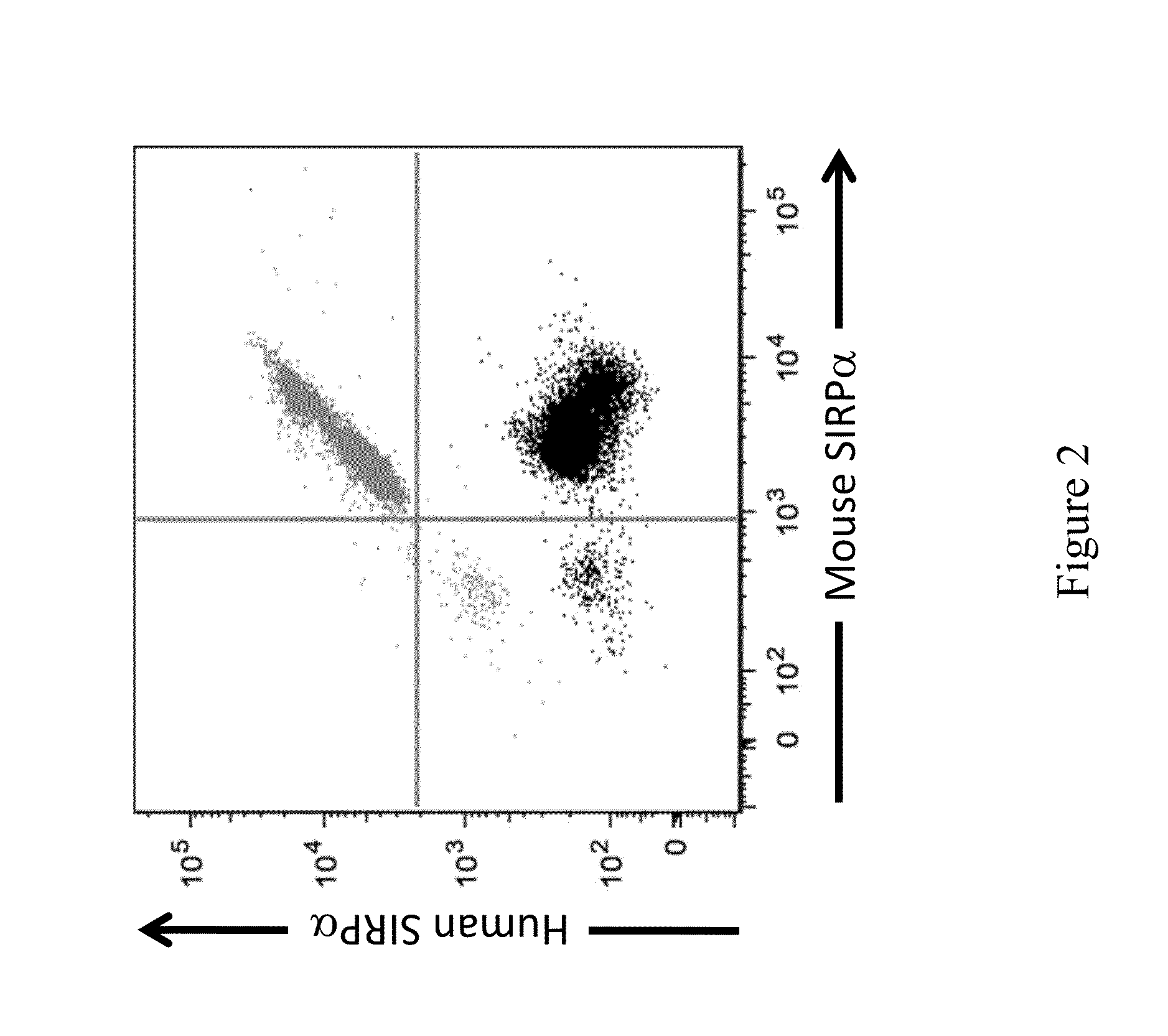
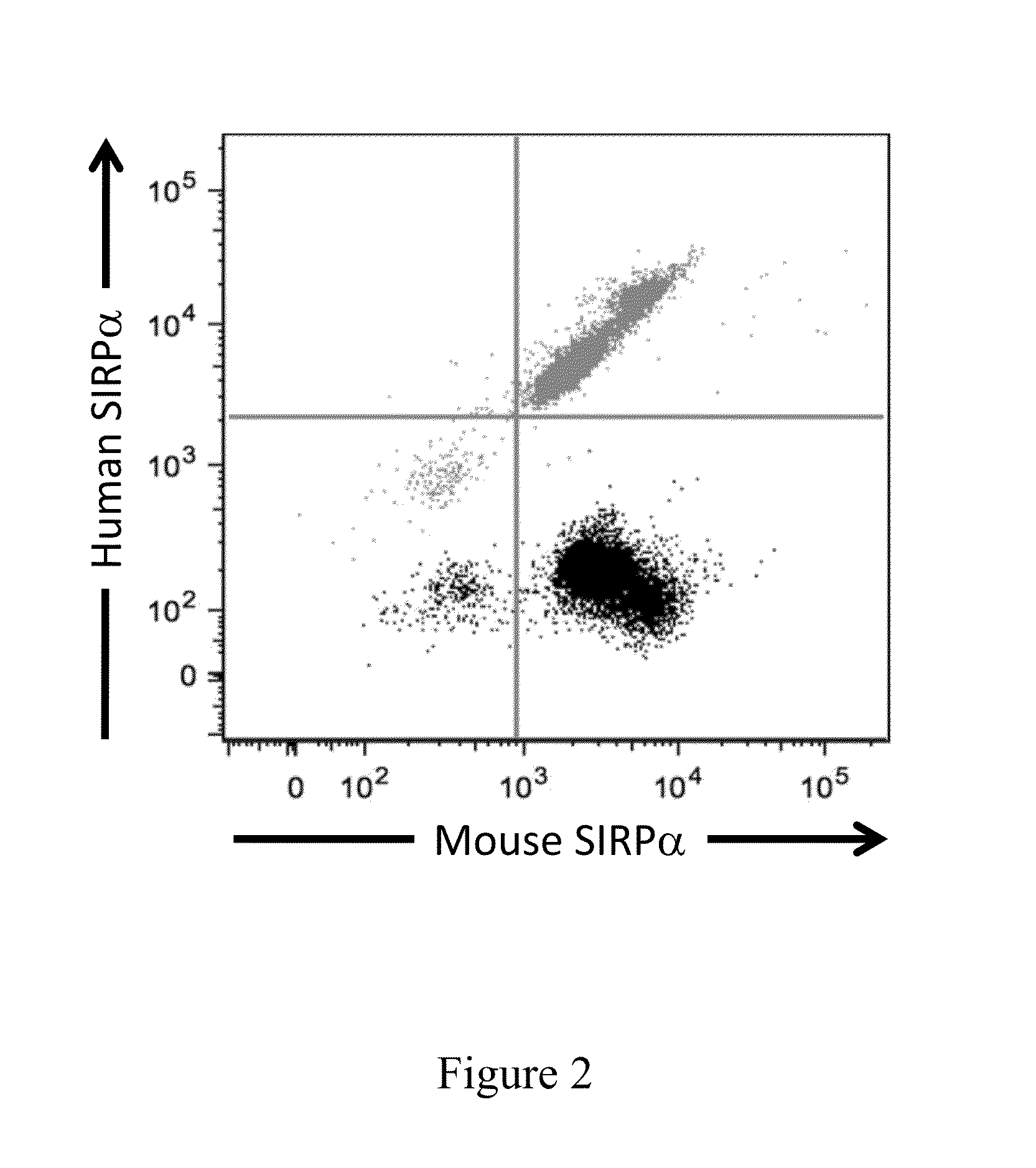
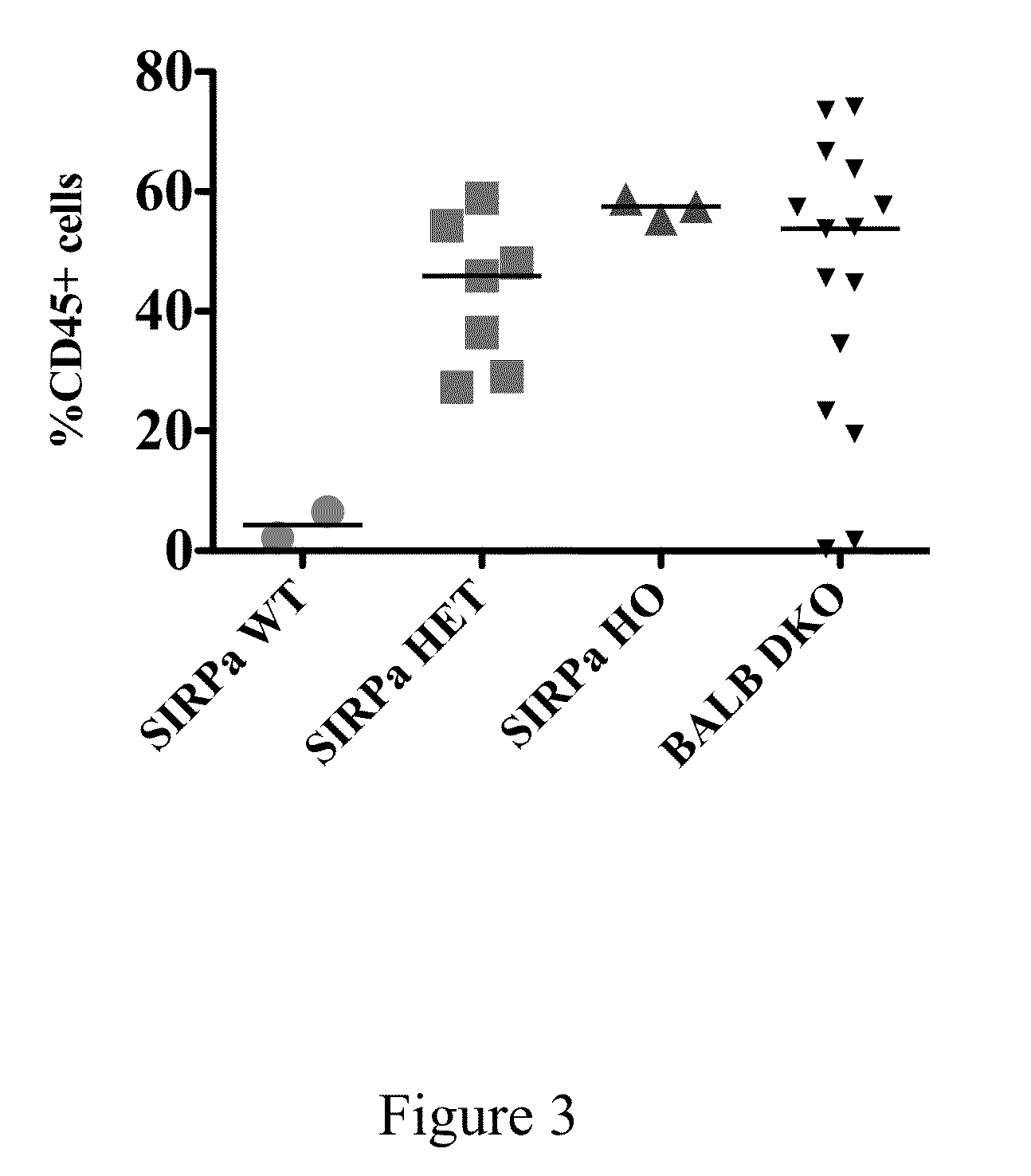
Non-human animals having a humanized signal-regulatory protein gene
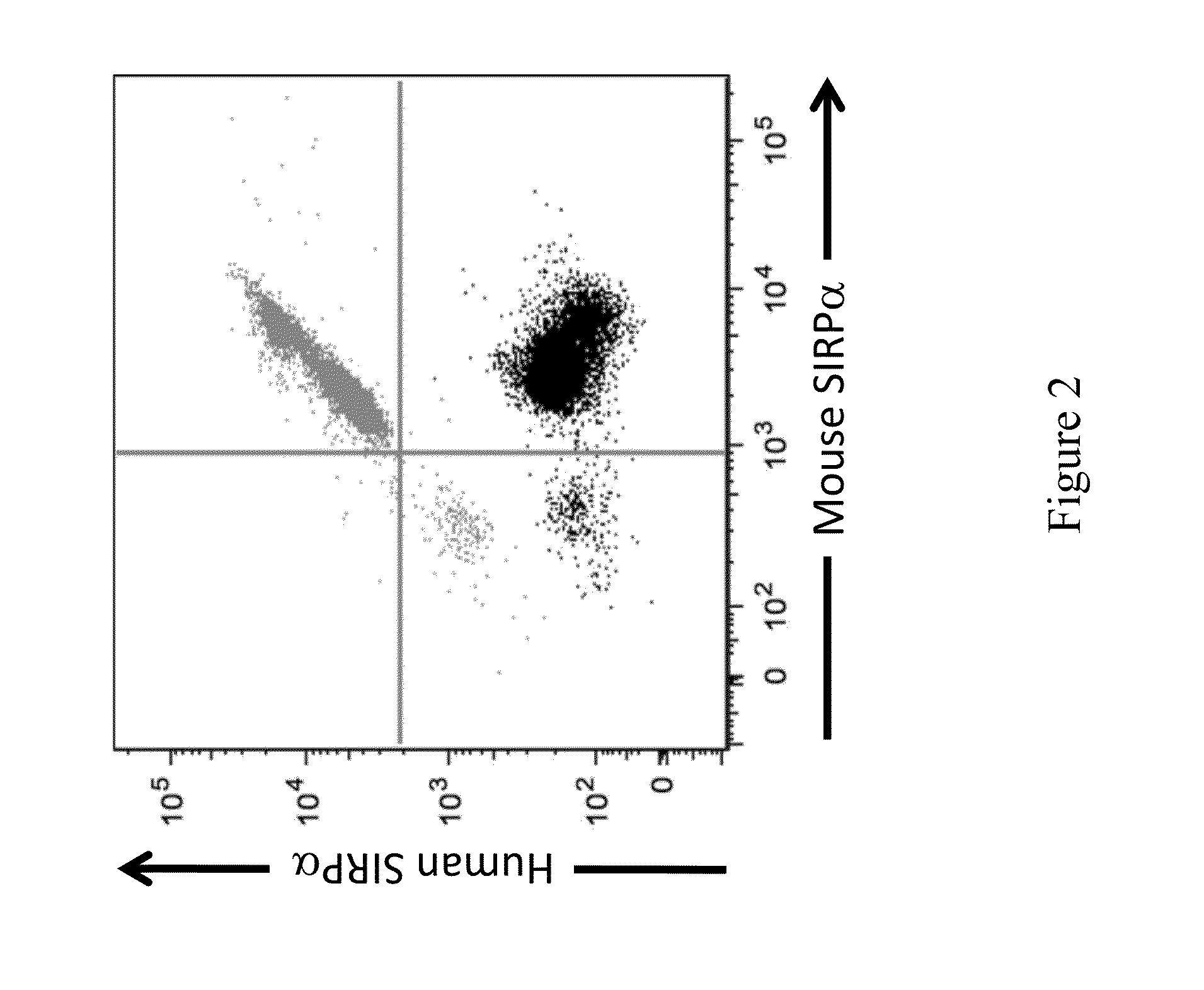
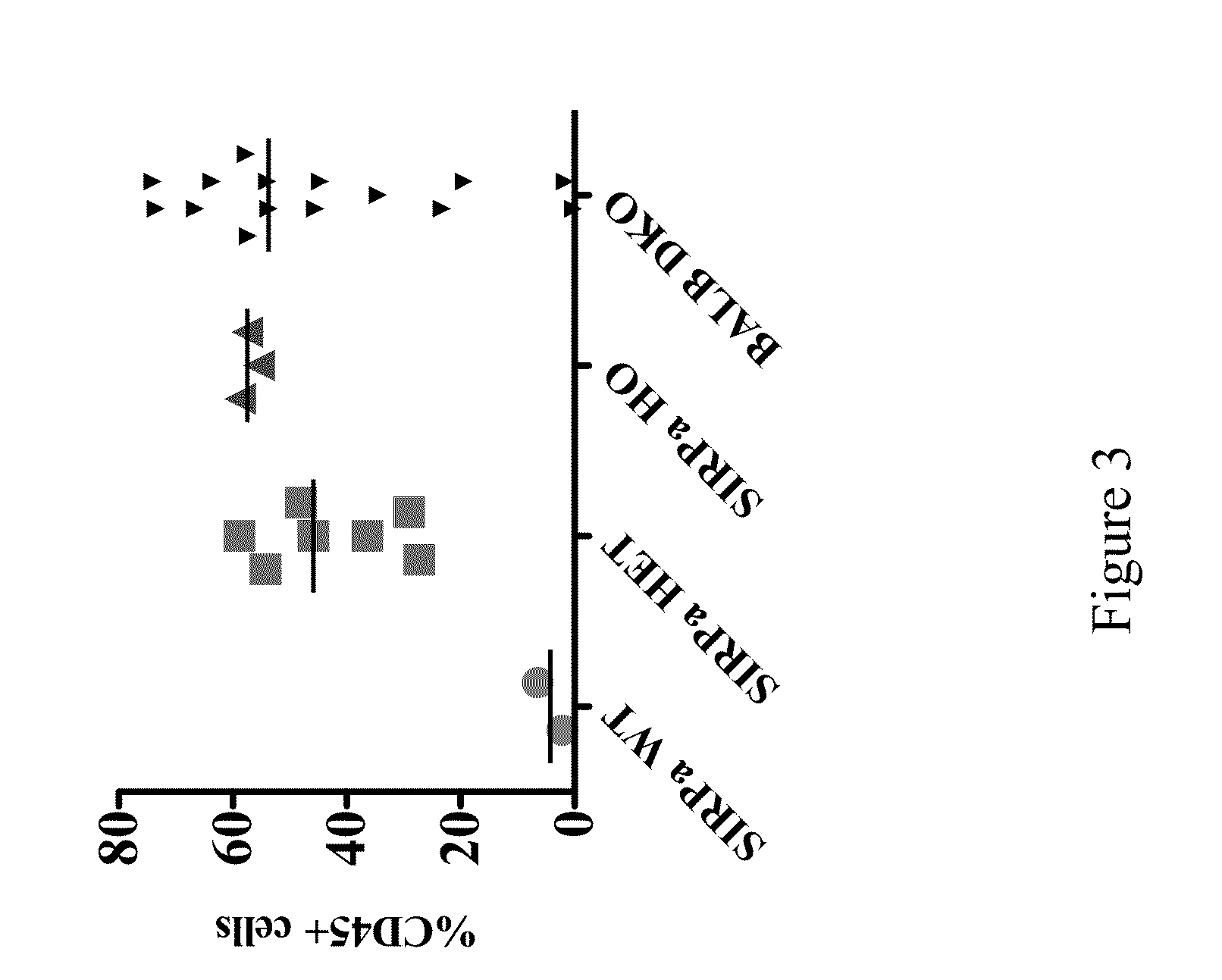
ActiveUS20150089678A1Increased susceptibilityIncrease and decrease in food consumptionCompounds screening/testingCompound screeningHuman animalGenetically modified mouse
Genetically modified non-human animals and methods and compositions for making and using the same are provided, wherein the genetic modification comprises a humanization of an endogenous signal-regulatory protein gene, in particular a humanization of a SIRPα gene. Genetically modified mice are described, including mice that express a human or humanized SIRPα protein from an endogenous SIRPα locus.
Owner:REGENERON PHARM INC
Transgenic mice expressing antibodies specific for genes of interest and uses thereof
The invention provides compositions and methods for the generation of novel non-human transgenic animals which contain an alteration in a gene of interest. These transgenic animals are capable of generating antibodies, e.g., human monoclonal antibodies, specific for the product of a gene of interest that has been functionally disrupted in the transgenic animal. Furthermore, the methods and compositions of the invention are suitable for use in the treatment, diagnosis, and imaging of disease.
Owner:ABBOTT GMBH & CO KG
Transgenic mouse expressing the human cyclooxygenase-2 gene and neuronal cell cultures derived therefrom
Owner:MT SINAI SCHOOL OF MEDICINE
Compositions and Methods Related to Controlled Gene Expression Using Viral Vectors
InactiveUS20090210952A1Significant advanceEfficient delivery and controlled expression of geneAntiviralsNucleic acid vectorViral vectorGenetically modified mouse
Provided herein are methods and compositions related to viral vectors. Also provided herein are methods and compositions for the efficient transfection of a host, for example through the highly efficient lentivector delivery system, and for the exquisite control of the timing and level of expression of the transferred sequence of interest by the simple administration of a modulator to the host harboring the transferred sequence of interest. Also disclosed are methods of making transgenic mice and transgenic mice made using compositions and methods relating to viral vectors.
Owner:UAB RES FOUND
