Selective glycosidase inhibitors, methods of making inhibitors, and uses thereof
An inhibitor, selective technology, applied in biochemical equipment and methods, sugar derivatives, pharmaceutical formulations, etc., can solve problems such as lack of selectivity, disrupting various cellular processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: Comparative analysis of O-GlcNAc enzyme and β-hexosaminidase catalytic mechanism
[0092] 1.1 Possible catalytic mechanism of O-GlcNAc enzyme
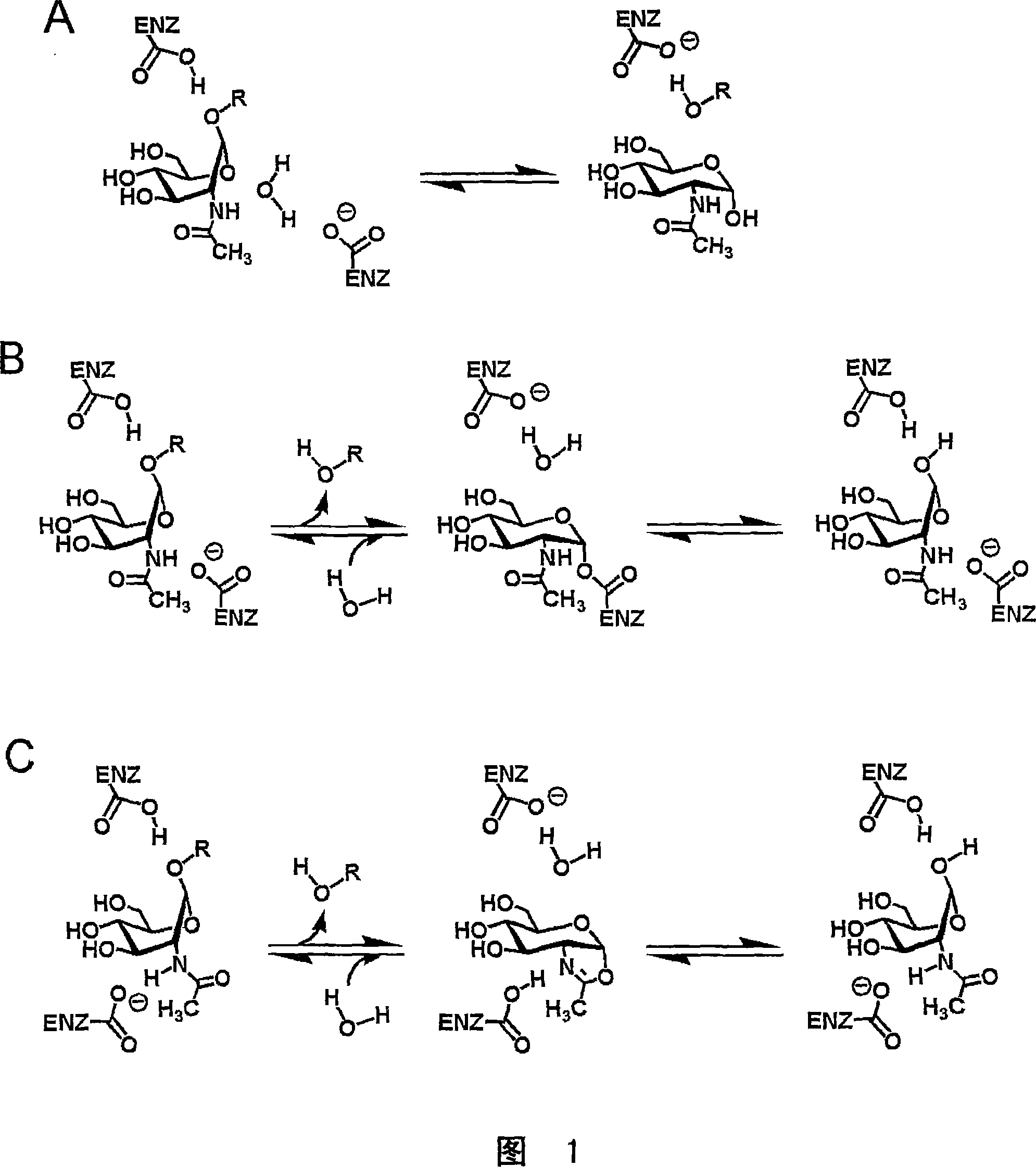
[0093] A logical starting point for designing O-GlcNAc enzyme inhibitors leads one to think about the catalytic mechanism of O-GlcNAc enzymes and β-hexosaminidases. Although the catalytic mechanism of action of human β-hexosaminidases A and B family 20 is well established [78] , the mechanism of O-GlcNAc enzyme family 84 is still unknown. Therefore, the present inventors first elucidated the catalytic mechanism of the human O-GlcNAc enzyme, and secondly used this information to design efficient, cell-permeable, and O-GlcNAc enzymes that are superior to lysosomal β-hexosaminidases. Simple inhibitor with high selectivity.
