Alcohol-soluble hole-transporting molecular material and preparation method thereof
A technology of hole transport and molecular materials, which is applied in the field of alcohol-soluble hole transport molecular materials and its preparation, can solve problems affecting device stability, device performance is difficult to control, and is difficult to overcome, so as to achieve convenient purification, avoid performance and lifespan , the effect of good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
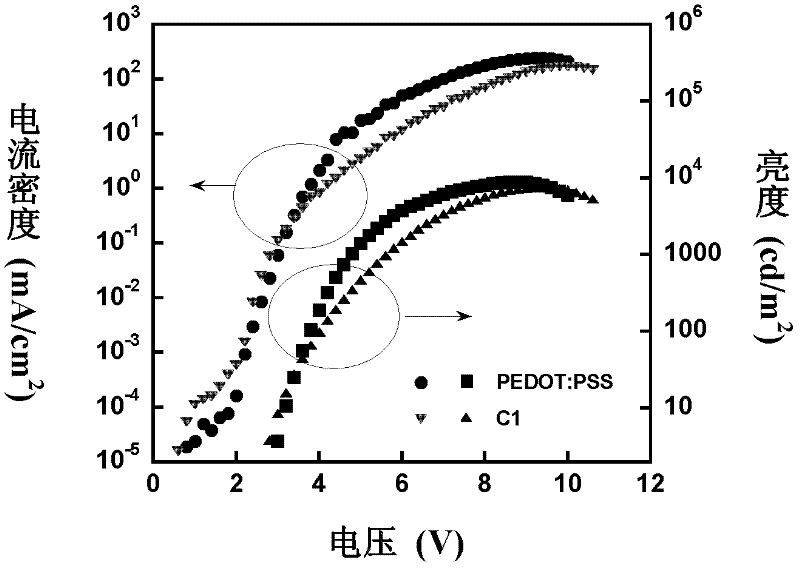
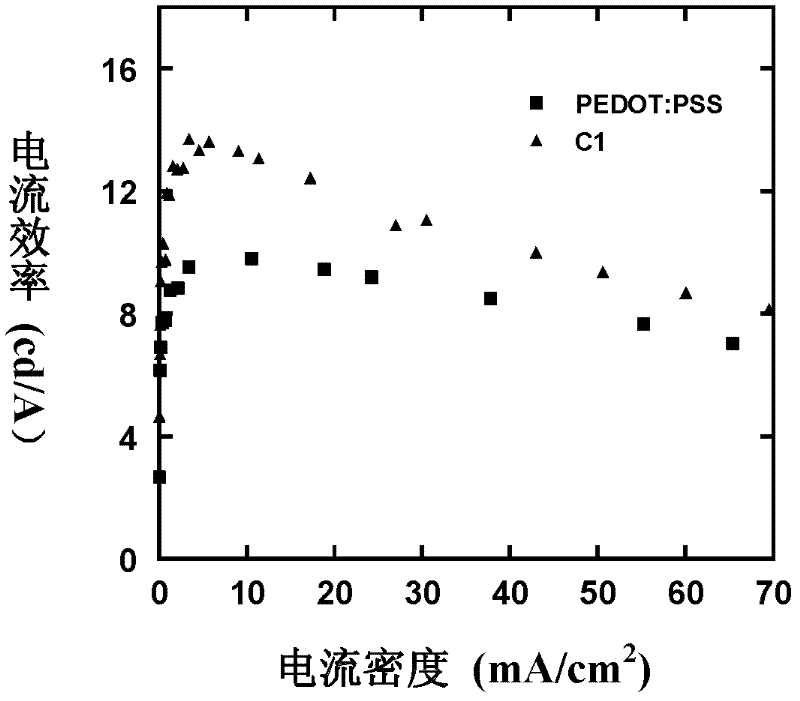
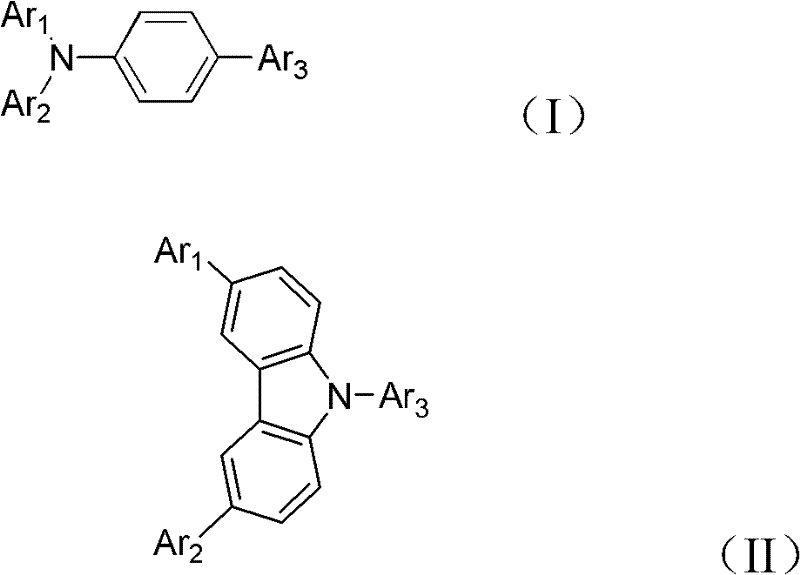
Image
Examples
Embodiment 1
[0040] As shown in the following formula, the preparation process of the alcohol-soluble hole transport molecular material of this embodiment is as follows:
[0041]
[0042] Step 1: slowly pour 2-bromoethanol (50g, 0.4mol) into dihydropyran (67g, 0.8mol) stirred in a flask, and cool with an ice-water bath. After pouring, add concentrated hydrochloric acid (12mol / L, 2mL), after 8h, the reaction solution turned black. After distillation under reduced pressure, a colorless oily liquid 1 was obtained.
[0043] Step 2: Pour 2,7-dibromofluorene (3.24g, 10mmol) into a flask, add dimethyl sulfoxide (30mL) and stir to exhaust until all solids are dissolved, then add KOH (5.8g, 104.1mmol), Reacted for 1 h under the protection of nitrogen, the solution gradually turned reddish brown. Then 1 (4.6g, 22mmol) was slowly added dropwise into the reaction flask, and reacted in an ice-water bath for 8h. The mixture was extracted and column chromatographed to give 2 as a white solid.
[...
Embodiment 2
[0056] As shown in the following formula, the preparation process of the alcohol-soluble hole transport molecular material of this embodiment is as follows:
[0057]
