Fluorine-substituted organic micro-molecular hole-transport material and application thereof
A hole transport material and small molecule technology, applied in the field of organic photoelectric functional materials, can solve the problems of cumbersome synthesis steps of Spiro-OMeTAD, increased complexity and cost of battery preparation process, low carrier mobility and conductivity, etc. Achieve the effects of improving photoelectric conversion efficiency and current density, good hole transport performance, high hole mobility and conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A fluorine-substituted organic small molecule hole transport material i-1, the synthesis route is as follows:
[0048]
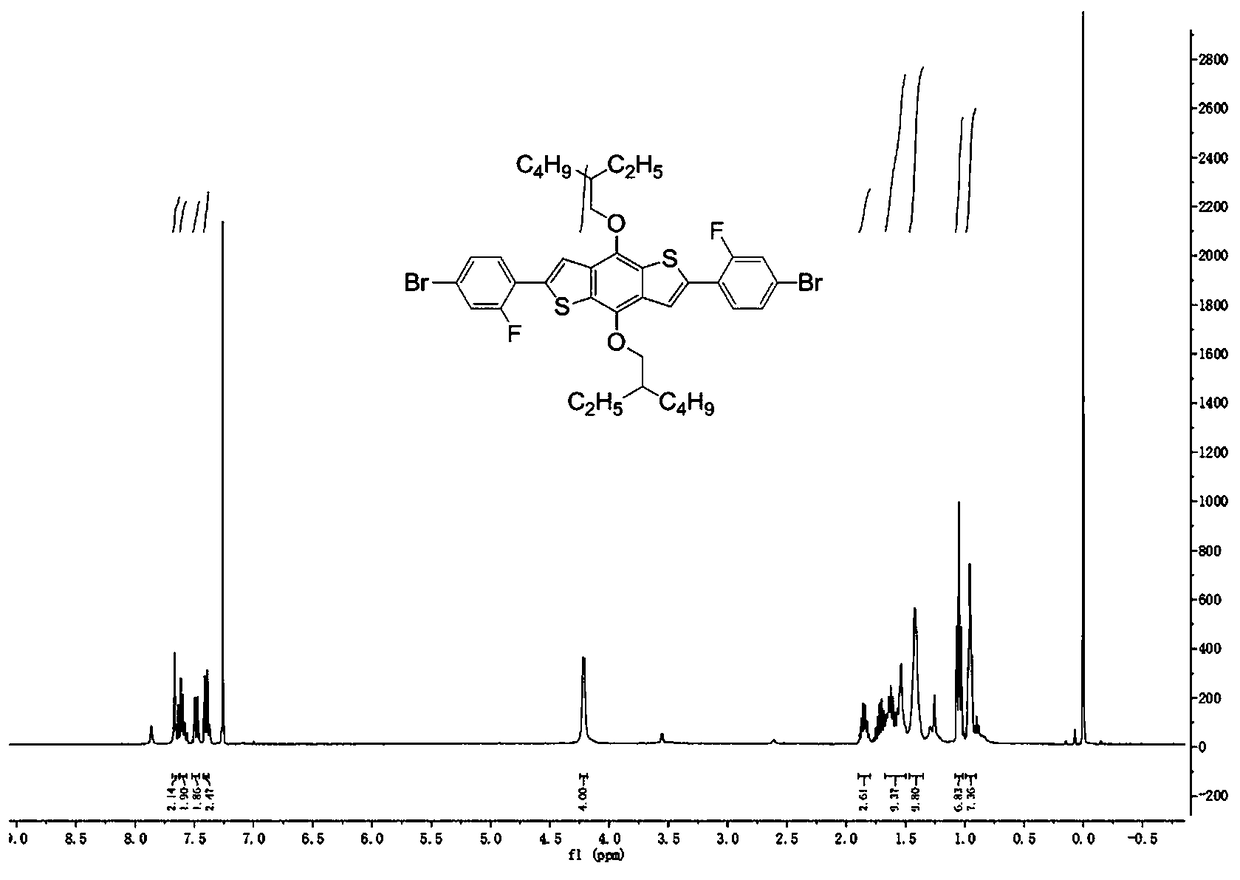
[0049] (1) Under nitrogen, 1,1'-[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b']dithiophene-2 ,6-diyl]bis[1,1,1-trimethyltin] (0.5g, 0.65mmol), 1,4-dibromo-2-fluorobenzene (1.0g, 3.9mmol), tetrakis(triphenyl Phosphine)palladium (Pd(PPh 3 ) 4 ) (22.5 mg, 19.5 μmol) and toluene (100 mL) were added to a 250 mL round bottom flask. React at 80°C for 6h, cool to room temperature after the reaction, dichloromethane (CH 2 Cl 2 ) extraction, the organic phase was washed with brine, anhydrous MgSO 4 Dry and concentrate by rotary evaporation to remove the solvent. Crude product with CH 2 Cl 2 and n-hexane column chromatography (hexane: CH 2 Cl 2 =4:1 (v / v)), a yellow-green intermediate (270mg, yield 53%) was obtained. 1 H NMR (400 MHz, CDCl 3 ): δ=7.655 (s, 2H), 7.634-7.561 (m, 2H), 7.499-7.471 (dd, 2H), 7.411-7.390 (d, 2H), 4.220-4.207 (d,4H), 1.88...
