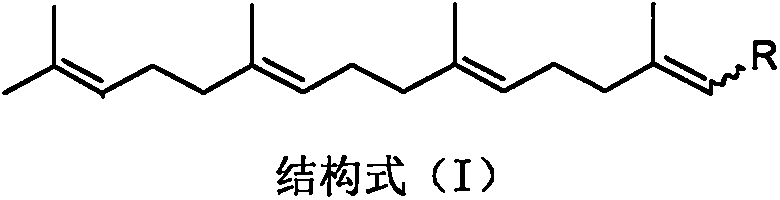
Teprenone and application of derivative of teprenone in preparing drug for treating drug addiction and preventing drug addiction recurrence
A technology of teprenone and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
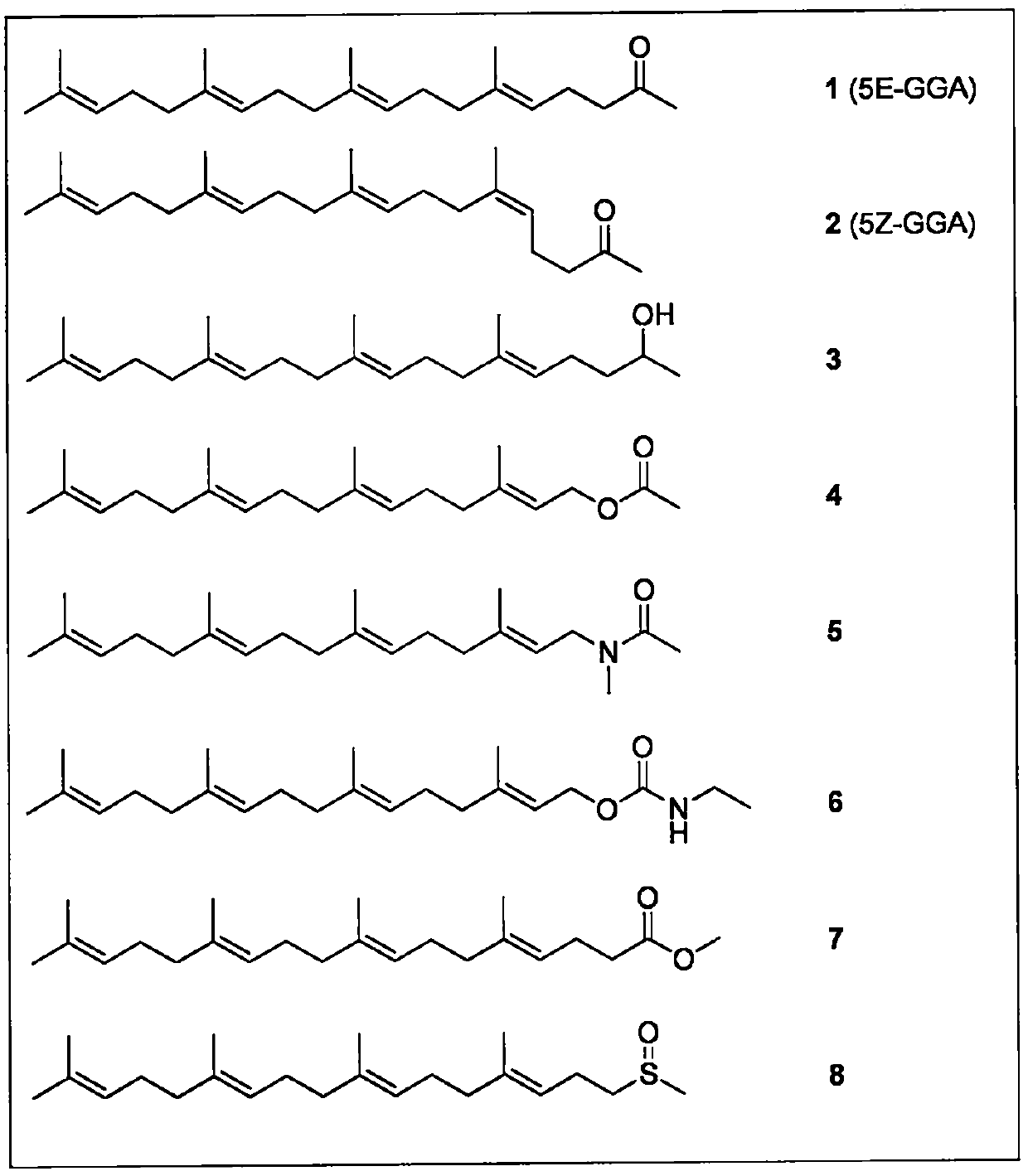
[0049] Embodiment 1: the preparation of all-trans and mono-cis teprenone
[0050] Mix the commercially available teprenone mixture (all-trans isomer: monocis isomer = 3:2) on a silica gel column with n-hexane / ethyl acetate or petroleum ether / ethyl acetate in an appropriate ratio Solvent elution, each component was concentrated on a rotary evaporator to remove the solvent to obtain all-trans teprenone and monocis-teprenone, LCMS: MS (m / z): 333 (MH+); 353 (M+Na). By H-NMR (CDCl 3 ) to determine whether it is an all-trans or a single-cis isomer.
Embodiment 2
[0051] Embodiment 2: the synthesis of all-trans teprenone (reaction formula one)
[0052] All-trans-teprenone can also be synthesized by the method reported in the literature [Bull.Korean Chem.Soc., 2009, 30(9):215-217]. The One-Pot synthesis method reported in this document is shown in the following reaction formula 1. In a round bottom flask was charged geranyllinalool (r-1) (0.30 mL, 0.91 mmol), compound r-2 (0.39 g, 2.28 mmol), p-xylene (4.63 mL) and aluminum isopropoxide (0.019g,
[0053] Reaction formula one,
[0054]
[0055] 0.09 mmol). The reaction flask was placed in an oil bath at 160° C., stirred and heated for 6 hours. The reaction was then cooled to room temperature and the solvent was spun off. A saturated aqueous sodium carbonate solution was added to the residue, followed by extraction with dichloromethane. The organic phase was dried over anhydrous magnesium sulfate, concentrated by filtration, and subjected to silica gel column chromatography, and t...
Embodiment 3
[0056] Embodiment 3: Preparation of all-trans and monocis-teprenone mixtures in various weight ratios
[0057] Mix the above-mentioned all-trans teprenone and commercially available teprenone in different proportions to prepare a mixture of teprenone isomers in the required weight ratio, such as all-trans / monocis The ratio of isomers is 7:3, 8:2, 9:1, 9.5:0.5, 9.8:0.2, 9.9:0.1 and so on. Among the four teprenone samples tested, the ratios of all-trans / mono-cis isomers were 10:0, 8:2, 6:4, and 0:10, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com