Preparation method and application of humanized gene modification animal model
ActiveCN107815467ACompounds screening/testingPolypeptide with localisation/targeting motifGene ModificationGenetically modified mouse
The invention relates to a humanized gene genetically modified non-human animal, particularly a genetically modified rodent, especially a genetically modified mouse, and in particular relates to a construction method of a humanized TIGIT gene animal model and application of the model in the biomedicine field.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
Non-human animals having a humanized signal-regulatory protein gene
Genetically modified non-human animals and methods and compositions for making and using the same are provided, wherein the genetic modification comprises a humanization of an endogenous signal-regulatory protein gene, in particular a humanization of a SIRPα gene. Genetically modified mice are described, including mice that express a human or humanized SIRPα protein from an endogenous SIRPα locus.
Owner:REGENERON PHARM INC
Animal model systems for viral pathogenesis of neurodegeneration, autoimmune demyelination, and diabetes
InactiveUS20060130161A1Nervous disorderPeptidesProgressive multifocal leukoencephalopathyAutoimmune disease
Provided are non-human animal model systems for viral pathogenesis of neurodegeneration, autoimmune demyelination, and autoimmune diseases such as diseases of the central nervous system, including multiple sclerosis (MS), and diabetes. Such non-human animal model systems may be suitably employed for the study of diseases such as MS and diabetes and for the identification and characterization of candidate therapeutic compounds and compositions for the treatment of such diseases. Also provided herein are markers and methods for the detection, in patients susceptible to autoimmune disease, of autoimmune diseases of the central nervous system such as progressive multifocal leukoencephalopathy (PML) following treatment with one or more therapeutic agent as exemplified herein by the therapeutic agent natalizumab. Exemplary animal model systems comprise marmosets infected with a herpesvirus such as HHV6-A and HHV6-B, transgenic mouse and zebrafish animal model systems wherein the transgene encodes CD46, and methods for monitoring the risks of patients having MS, diabetes and other auto-immune disorders treated with anti-adhesion molecules such as natalizumab.