[0058] Step 1: Preparation 6. The process of preparation 6 in this example is the same as that in Example 1, and will not be repeated here.
[0059] Step 2: Dissolve 6 (1.6g, 1.6mmol) in dry THF (60mL), protect with nitrogen, and cool to -78°C. Slowly add n-BuLi (1.6M, 1 mL, 1.6 mmol) dropwise into the reaction flask. After the dropwise addition was completed, stirring was continued at -78°C for 1 h, and then 4,4,5,5-tetramethyl-1,3,2-isopropanol borate (0.3 mg, 1.6 mmol) was added with a syringe, Then naturally warmed to room temperature and stirred for 24h. After the mixture was terminated with ethanol, ethanol and THF were removed by rotary distillation, and the remaining solid was extracted and column chromatographed to obtain 8 as a yellow solid.
[0060] Step 3: Add 6 (0.5g, 0.5mmol), 8 (0.5g, 0.5mmo...
Embodiment 3
[0063] As shown in the following formula, the preparation process of the alcohol-soluble hole transport molecular material of this embodiment is as follows:
[0064]
[0065] Step 1: Slowly pour 6-bromo-1,2-hexanediol (50g, 254mmol) into dihydropyran (64g, 760mmol) stirred in a flask, and cool it in an ice-water bath. Concentrated hydrochloric acid (0.63mL, 12mol / L) was added after 8h. After distillation under reduced pressure, 10 was obtained as a colorless oily liquid.
[0066] Step 2: Pour 2,7-dibromofluorene (1.62g, 5mmol) into a flask, add dimethyl sulfoxide (30mL) and stir to exhaust until the solids are completely dissolved, then add KOH (2.8g, 50mmol), in Under the protection of nitrogen, the reaction was carried out for 1 h, and the solution gradually turned reddish brown. Then 1 (4.0g, 11mmol) was slowly added dropwise into the reaction flask, and reacted in a 50°C water bath for 36h. The mixture was extracted and column chromatographed to give 11 as a colorles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com