Embodiment 2
[0052] A fluorine-substituted organic small molecule hole transport material i-2, the synthesis route is as follows:
[0053]
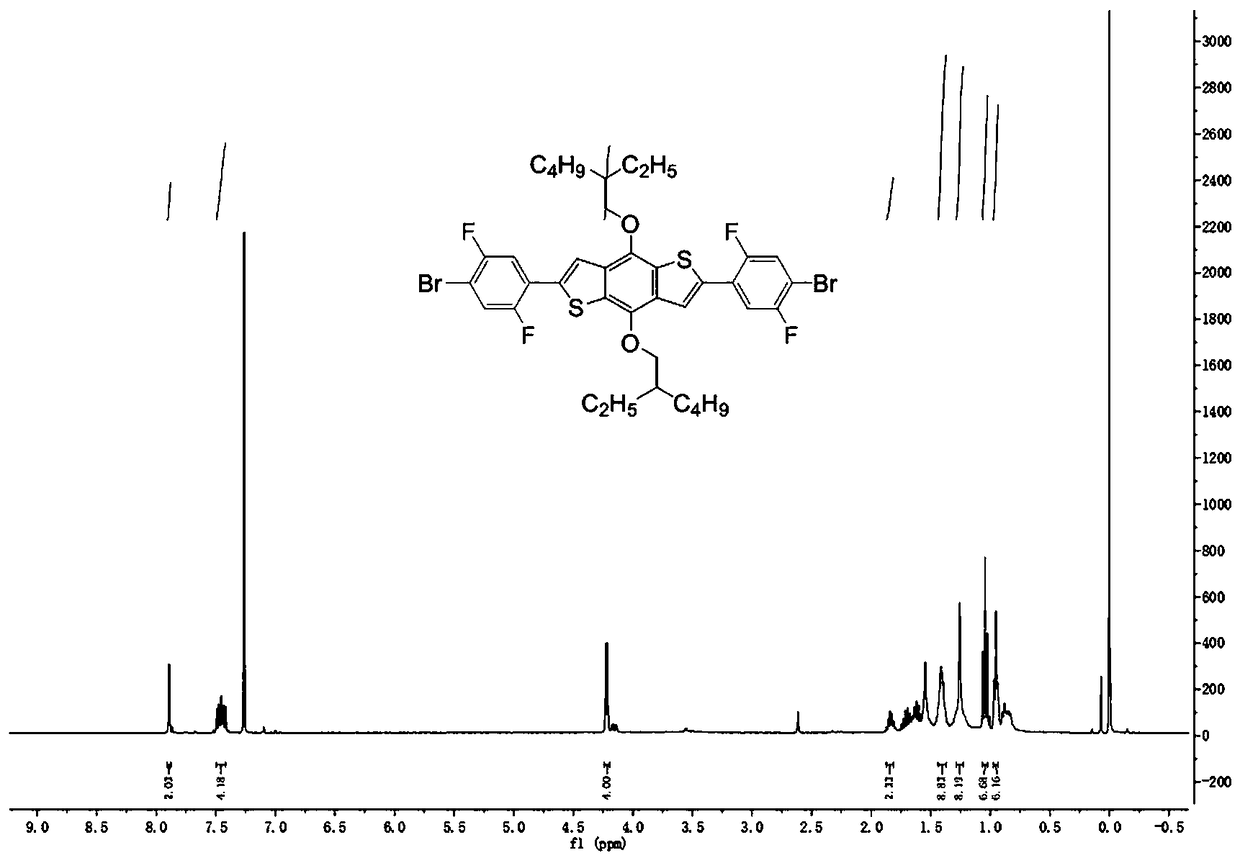
[0054] (1) The process is the same as in Example 1, but the raw material 1,4-dibromo-2-fluorobenzene is replaced by 1,4-dibromo-2,5-fluorobenzene to obtain a yellow intermediate product (266mg, yield 50% ). 1 H NMR (400 MHz, CDCl 3 ): δ=7.655 (s, 2H), 7.634-7.561(m, 2H), 7.499-7.471 (dd, 2H), 7.411-7.390 (d, 2H), 4.220-4.207 (d, 4H), 1.885-1.824 (m, 2H), 1.753-1.535 (m, 8H) , 1.424 (m, 8H), 1.068-1.031 (t,6H), 0.959 (t, 6H).