Owner:CARANTECH
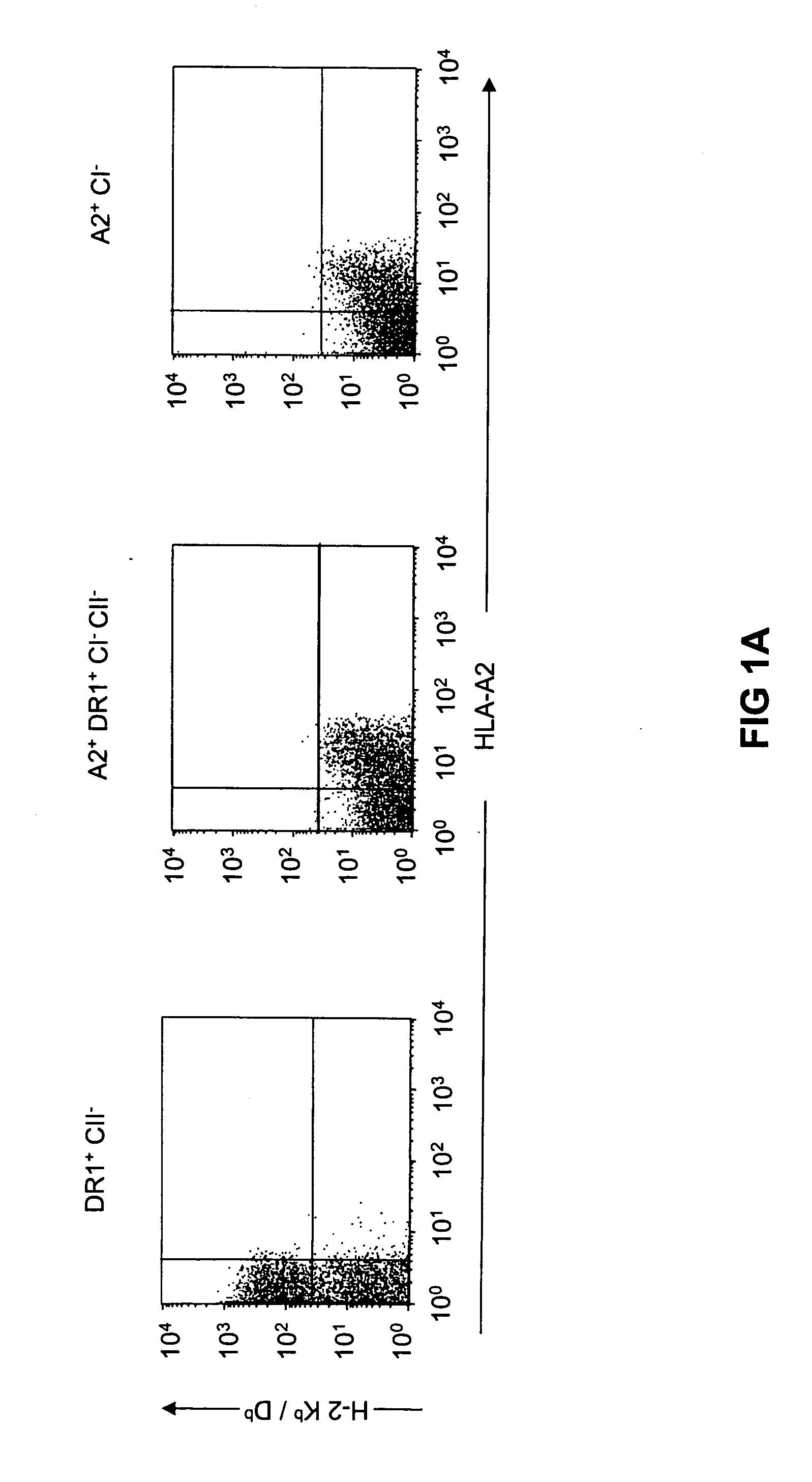
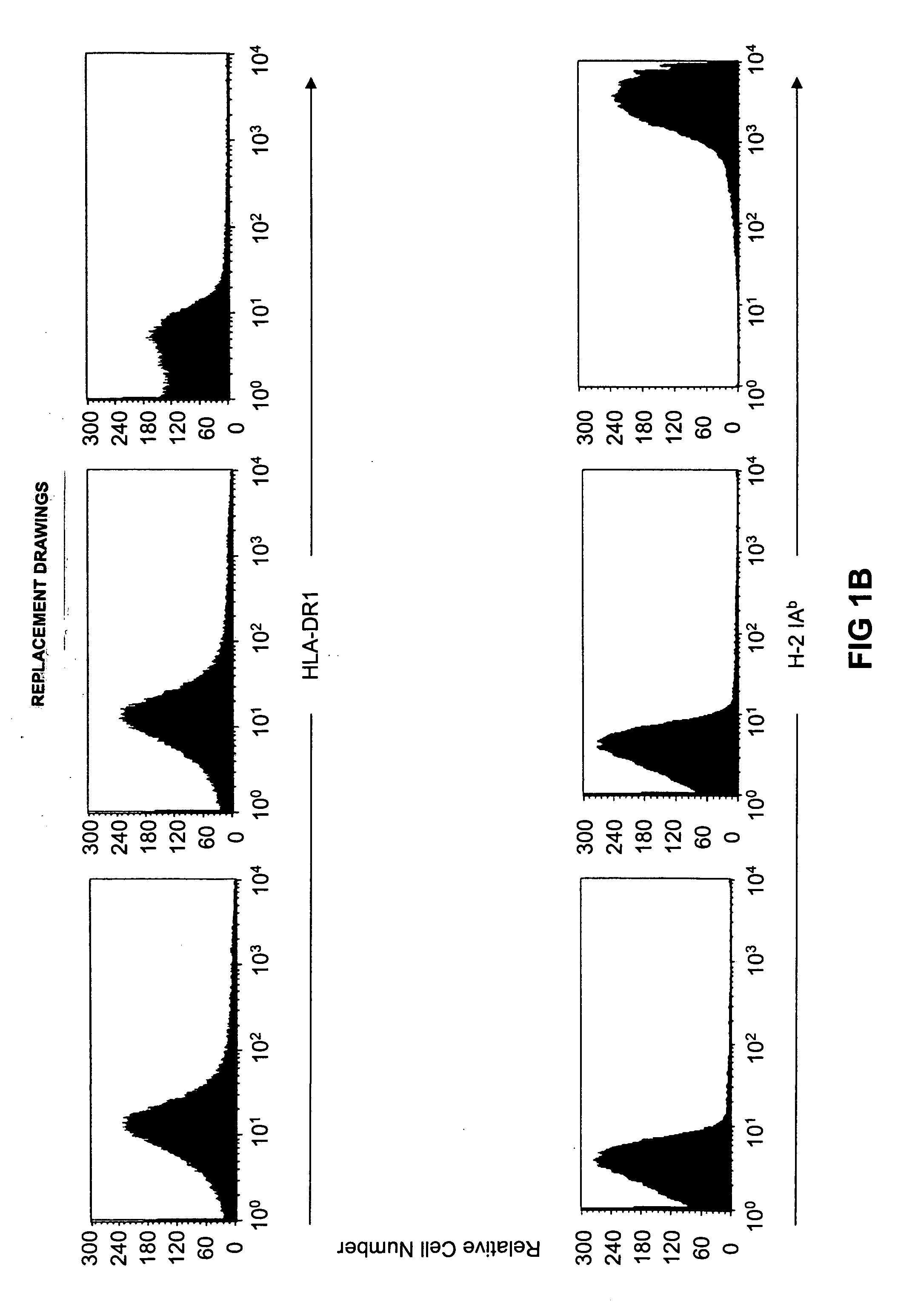
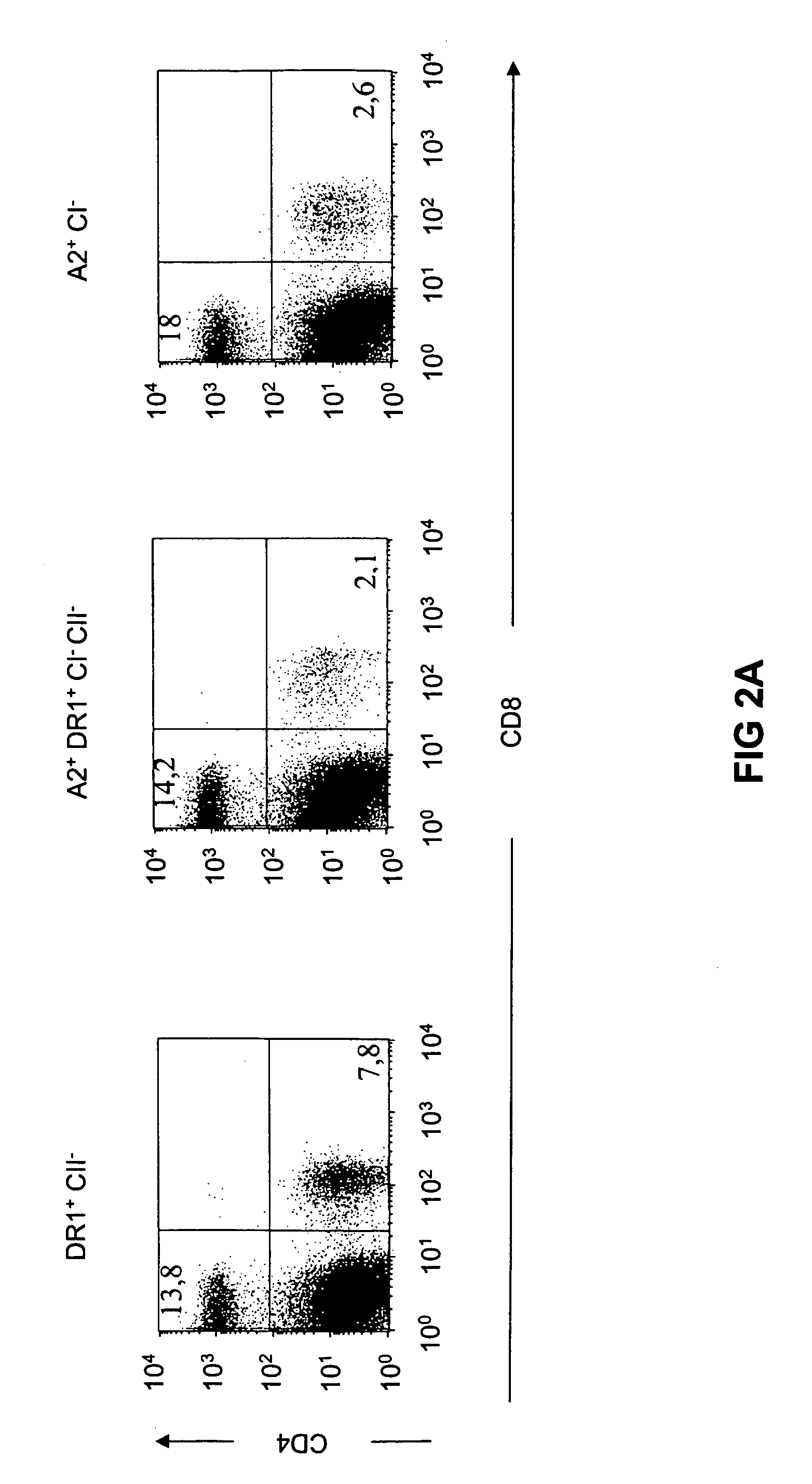
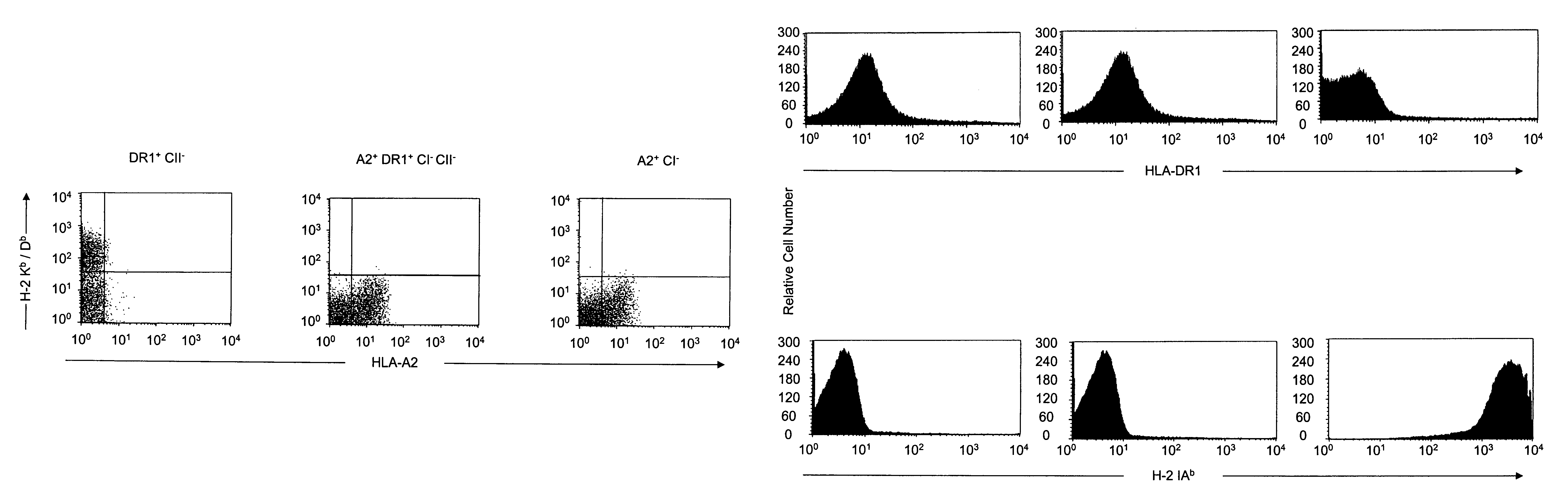
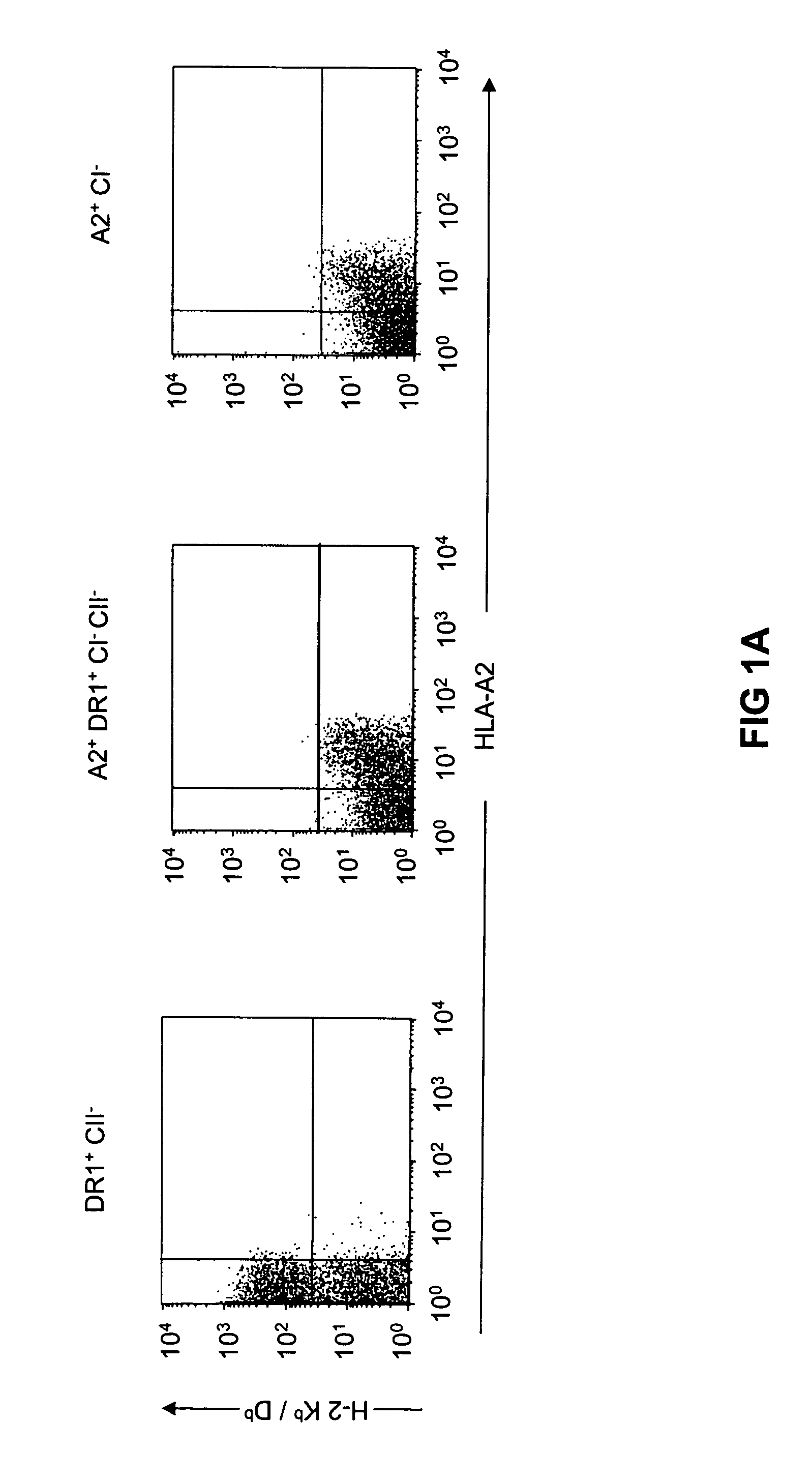
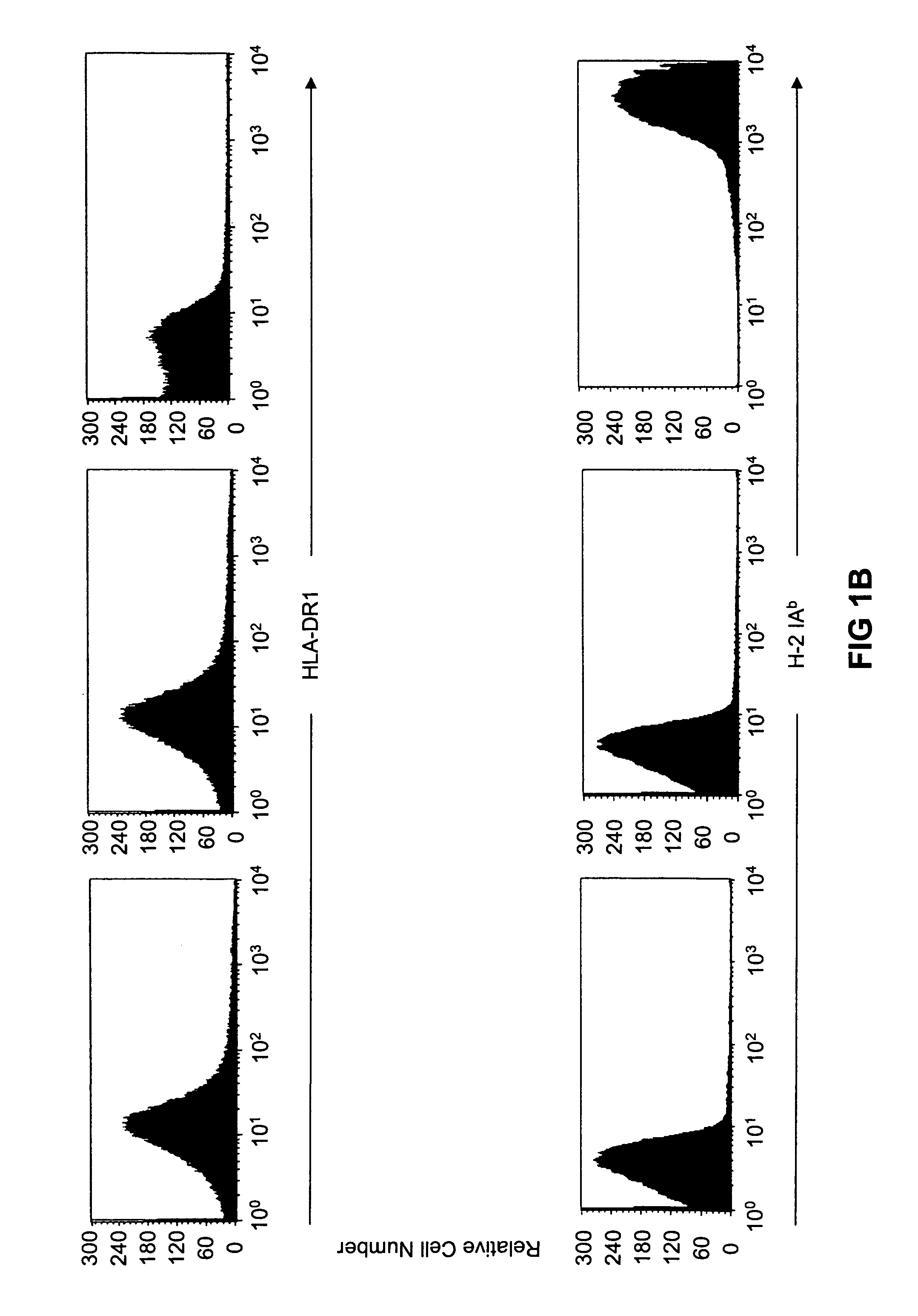
Transgenic mice having a human major histocompatibility complex (MHC) phenotype, experimental uses and applications
The present invention relates to transgenic mice and isolated transgenic mouse cells, the mice and mouse cells comprising a disrupted H2 class I gene, a disrupted H2 class II gene, a functional HLA class I transgene, and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cells are deficient for both H2 class I and class II molecules, wherein the transgenic mouse comprises a functional HLA class I transgene and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cell has the genotype HLA-A2+HLA-DR1+β2m°IAβ°. The invention also relates to methods of using a transgenic mouse of the invention.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Transgenic mice having a human major histocompatability complex (MHC) phenotype, experimental uses and applications
