Application of four kinds of kaurane diterpene compounds in the preparation of glycosidase inhibitor drugs
A glycosidase inhibitor, a kaurine-type technology is applied to the application field of four kaurane diterpenoid compounds in the preparation of glycosidase inhibitor drugs, achieving the effects of abundant sources, high safety, and easy operation in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
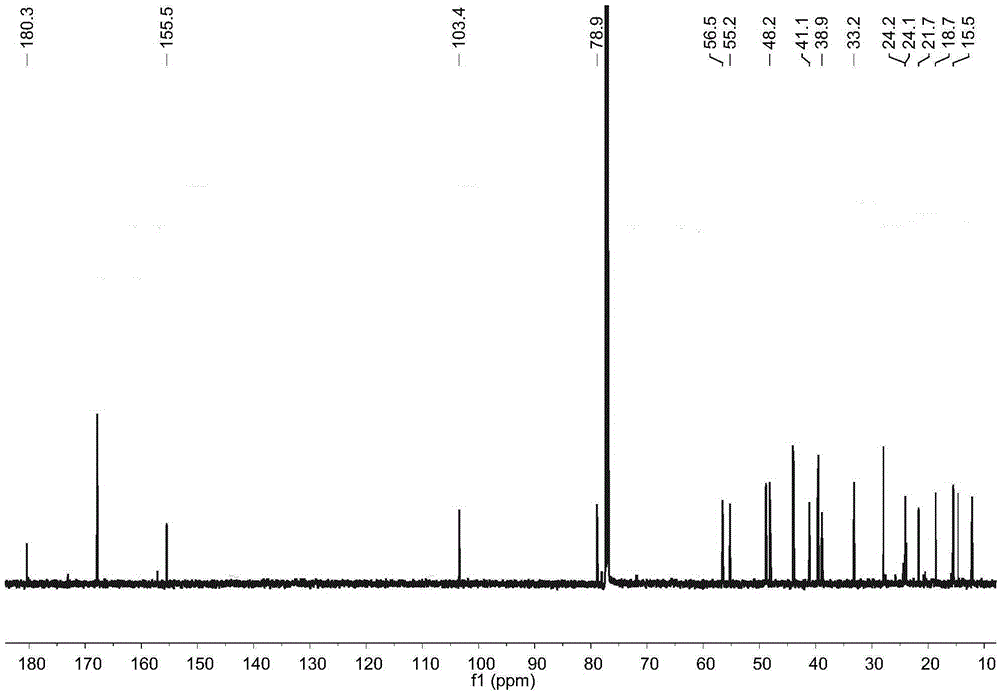
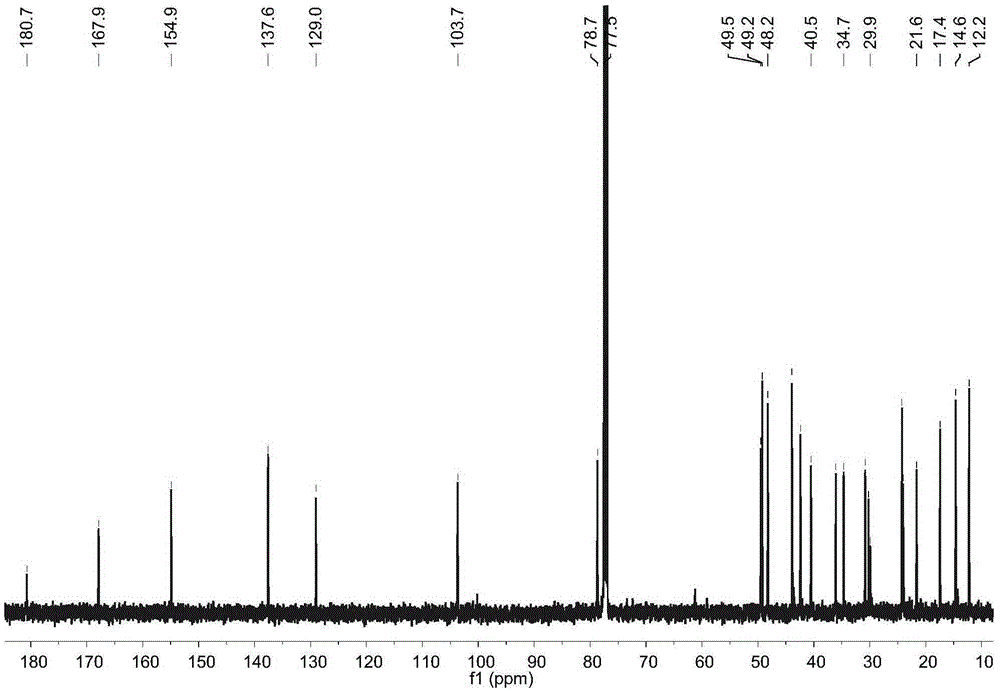
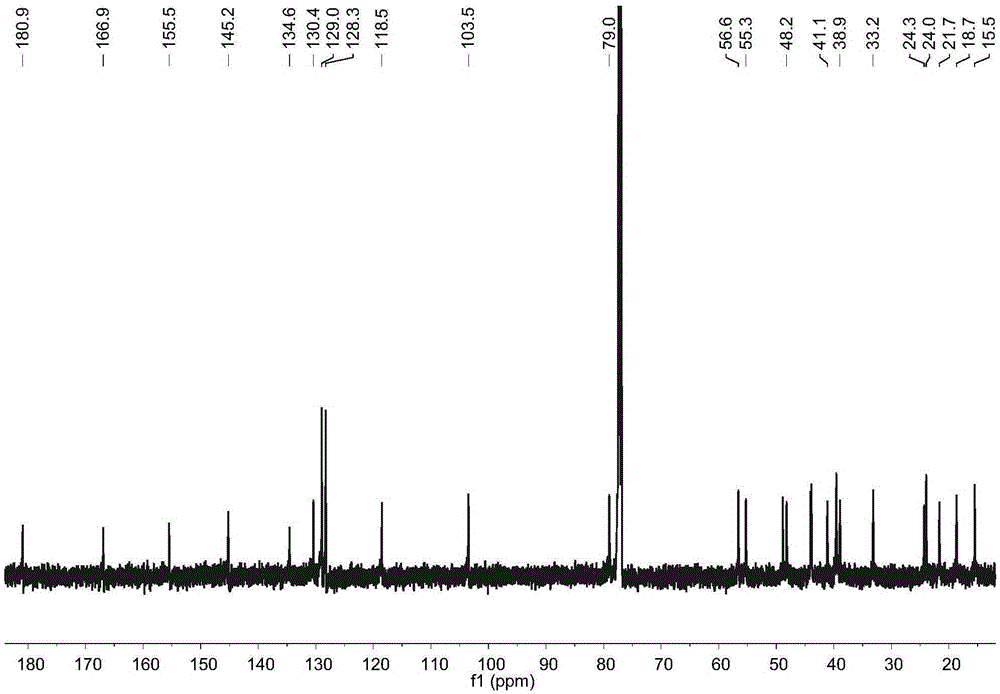
[0017] Example 1: Preparation of four diterpene compounds in Wedelia chinensis
[0018] 1.1 Plant source and identification
[0019] The plant material for extraction, Sphagneticolatrilobata (L.) Pruski plant samples were collected from the South China Botanical Garden in Guangdong Province in September 2009, and were identified by researcher Xing Fuwu, South China Botanical Garden, Chinese Academy of Sciences.
[0020] 1.2 Extraction and separation
[0021] The whole South American Wedelia chrysanthemum plant (dry weight 2kg) is smashed and extracted with 95% ethanol at room temperature for 3 times, 2 days each time, and the extracts are combined; the ethanol in the extract is drained by vacuum concentration and an appropriate amount of water is added to make It became a suspension and was extracted with petroleum ether 4 times; a petroleum ether fraction (45 g) was obtained after concentration under reduced pressure. The obtained petroleum ether extraction part (45g) was subjected ...
Embodiment 2
[0026] Example 2: Detection of α-glycosidase inhibitory activity of the four diterpene compounds
[0027] 2.1 Instruments and reagents
[0028] Experimental instrument: microplate reader Genoismicroplatereader (TecanGENios, Swizerland)
[0029] Reagent samples: α-glucosidase was purchased from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, USA); Acarbose was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); 4-nitrophenol-α- D-glucopyranoside (PNPG) was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); four diterpene compounds α-hydroxy-ent-kauran-19-oicacid (compound 1), 3α-tigloyloxy-9β-hydroxy-ent -kaur-16-en-19-oicacid (compound 2), 3α-cinnamoyloxy-ent-kaur-16-en-19-oicacid (compound 3), 3α-cinnamoyloxy-9β-hydroxy-ent-kaur-16-en -19-oicacid (compound 4) is prepared by the above experimental example 1 or can also refer to the literature Qiangetal., Helvetica Chimica Acta 2011, 94: 817-823; Bohlmannetal., Phytochemistry 1981, 20: 751-756; Maetal., Natural Products a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com