Preparation method of spermacoce latifolia triterpenoids and application of spermacoce latifolia triterpenoid in preparation of glycosidase inhibitor medicine
A technology of glycosidase inhibitors and triterpenes, which is applied in the preparation of triterpenoids and its application in the preparation of glycosidase inhibitors, and achieves the effects of high safety, easy storage and abundant sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of six kinds of triterpenoids in broad-leaved abundant flowers and plants
[0025] 1.1 Plant source and identification
[0026] The whole plant of Spermacoce latifolia Aubl.K.Schum. was collected from South China Botanical Garden, Guangzhou City, Guangdong Province in September 2012, and was identified by Xing Fuwu, a researcher of South China Botanical Garden, Chinese Academy of Sciences.
[0027] 1.2 Extraction and separation
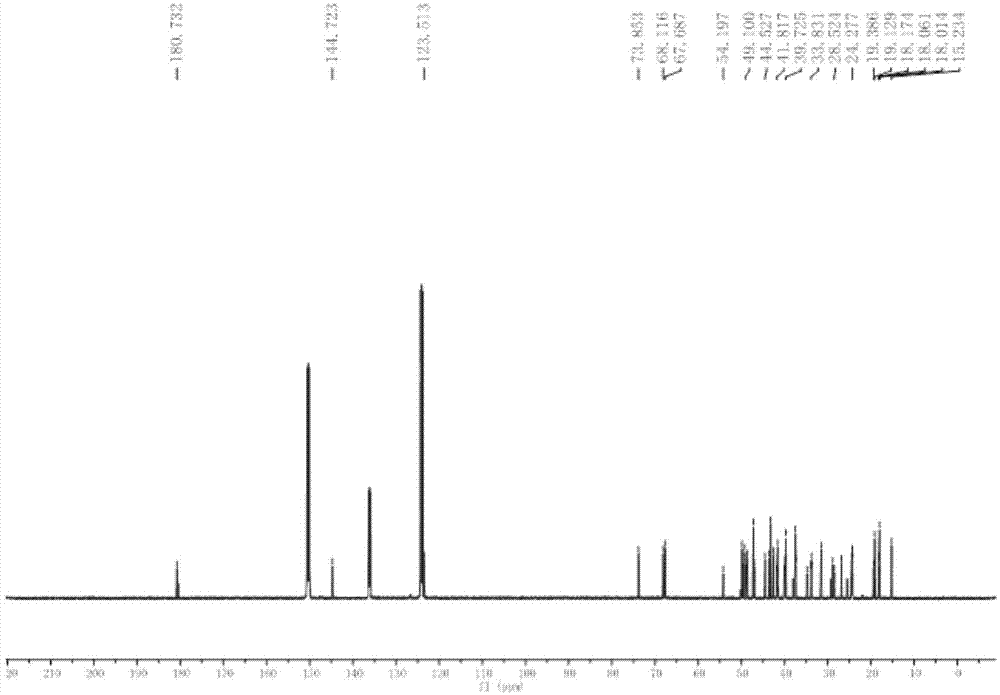
[0028] The whole plant (5.0Kg) of the dried Herba chinensis (5.0Kg) was crushed and extracted at room temperature with 95% ethanol aqueous solution, the extracts were combined and concentrated under reduced pressure to obtain an alcohol-free extract. The extract was suspended in water, and extracted four times with petroleum ether and ethyl acetate in turn; after concentration under reduced pressure, petroleum ether extract (330 g) and ethyl acetate extract (202 g) were obtained. The obtained petroleum ether extract ...
Embodiment 2
[0036] Embodiment 2: α-glucosidase inhibitory activity detection of described six kinds of triterpenoids
[0037] 2.1 Instruments and reagents
[0038] Experimental instrument: Genois microplate reader (Tecan GENios, Switzerland).
[0039]Reagent samples: α-glucosidase was purchased from Sigma Chemical Co. (Sigma-Aldrich, St.Louis, USA); Acarbose was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); Phenol-α-D-glucopyranoside (PNPG) was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); six triterpenoid mesembryanth emoidigenic acid (compound 1), 29-hydroxyhederagenin (compound 2), 3β ,6β-dihydroxy-olean-12-en e-28-oic acid (compound 3), scutellaric acid (compound 4), arjunic acid (compound 5), 3β,6β,23-tr ihydroxy-olean-12-en- 28-oic acid (compound 6) is prepared by the method of Experimental Example 1 above, and can also be prepared according to the literature Magina et al., Quim Nova, 2012, 35:1184-1188; Huu et al., J Chem, 2007, 45: 363-367; Ku o et al., ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com