The present invention relates to transgenic mice and isolated transgenic mouse cells, the mice and mouse cells comprising a disrupted H2 class I gene, a disrupted H2 class II gene, a functional HLA class I transgene, and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cells are deficient for both H2 class I and class II molecules, wherein the transgenic mouse comprises a functional HLA class I transgene and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cell has the genotype HLA-A2+HLA-DR1+β2m°IAβ°. The invention also relates to methods of using a transgenic mouse of the invention.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
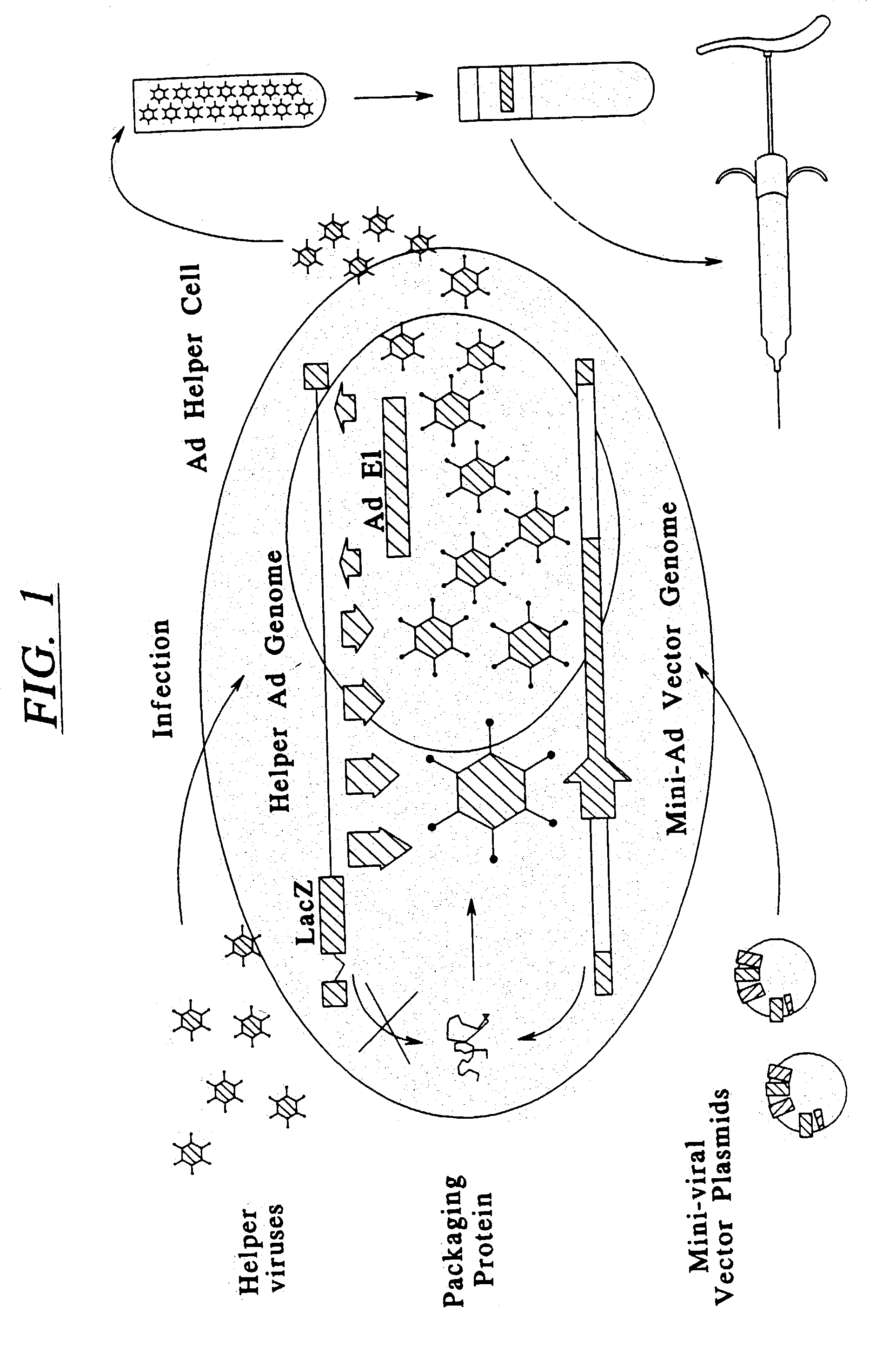
Minimal adenoviral vector
InactiveUS20030192066A1Accurate assessmentComplicate studies of long-term delivery of FVIIIBiocideFactor VIIVaccinationGene transfer
This invention is related to adenoviral (Ad) vectors and their applications in the field of genetic medicine, including gene transfer, gene therapy, and gene vaccination. More specifically, this invention is related to the Ad vectors that carry the minimal cis-element of the Ad genome (mini-Ad vector) and are capable of delivering transgenes