[0094] There are three real alternative mechanisms for O-GlcNAc enzymes and the glycoside hydrolase family84. The first is an inversion mechanism such as that found for the goose lysozyme ...
Embodiment 2
[0136] Example 2: Design and Synthesis of a First Embodiment of a Selective O-GlcNAc Enzyme
[0137] 2.1 Design of the first embodiment of selective O-GlcNAc enzyme inhibitor
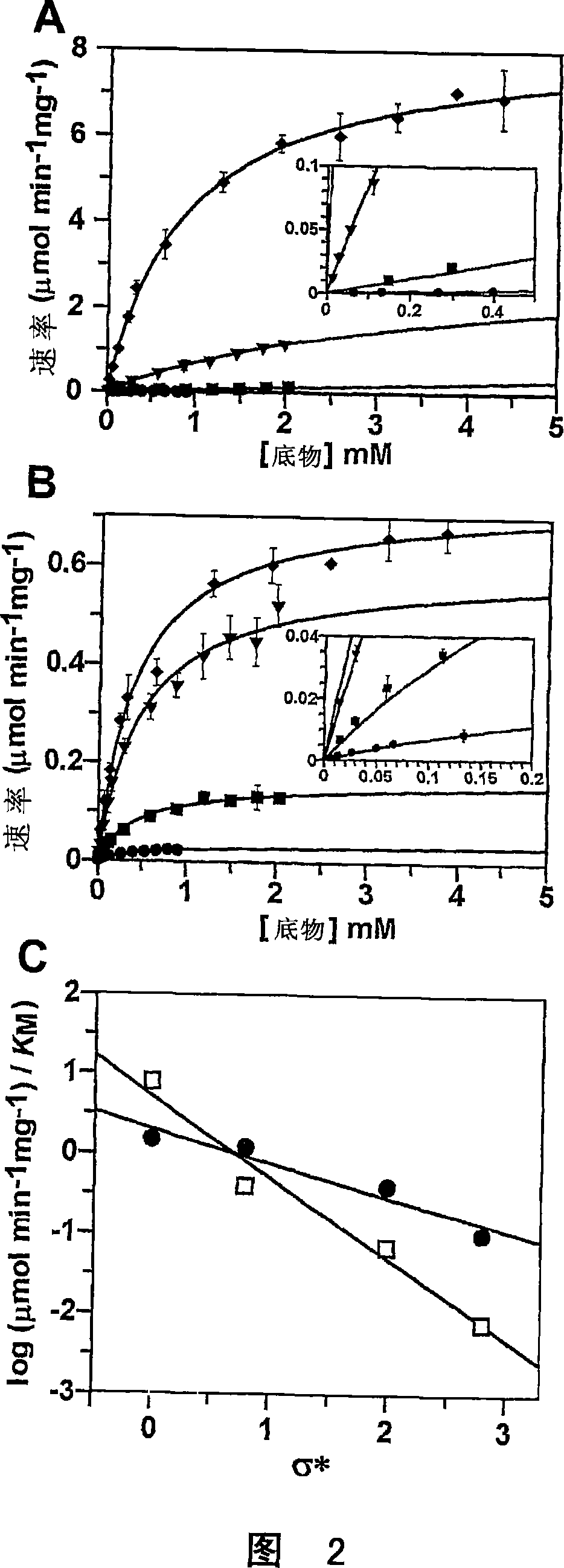
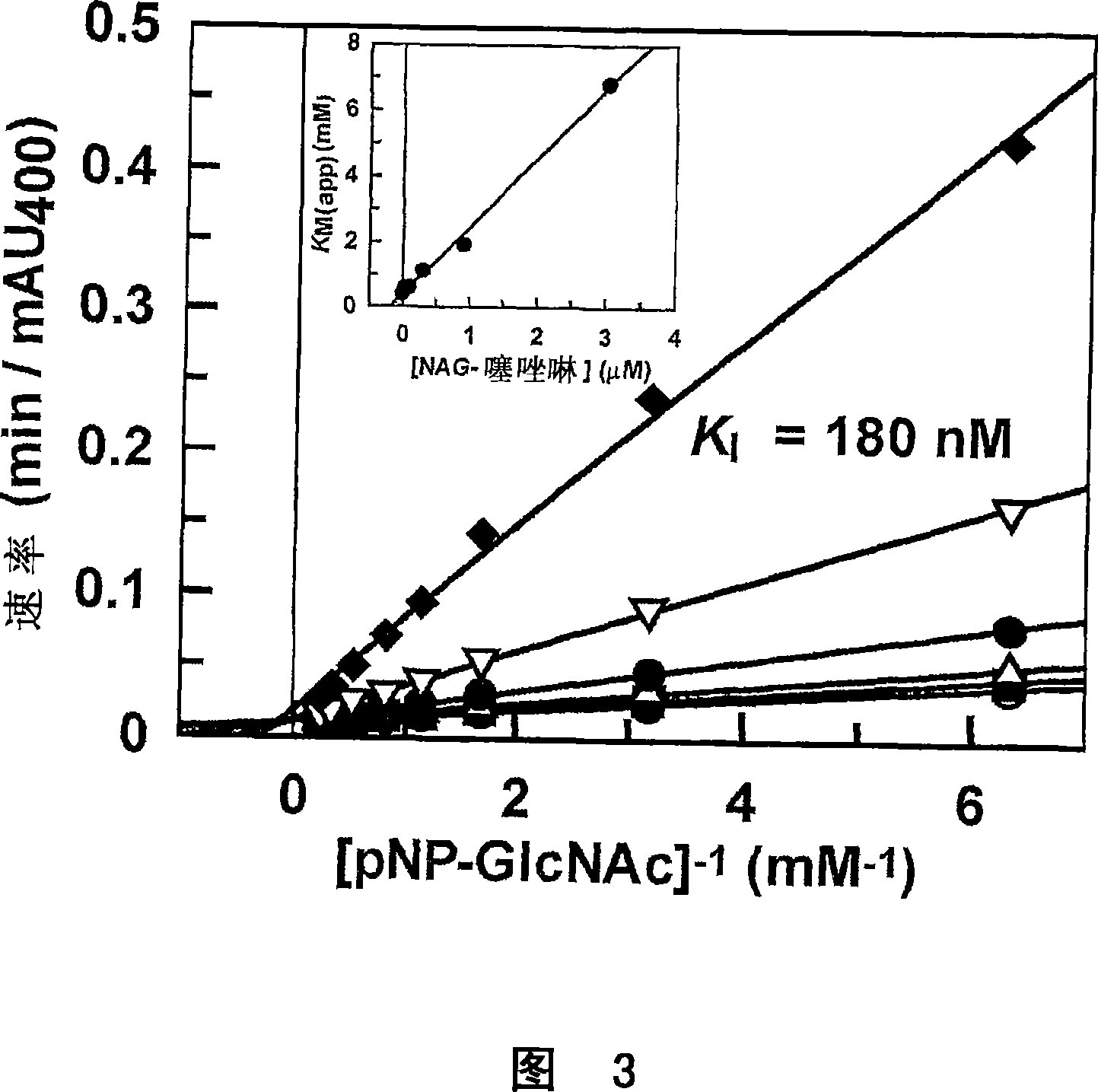
[0138] With the mechanism of the human O-GlcNAc enzyme and by extension other glycoside hydrolase family 84 members established, attention has shifted to using this information in the design of inhibitors with specific properties for this enzyme. Superior selectivity to human lysosomal β-hexosaminidase. Since both β-hexosaminidase and O-GlcNAc enzymes employ a mechanism involving ortho-promotion, NAG-thiazoline was chosen as the backbone, which can be modified to yield the desired selectivity. Three observations provide a starting point for the design of such inhibitors. The first is that the slope of the Taft-like assay for lysosomal enzymes is much larger than that measured for O-GlcNAc enzymes, thus suggesting that the bulk of the N-acyl group may be a determining factor in substrate recognitio...
Embodiment 3
[0180] Example 3: Design and implementation of a second embodiment of a selective O-GlcNAc enzyme inhibitor synthesis
[0181] 3.1 Design of the second class of selective O-GlcNAc inhibitors
[0182] Based on the above observations, modifications were made on the N-acyl side chain of PUGNAc to generate a second embodiment of a potent and selective O-GlcNAc enzyme inhibitor based on another inhibitor backbone. Such inhibitors can have different pharmacokinetic properties and are an important tool for analyzing the effects of O-GlcNAc post-translational modifications at the cellular and organismal level.
[0183] 3.2 Synthesis of a second embodiment of a selective O-GlcNAc enzyme inhibitor
[0184] The synthesis of a series of six inhibitors using PUGNAc as the backbone is outlined in Scheme 3.
[0185] Start with readily available hydrochloride 10 [93] , introducing a Boc group for protecting the amine moiety, to generate the tetraacetate 11 [94] (Route 3). With the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com