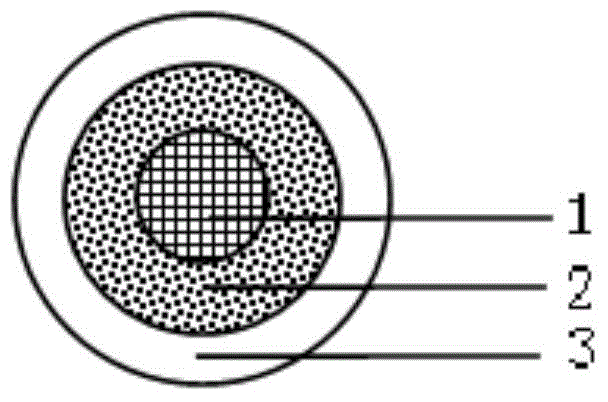
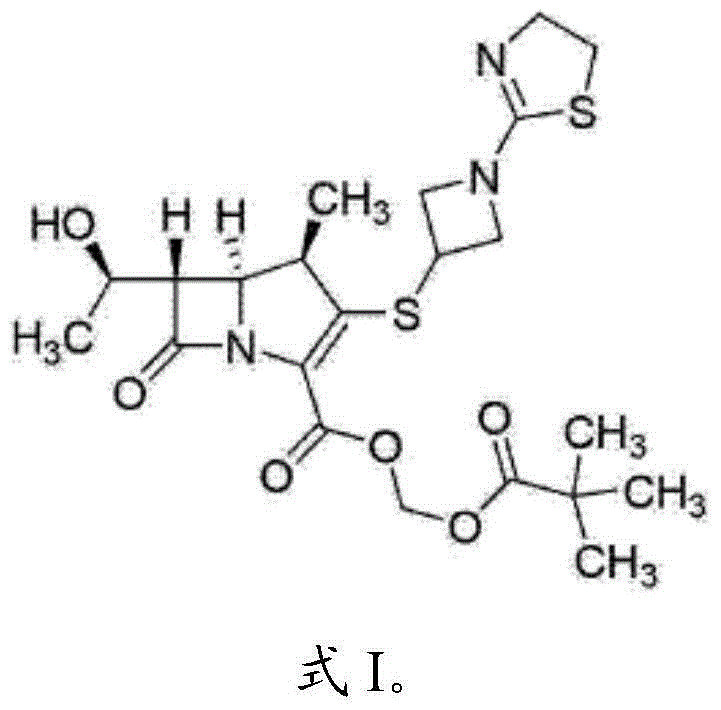
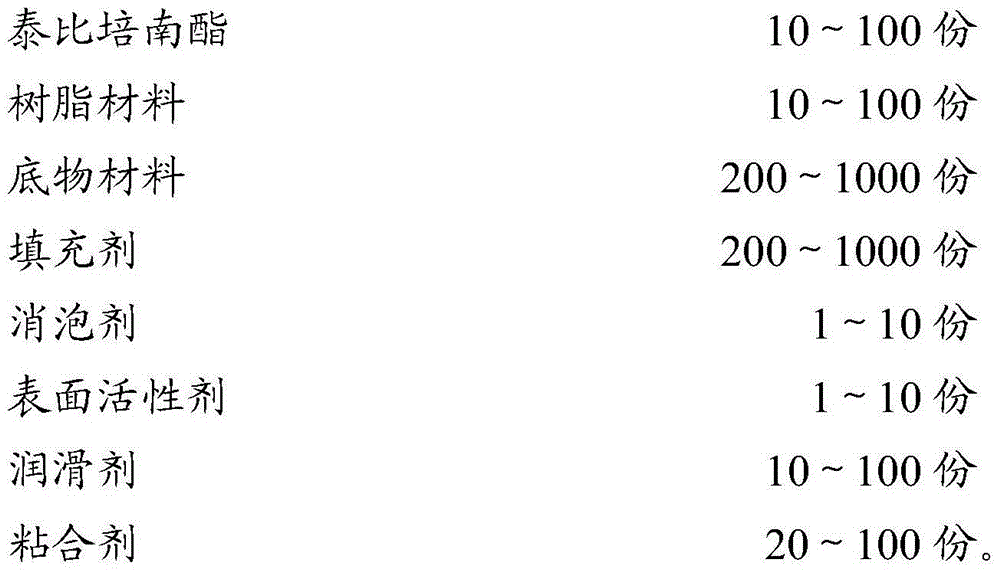Pharmaceutical preparation of tebipenem pivoxil composition and preparation method of pharmaceutical preparation
A technology of tibipenem and pharmaceutical preparations, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of low solubility, increased cost, and adverse effects of tibipenem Problems such as promotion and use to achieve good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of tibipenem compound granules
[0075] Take the raw and auxiliary materials of the following weight, and set aside;
[0076]
[0077] Pass the raw materials through an 80-mesh sieve respectively, and set aside.
[0078] Preparation of resin suspension: Take Eudragit EPO (acrylic resin type E) and Eudragit RSPO (acrylic resin RS type) into a beaker, add 760g of purified water, add simethicone, lauryl sulfuric acid Sodium and talcum powder, stirred at a high speed, and mixed evenly to obtain a resin suspension, set aside.
[0079] Preparation of medicinal solution: Take 400g of the prepared resin suspension, add tibipenem ester, stir evenly, and get ready for use.
[0080] Preparation of adhesive: Take another beaker, add 437g of water into the beaker, add hypromellose, stir until dissolved, and get ready for use.
[0081] Take microcrystalline cellulose and add it to the fluidized bed for preheating for 3 minutes at a temperature of 31°C; the...
Embodiment 2
[0083] Example 2 Preparation of tibipenem compound granules
[0084] Take the raw and auxiliary materials of the following weight, and set aside;
[0085]
[0086]
[0087] Pass the raw materials through an 80-mesh sieve respectively, and set aside.
[0088] Preparation of resin suspension: add 1000g of water to a beaker, add the above-mentioned simethicone, sodium lauryl sulfate, Eudragit EPO (acrylic resin type E), talcum powder, and stir well to obtain 1220g of the first product ,spare.
[0089] Preparation of the medicinal solution: Weigh 488g of the prepared resin suspension, pour it into another beaker, add tibipenem, stir evenly, and get ready for use.
[0090] Preparation of adhesive: take another beaker, add 5000g of water into the beaker, add hypromellose, stir until dissolved, and get ready for use.
[0091] Take microcrystalline cellulose and add it to the fluidized bed for preheating for 2 minutes at a temperature of 25°C; then spray the prepared medicina...
Embodiment 3
[0092] Example 3 Preparation of tibipenem composition granules
[0093] Take the raw and auxiliary materials of the following weight, and set aside;
[0094]
[0095]
[0096] Pass the raw materials through an 80-mesh sieve respectively, and set aside.
[0097] Preparation of resin suspension: Add 400g of water into a beaker, add the above-mentioned simethicone, sodium stearyl sulfonate, Eudragit RSPO (acrylic resin RS type), micropowder silica gel, stir well, and get 422g of resin suspension liquid, spare.
[0098] Preparation of the medicinal solution: Weigh 253.2 g of the prepared resin suspension, pour it into another beaker, add tibipenem, stir evenly, and get ready for use.
[0099] Preparation of adhesive: take another beaker, add 250g of water and hydroxypropyl cellulose into the beaker, stir until dissolved, ready to use.
[0100] Take microcrystalline cellulose and add it to the fluidized bed for preheating for 5 minutes at a temperature of 35°C; then spray ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com