and / or heterologous DNA up to 36 kb. The generation and propagation of the mini-Ad vectors require trans-complementation of a packaging-attenuated and replication-defective helper Ad (helper) in an Ad helper cell line. This invention further comprises a methodology for generating a mini-adenoviral (mini-Ad) vector for use in gene therapy of hemophilia and animal test systems for in vivo evaluation of the Ad vectors. More specifically, this invention describes factor VIII (FVIII) Ad vectors that only contain minimal cis-elements of the Ad genome (so called mini-Ad) and comprise a human FVIII cDNA with other supporting DNA elements up to 36 kb. The FVIII mini-Ad can be generated and preferentially amplified through the assistance of a packaging-attenuated helper Ad and a helper cell line. This invention also reports designs and methods for producing transgenic mouse models that can be used for in vivo testing the mini-Ad.
Owner:GENSTAR THERAPEUTICS
Adenosine deaminase deficient transgenic mice and methods for the use thereof
The present invention relates to the production of adenosine deaminase (ADA) deficient mice and the use of such mice as an animal model for dysfunctions associated with elevated adenosine levels. Also, provided by the present invention are methods of treating dysfunctions associated with elevated adenosine levels and methods of screening compounds for pharmaceutical activity in the treatment of dysfunctions associated with elevated adenosine levels.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
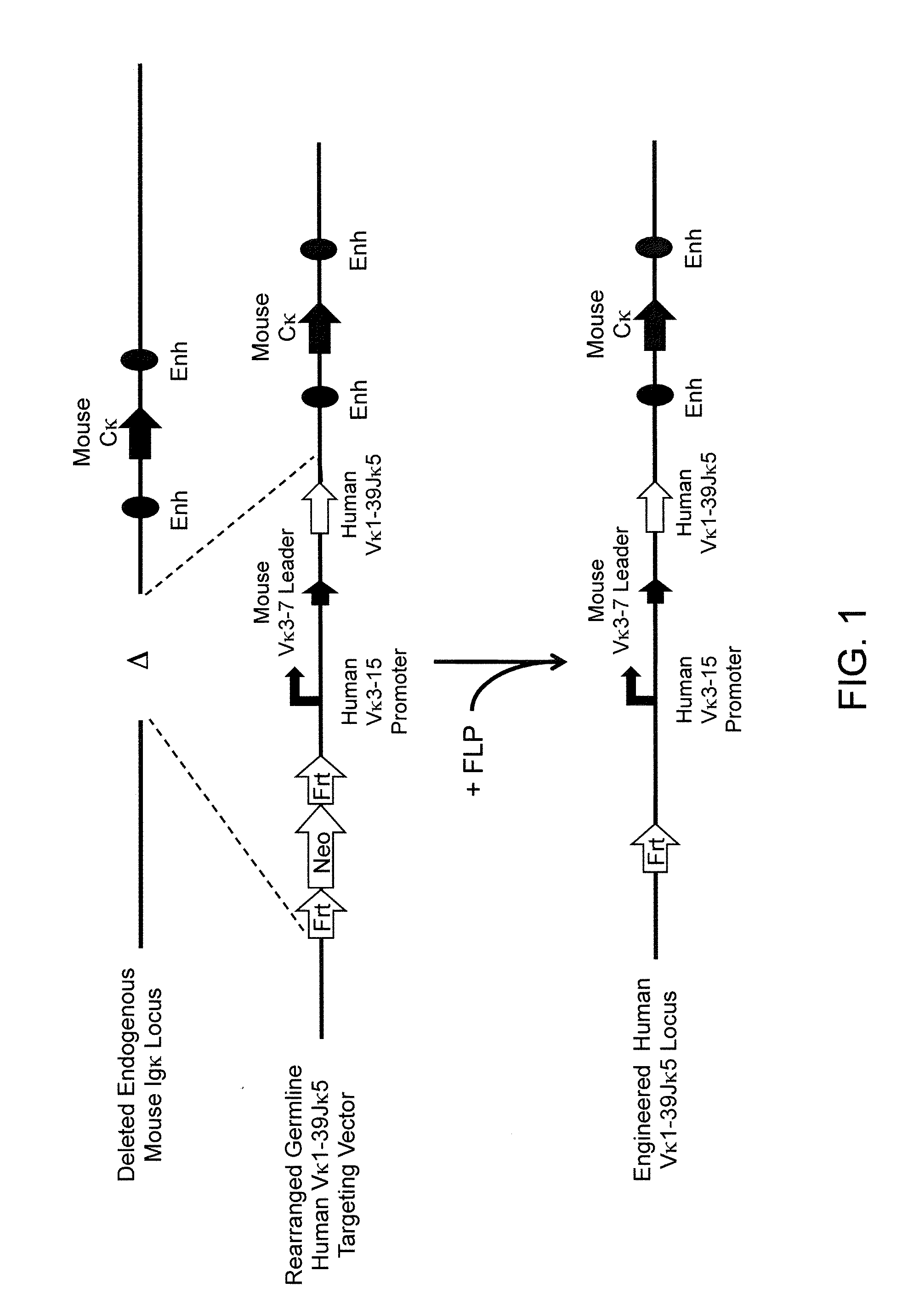
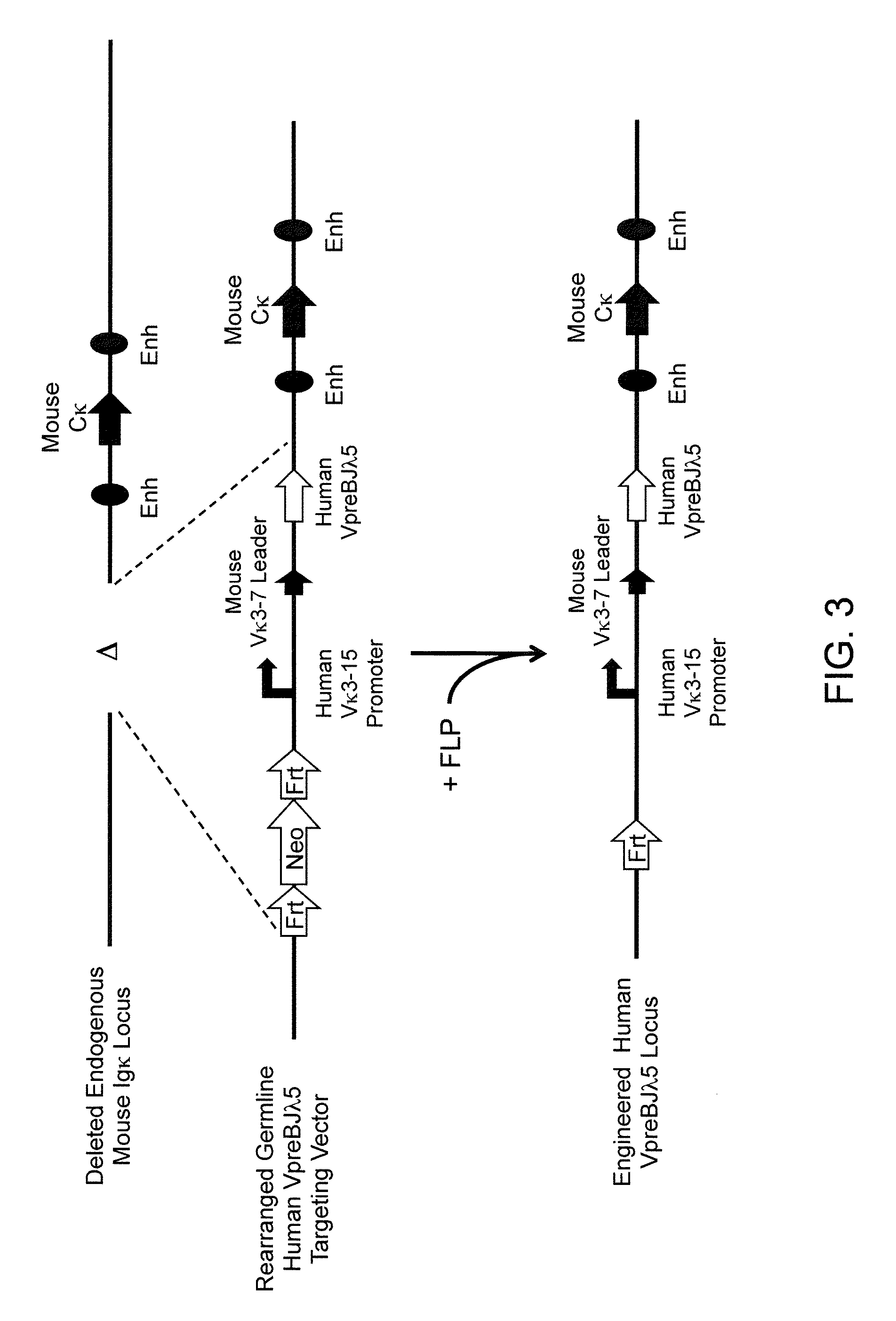
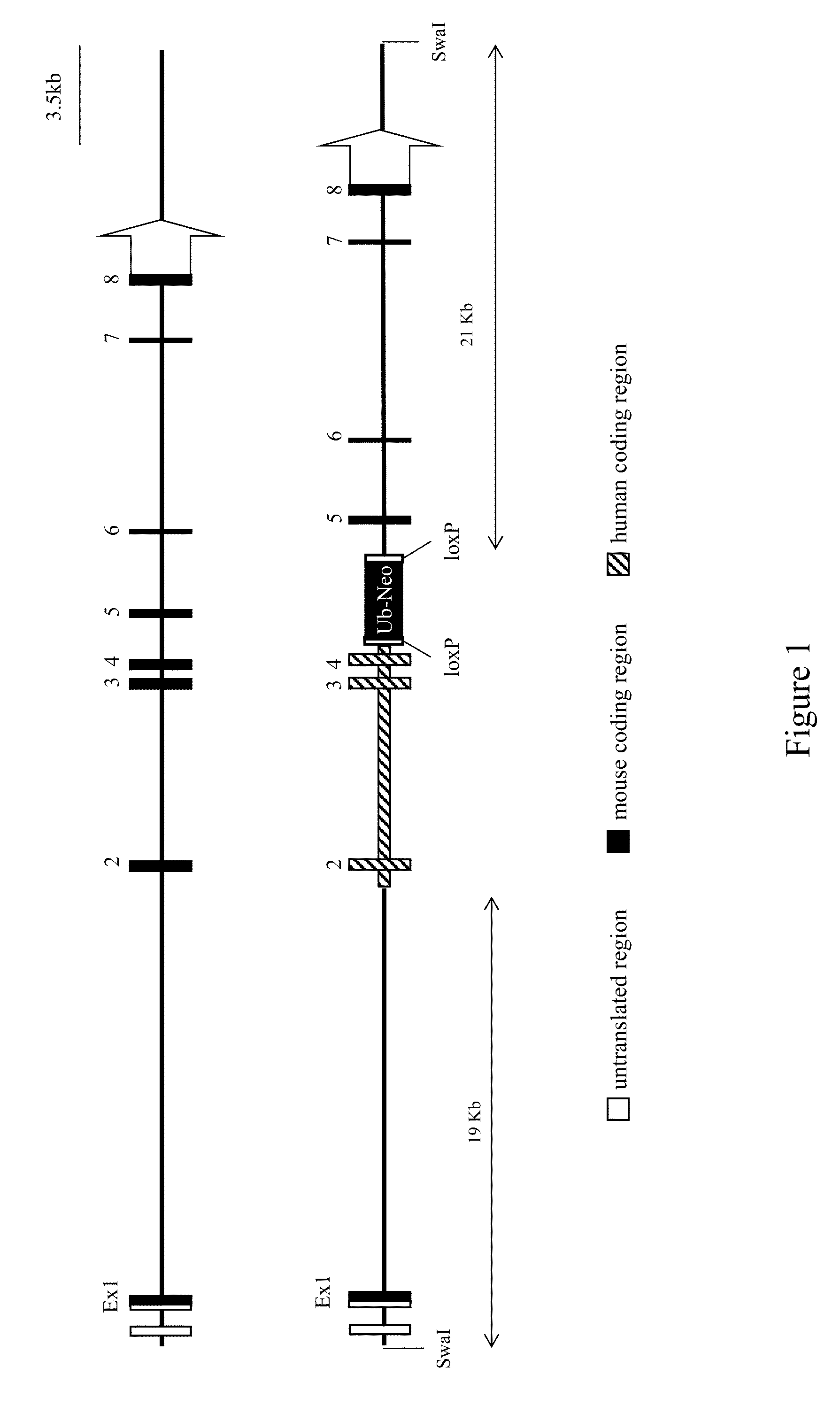
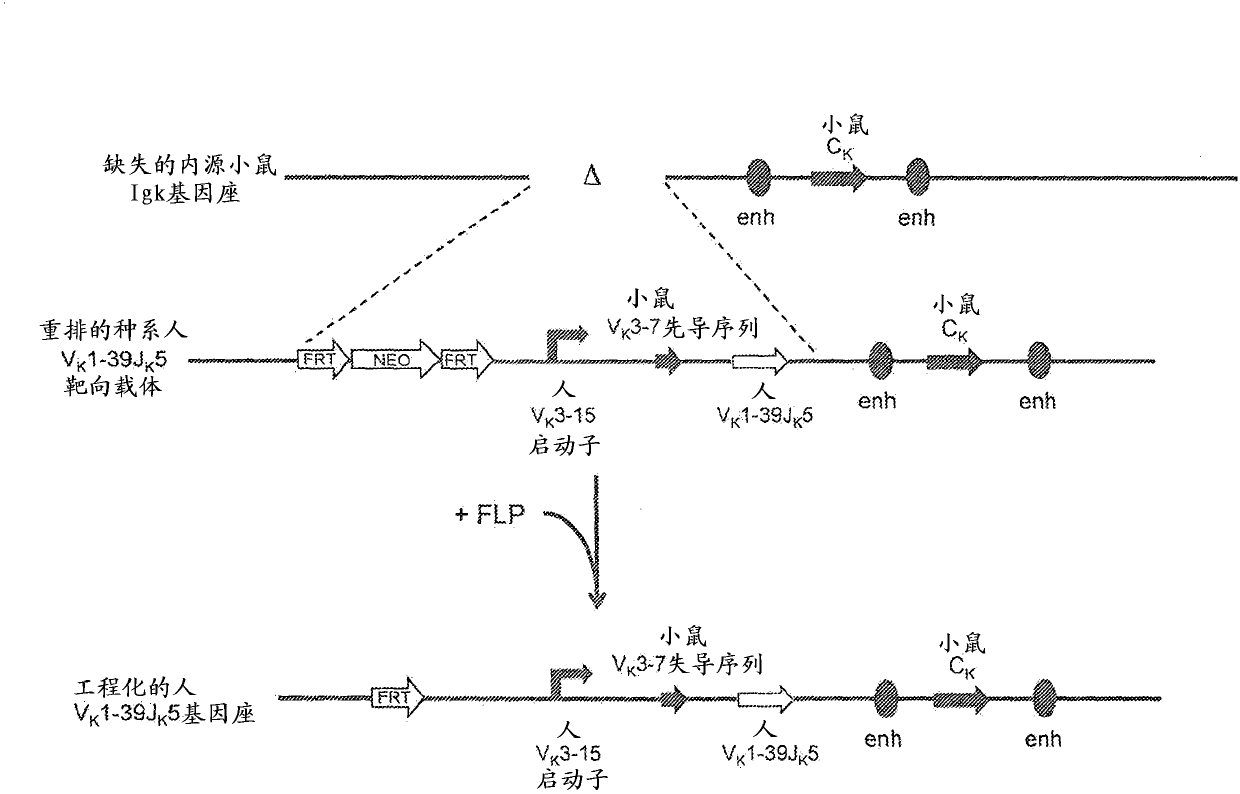
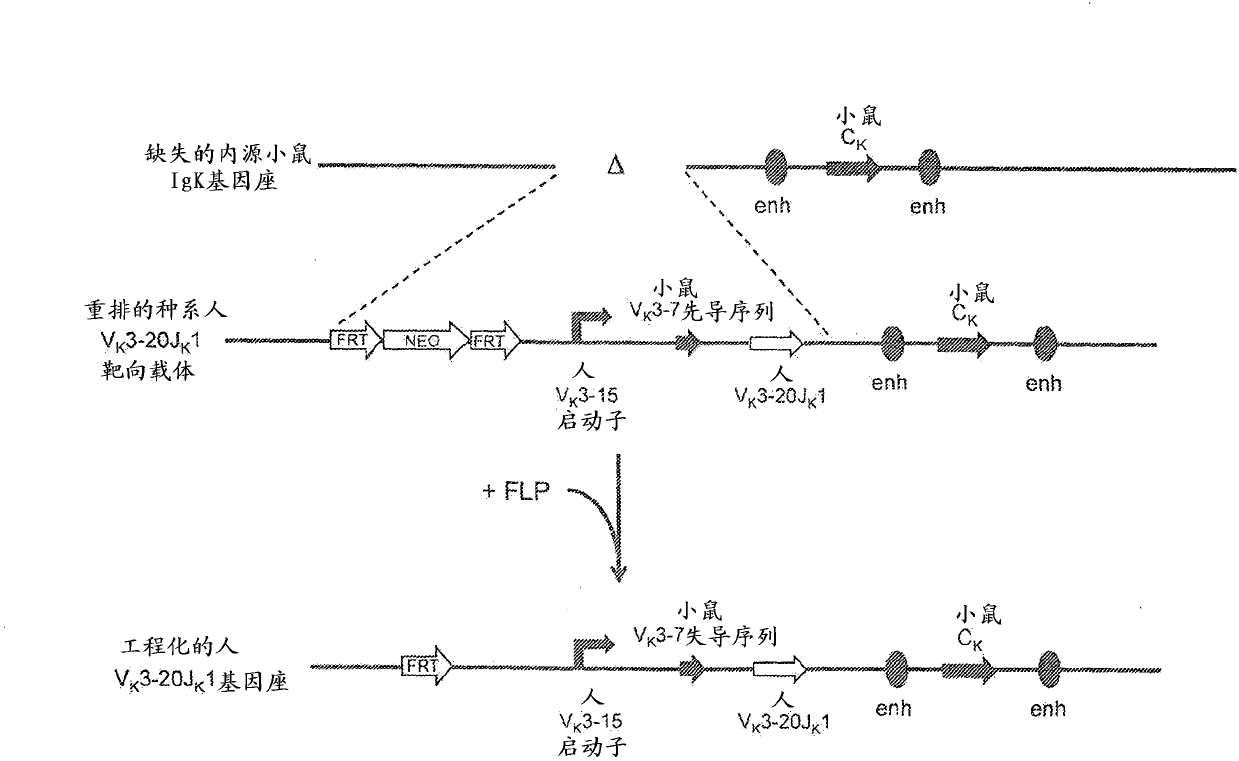
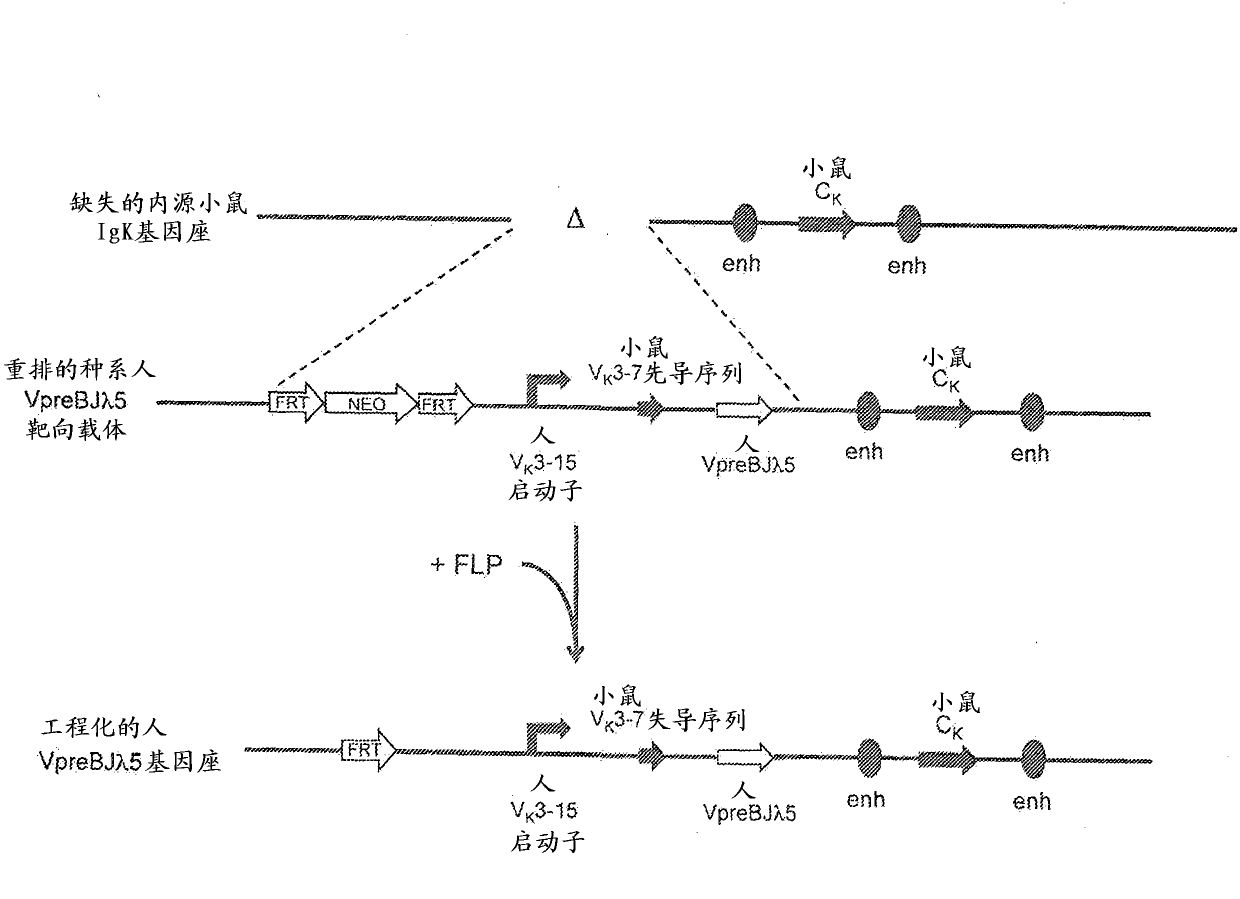
Common light chain mouse
A genetically modified mouse is provided, wherein the mouse is incapable of rearranging and expressing an endogenous mouse immunoglobulin light chain variable sequence, wherein the mouse expresses only one or two human light chain variable domains encoded by human immunoglobulin sequences operably linked to the mouse kappa (K) constant gene at the endogenous mouse K locus, wherein the mouse expresses a reverse chimeric antibody having a light chain variable domain derived from one of only two human light chain variable