A kind of refining method of teprenone and its intermediate
A refining method and technology of teprenone, applied in the field of teprenone refining method and its intermediates, can solve the problems of farnesyl acetone separation and removal difficulties, column chromatography is not suitable for large-scale industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
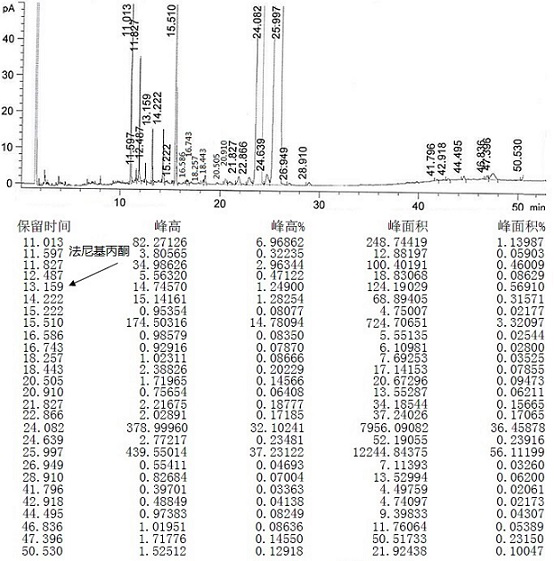
[0073] Mix 1 mol of crude teprenone {the ratio of monocis-isomer (5Z, 9E, 13E) to all-trans-isomer (5E, 9E, 13E) is 0.61-0.68}, 1w / w methanol (density 0.8 g) and 5w / w water (20% methanol) were added into the flask and stirred evenly, 2mol potassium bisulfite was added, and the reaction was completed under reflux with stirring. The reaction solution was centrifuged to discard the filtrate, and the granular solid in the centrifuge bag was taken out to obtain the intermediate 2-hydroxy-6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraene- Potassium 2-sulfonate.
[0074] Add 3w / w water and 2mol potassium hydroxide to the flask, stir until dissolved, then add 3w / w n-heptane, add the intermediate 2-hydroxy-6,10,14,18-tetramethyl obtained in the previous step while stirring base-5,9,13,17-nonadecatetraene-2-sulfonate potassium, and then reacted with stirring at 25~35°C. Stand to separate the layers, discard the water phase, and wash the organic phase with 3×3 w / w water, and let stand ...
Embodiment 2
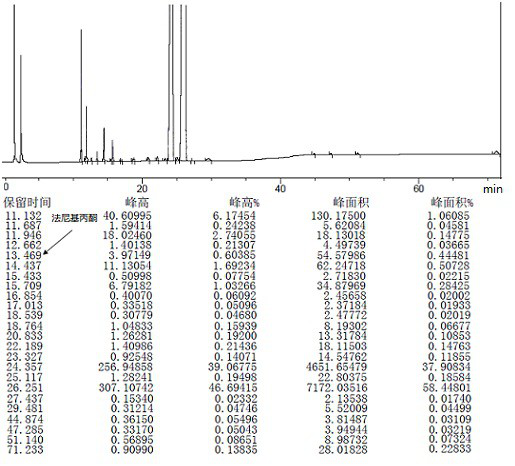
[0076] 1 mol of crude teprenone {the ratio of monocis-isomer (5Z, 9E, 13E) to all-trans-isomer (5E, 9E, 13E) is 0.61-0.68}, 8.5w / w / tetrahydrofuran ( Density is calculated as 0.9) and 0.5w / w water (95% tetrahydrofuran) are added into the flask and stirred evenly, 2mol sodium bisulfite is added, and the reaction is completed by stirring at -20°C. Centrifuge the reaction solution to discard the filtrate, and take out the granular solid in the centrifuge bag to obtain the intermediate 2-hydroxy-6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraene-2 - sodium sulfonate.
[0077] Add 3w / w water and 2mol hydrochloric acid to the flask, stir until dissolved, then add 3w / w n-heptane, and add the intermediate 2-hydroxy-6,10,14,18-tetramethyl- 5,9,13,17-Nadecatetraene-2-sodium sulfonate, then stirred and reacted at 25~35℃. Stand to separate the layers, discard the water phase, and wash the organic phase with 3×3 w / w water, and let stand overnight. The next day, it was concentrated under r...
Embodiment 3
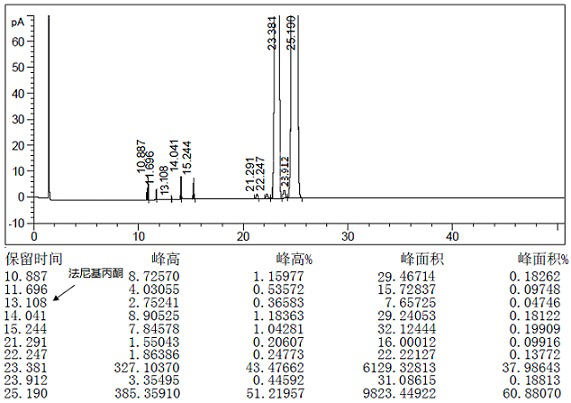
[0079] 1mol teprenone crude product {the ratio of monocis isomer (5Z, 9E, 13E) to all trans isomer (5E, 9E, 13E) is 0.61-0.68}, 9w / w / DMF (density 0.95) and 0.5w / w water (95% DMF) were added into the flask and stirred evenly, 1mol of potassium metabisulfite was added, and the reaction was completed with stirring at -20°C. The reaction solution was centrifuged to discard the filtrate, and the granular solid in the centrifuge bag was taken out to obtain the intermediate 2-hydroxy-6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraene- Potassium 2-sulfonate.
[0080] Add 3w / w water and 1mol barium hydroxide to the flask, stir until dissolved, then add 3w / w n-heptane, add the intermediate 2-hydroxy-6,10,14,18-tetramethyl obtained in the previous step while stirring base-5,9,13,17-nonadecatetraene-2-sulfonate potassium, and then reacted with stirring at 25~35°C. Stand to separate the layers, discard the water phase, and wash the organic phase with 3×3 w / w water, and let stand overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com