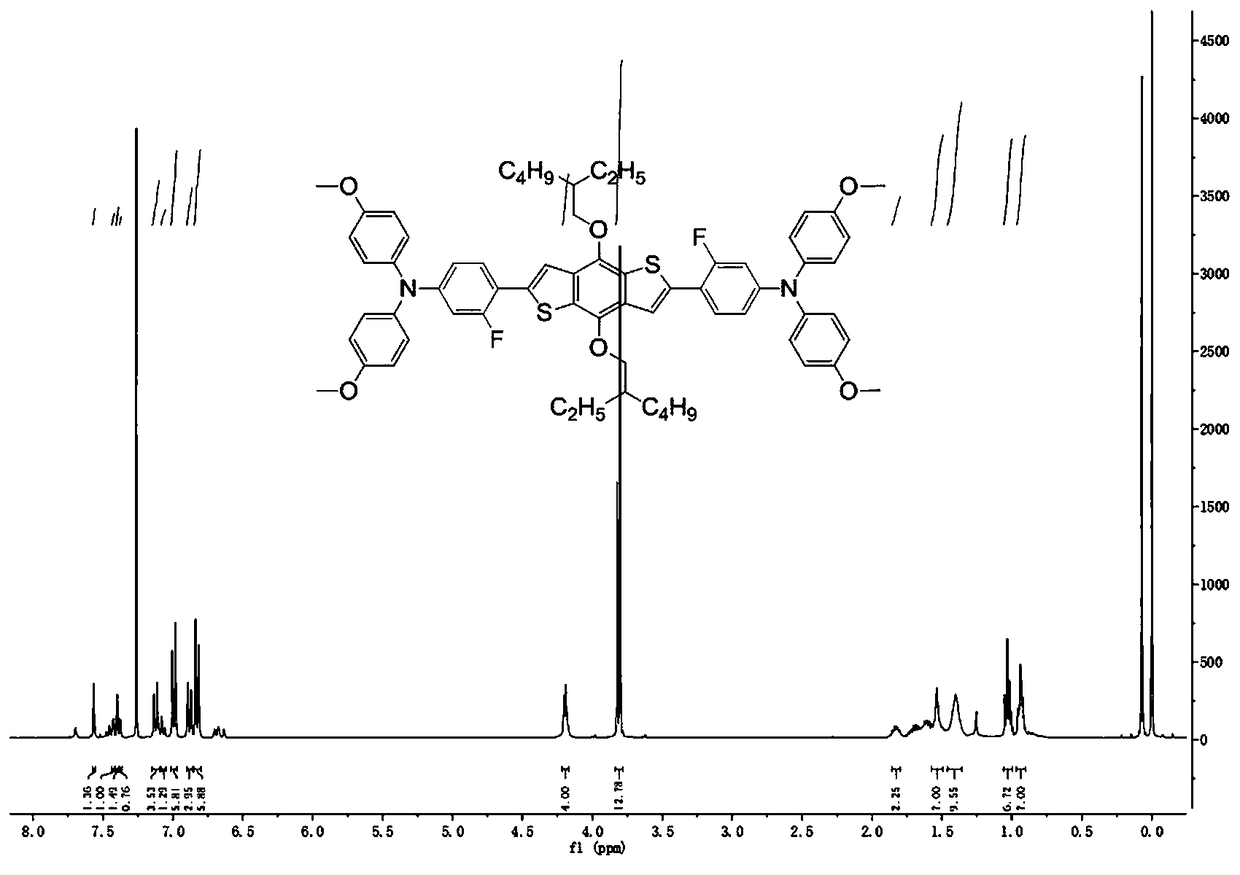
[0055] (2) The process was the same as in Example 1, and an orange-yellow powder (847 mg, yield 80%) was finally obtained. 1 H NMR (400MHz, CDCl 3 ): δ=7.565 (s, 1H), 7.431-7.425 (d, 1H), 7.393 (s, 1H), 7.378-7.374(d, 1H), 7.135-7.112 (d, 3H), 7.101-7.058 (t , 2H), 7.004-6.982 (d, 6H), 6.893-6.871 (d, 3H), 6.837-6.815 (d, 6H), 4.204-4.190 (d, 4H), 3.822-3.802(d, 12H), 1.869 -1.800 (m, 2H), 1.755-1.570 (m, 8H) , 1.404 (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com