region gene segments and a mouse K constant domain,. and a human heavy chain variable domain and a mouse heavy chain constant domain, from an endogenous mouse heavy chain locus. Bispecific epitope-binding proteins that are fully human are provided, comprising two different heavy chains that associate with an identical light chain that comprises a variable domain derived from one of two different human light chain variable region gene segments.
Owner:REGENERON PHARM INC
Non-human animals having a humanized signal-regulatory protein gene
ActiveUS20150089679A1Increased susceptibilityIncrease and decrease in food consumptionCompounds screening/testingCompound screeningGenetically modified mouseGenetically modify
Genetically modified non-human animals and methods and compositions for making and using the same are provided, wherein the genetic modification comprises a humanization of an endogenous signal-regulatory protein gene, in particular a humanization of a SIRPα gene. Genetically modified mice are described, including mice that express a human or humanized SIRPα protein from an endogenous SIRPα locus.
Owner:REGENERON PHARM INC
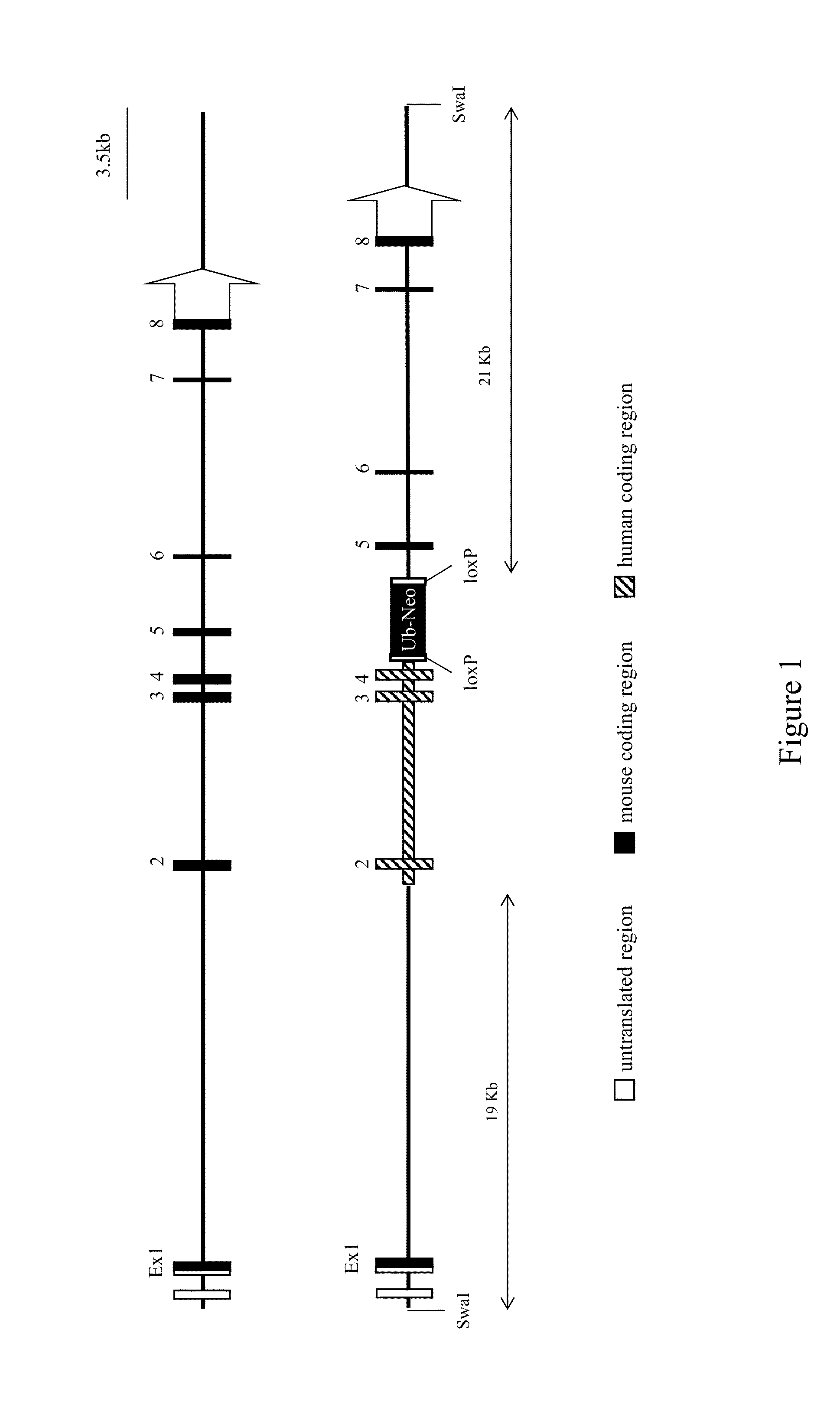
