Preparation method of all-trans-teprenone
A teprenone, all-trans technology, applied in the field of chemical synthesis of all-trans teprenone, can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthetic geranylgeranyl bromide
[0024] Add geranylgeraniol (200g, 0.688mol, Beijing Bailingwei Technology Co., Ltd.), pyridine (48g, 0.607mol) and methyl tert-butyl ether (1000ml) into the flask, cool to -5°C under nitrogen protection, drop Add phosphorus tribromide (260g, 0.961mol), after the dropwise addition, heat up to room temperature for reaction, keep warm for 2h, filter with suction, wash the organic phase once with 5% sodium carbonate, wash once with water, dry over anhydrous sodium sulfate, and filter with suction. The mother liquor was evaporated to dryness at 20°C to obtain 230 g of geranylgeranyl bromide liquid with a yield of 94.6%. 1 HNMR (CDC13), δ: 1.56(S,9H); 1.66(S,6H); 1.9-2.08(m,12H); 4.09(d,2H); 5.07(t,3H); 5.40(t,1H) ).
Embodiment 2
[0026] Synthesis of teprenone
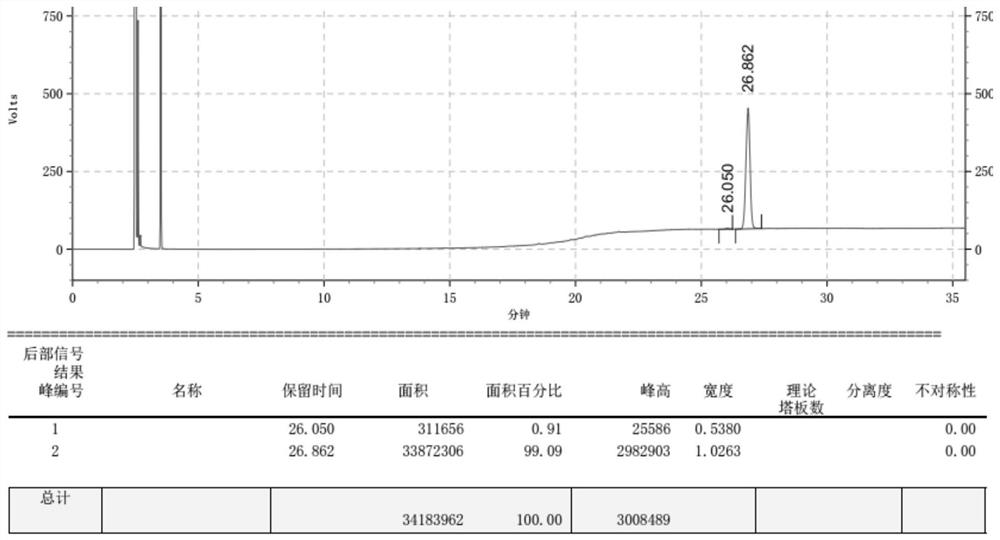
[0027] Add geranylgeranyl bromide 100g (283mmol) into the flask, then add acetone 500ml, cuprous iodide 53.9g (283mmol), stir to dissolve, cool to -10°C, add dropwise LDA36.4g (339.6mmol), control Temperature below 0°C, rise to room temperature after dripping, keep warm for 12 hours, post-treatment, distill off excess acetone, add 500ml of n-hexane to dissolve, wash twice with saturated ammonium chloride aqueous solution, then wash once with saturated sodium chloride, Dry over anhydrous sodium sulfate, filter with suction, and evaporate the mother liquor to dryness at 40°C to obtain 90.1 g of the crude product, with a yield of 96.3%. The gas chromatogram is as follows: image 3 , with the commercially available preparation teprenone (Eisai (China) Pharmaceutical Co., Ltd.) as a contrast, the gas chromatogram is as follows Figure 4 (The cis-trans ratio is cis:trans=2:3), the gas phase spectrum of the all-trans teprenone crude product and the co...
Embodiment 3
[0029] Synthesis of teprenone
[0030] Add geranylgeranyl bromide 100g (283mmol) into the flask, then add acetone 500ml, cuprous iodide 53.9g (283mmol), stir to dissolve, cool to -10°C, add dropwise LDA42.5g (396.2mmol), control Temperature below 0°C, rise to room temperature after dripping, keep warm for 12 hours, post-treatment, distill off excess acetone, add 500ml of n-hexane to dissolve, wash twice with saturated ammonium chloride aqueous solution, then wash once with saturated sodium chloride, Dry over anhydrous sodium sulfate, filter with suction, and evaporate the mother liquor to dryness at 40°C to obtain 88 g of crude product, with a yield of 94.1%. The crude product was rectified with a thorn rectifier to obtain 75.6g of teprenone finished product, with a yield of 80.8%, and a purity of 100% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com