Solid preparation for treating pediatric epilepsy and preparation method thereof
A technology for solid preparations and epilepsy, applied in the field of medicine, can solve problems such as poor disintegration and dissolution effects, inaccurate dosage, and difficulty in using it on time, so as to enhance the stability of drugs and solve the effects of swallowing difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The method for preparing a spray-type preparation includes the following steps: coating the core particles containing only epilepsy drug particles or crystals or one or more excipients with a taste-masking mixture, and then drying. The preparation method of core particle comprises:
[0025] The first method, the epilepsy drug raw material powder is placed in the fluidized bed equipment earlier, and then excipients known to those skilled in the art such as PVP, starch, sucrose, syrup, HPMC in a pharmaceutically acceptable solvent (such as water , ethanol, acetone, etc.) is sprayed onto the powder to form granules, and then dried until the solvent evaporates to obtain core particles.
[0026] In the second method, powdered or granular epilepsy drugs and diluents or fillers are mixed with water or pharmaceutically acceptable solvents (such as water, ethanol) to form a wet material. Mix the mixture until it forms a wet mass, ie a kneaded mass. The wet material is then ext...
Embodiment 1
[0029] The influence of embodiment 1 taste-masking coating content on pharmaceutical preparation
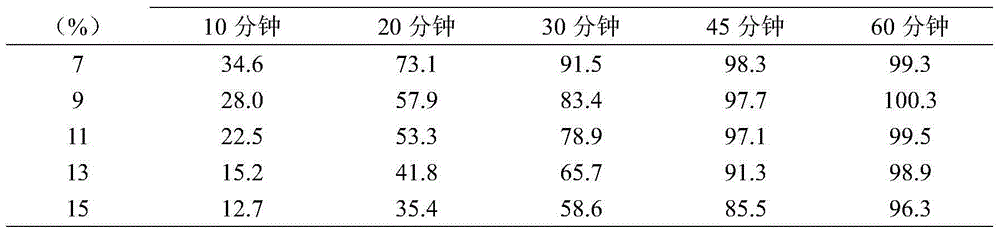
[0030] The magnesium valproate dispensing preparation is prepared by the method of the present invention, wherein the medicine contains 7-15% by weight of a taste-masking coating, and the results of the dissolution test in water of the bioavailability of the pharmaceutical composition are shown in Table 1.
[0031] Table 1 Dissolution test results in water
[0032]
[0033]
[0034] The following are specific examples of the present invention, but the scope of the present invention is not limited to the following examples.
Embodiment 2
[0035] The preparation of embodiment 2 core particles
[0036]
[0037] Accurately weigh batches of each core bead ingredient. Place an appropriate batch of pure water in a jacketed pot equipped with a purger, homogenizer, and mixer. Bulk PVP was added and the resulting mixture was allowed to disperse in the water for at least 15 minutes. Sodium valproate (75 kg) was added and the mixture was mixed for at least 15 minutes to disperse. Water is passed through the interlayer. The sodium valproate suspension was homogenized with a mixer and homogenizer for about 90 minutes (range: 80-100 minutes). Agitation is continued in the subsequent steps of preparing the core beads.
[0038] Prepare a pump with three spray pump heads. Load the batch of sugar spheres, NF into the fluidized bed. The sugar balls are fluidized, and the sodium valproate suspension is sprayed through the nozzle according to certain parameters. The core particles were dried at 60°C for at least 15 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com