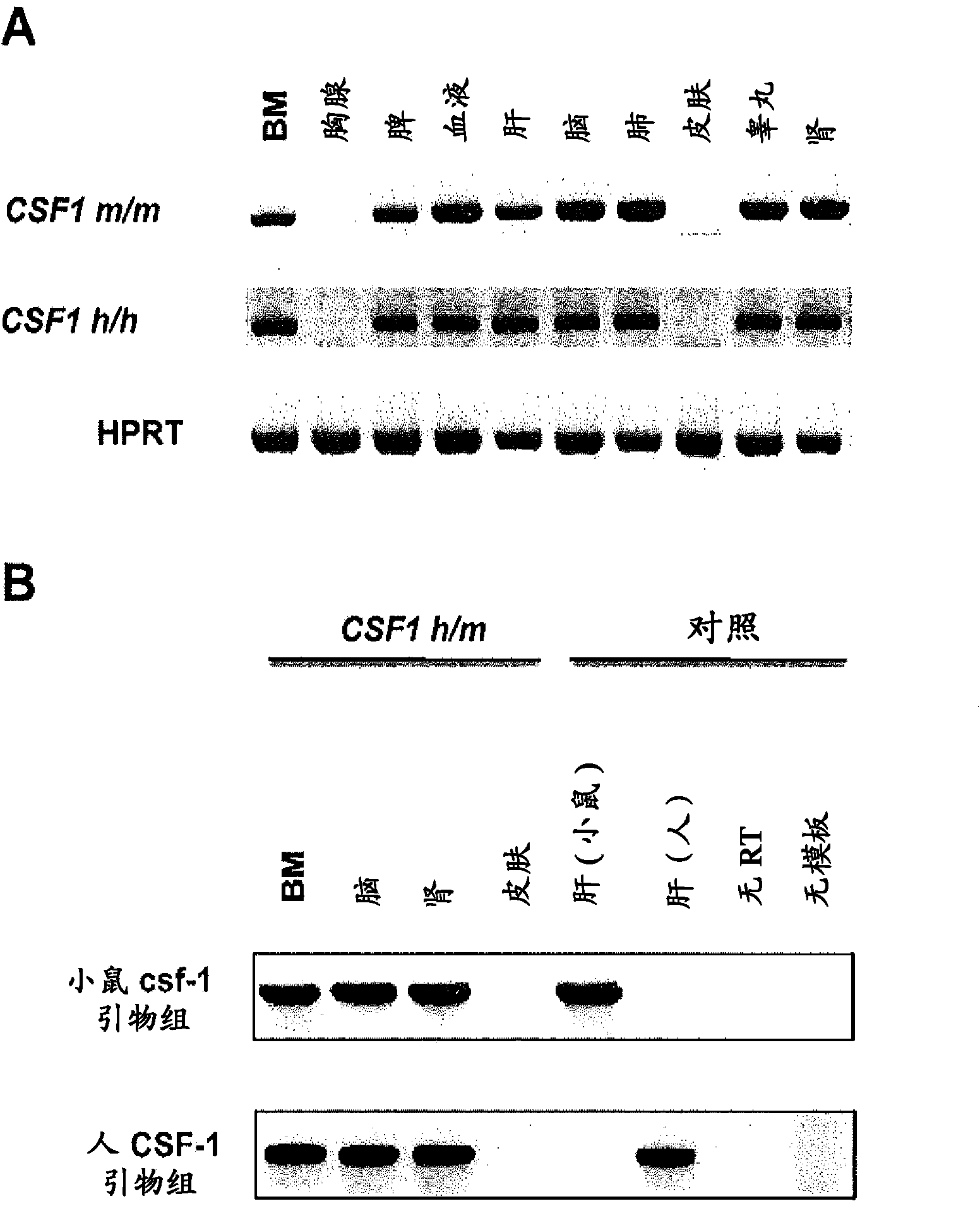
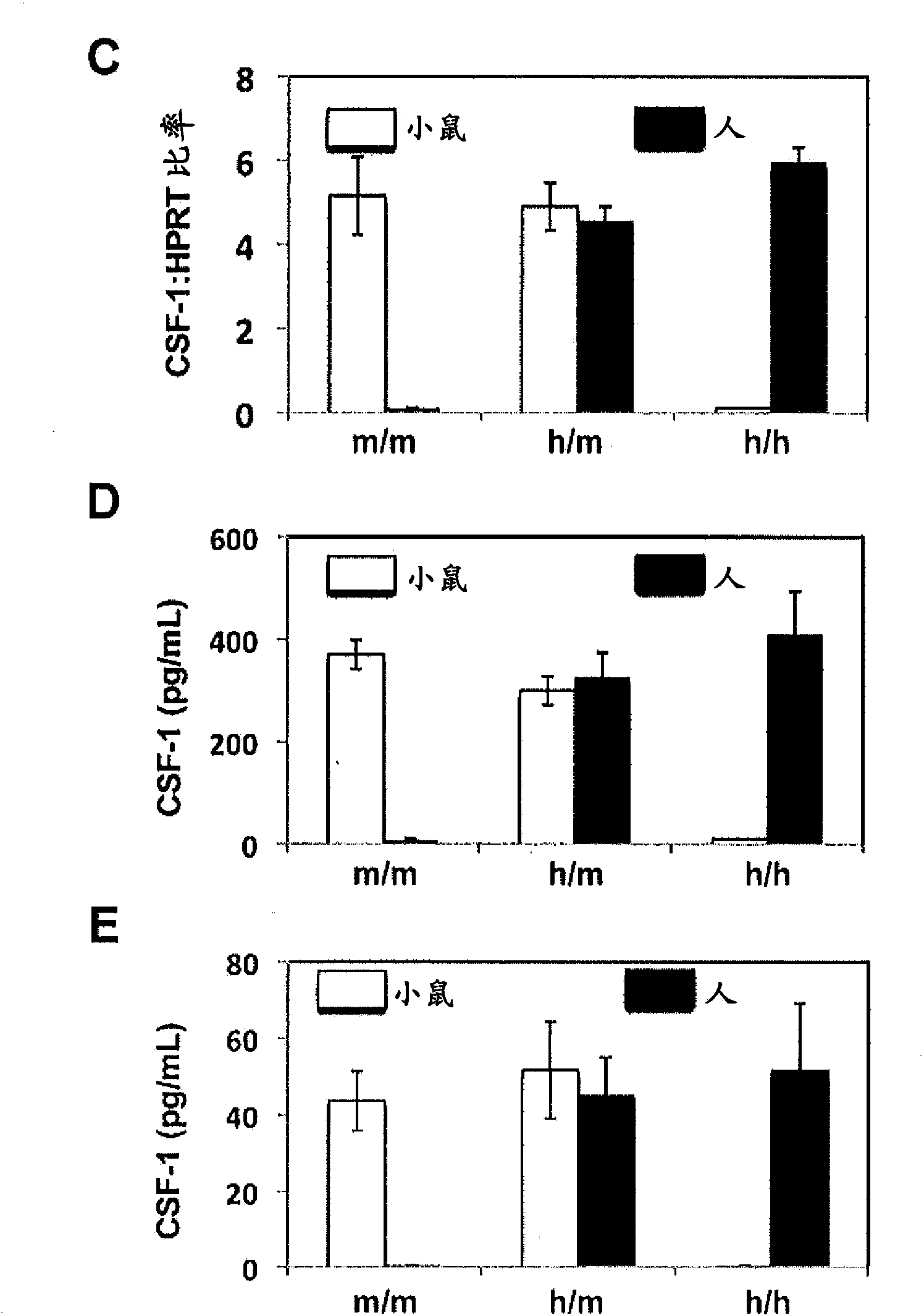
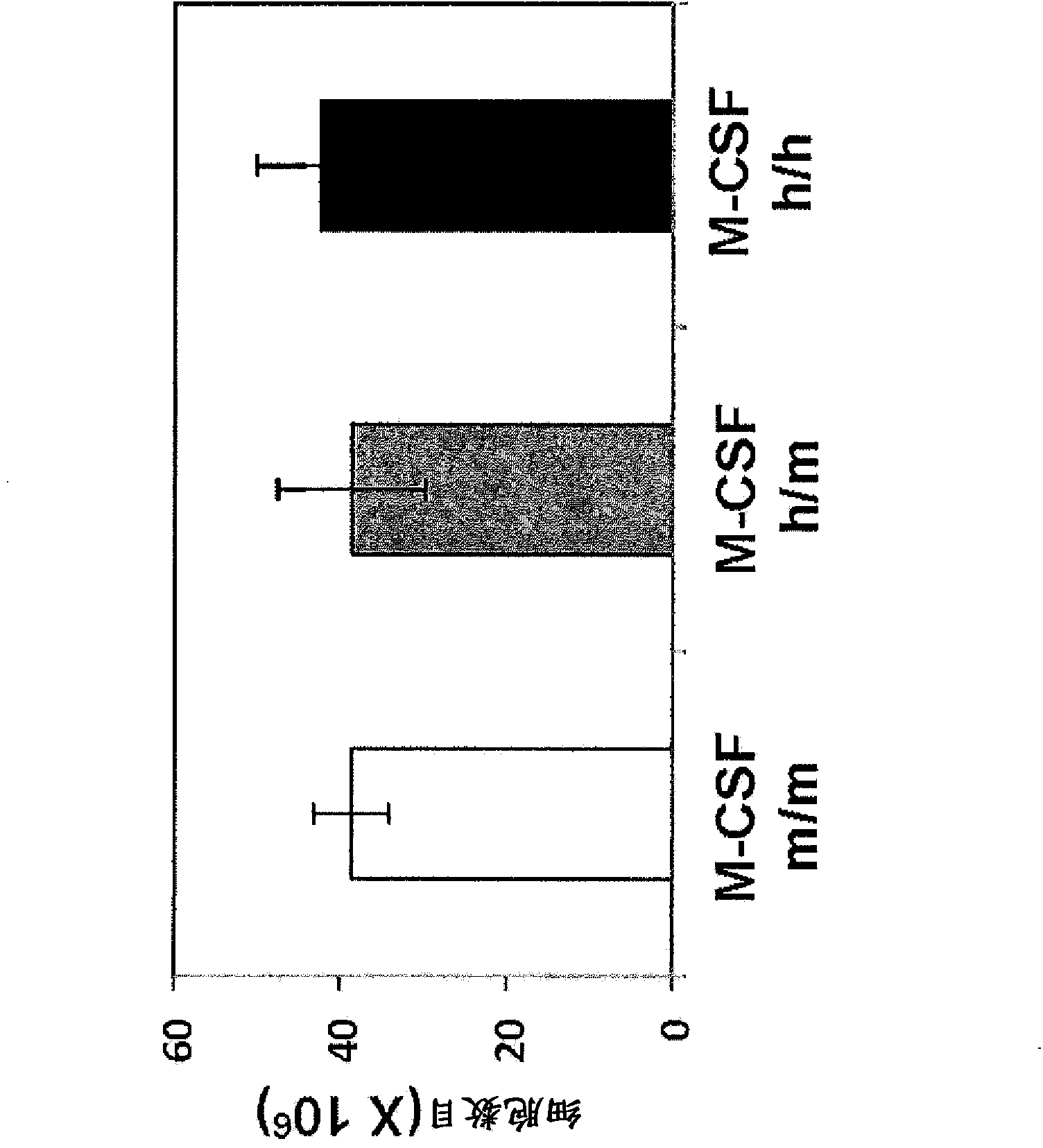
Humanized M-CSF mice
Genetically modified mice comprising a nucleic acid sequence encoding a human M-CSF protein are provided. Also provided are genetically modified mice comprising a nucleic acid sequence encoding a human M-CSF protein that have been engrafted with human cells such as human hematopoietic cells, and methods for making such engrafted mice. These mice find use in a number of applications, such as in modeling human immune disease and pathogen infection; in in vivo screens for agents that modulate hematopoietic cell development and / or activity, e.g. in a healthy or a diseased state; in in vivo screens for agents that are toxic to hematopoietic cells; in in vivo screens for agents that prevent against, mitigate, or reverse the toxic effects of toxic agents on hematopoietic cells; in in vivo screens of human hematopoietic cells from an individual to predict the responsiveness of an individual to a disease therapy, etc.
Owner:REGENERON PHARM INC +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com