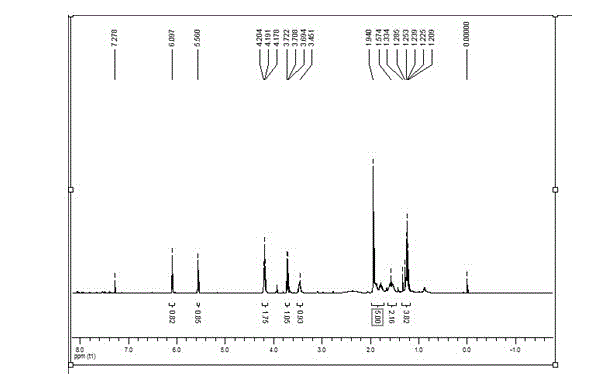
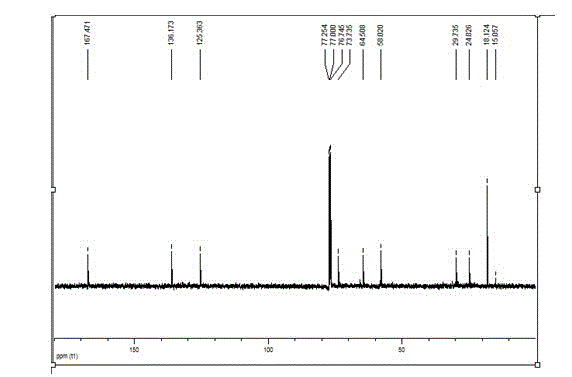
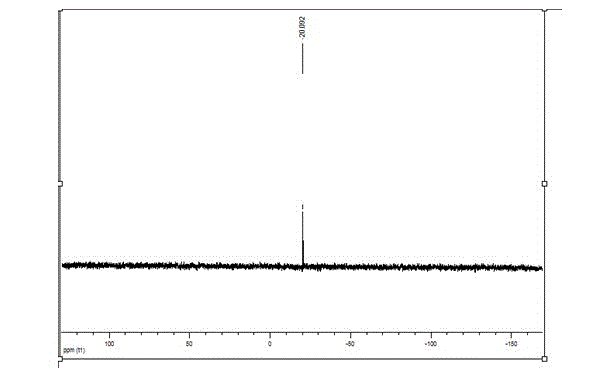
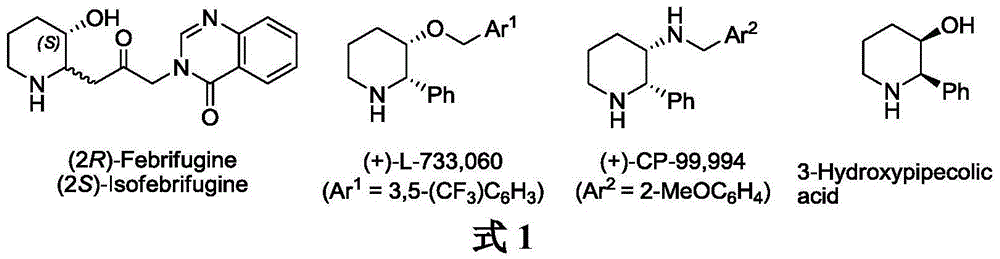
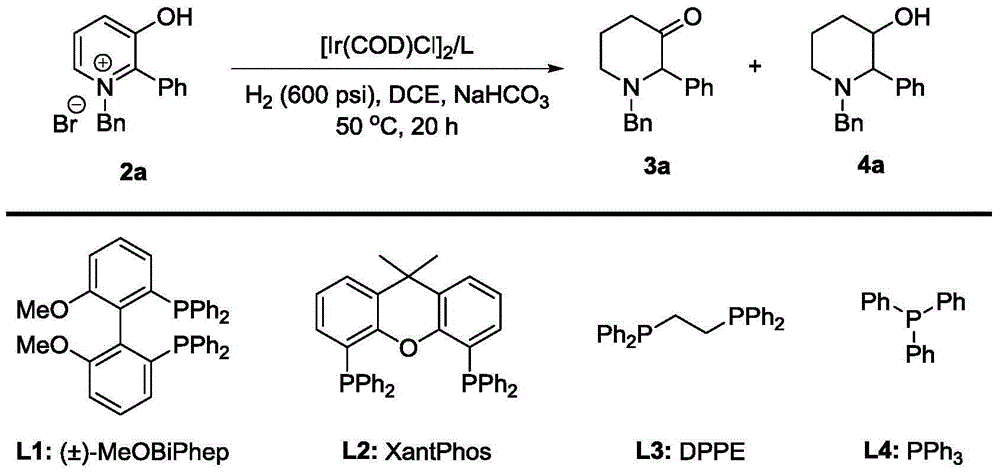
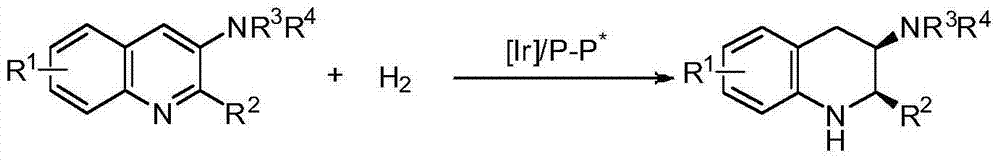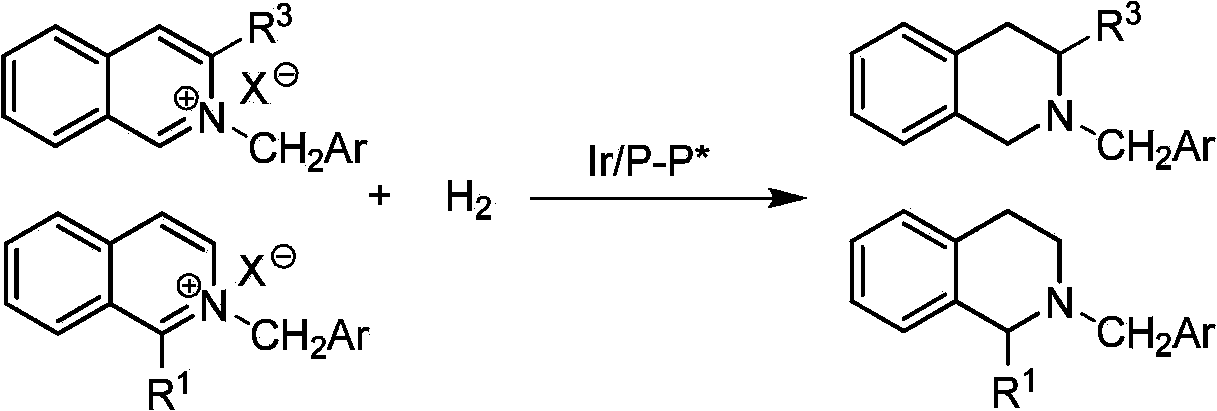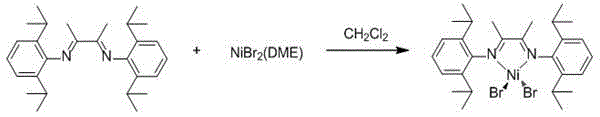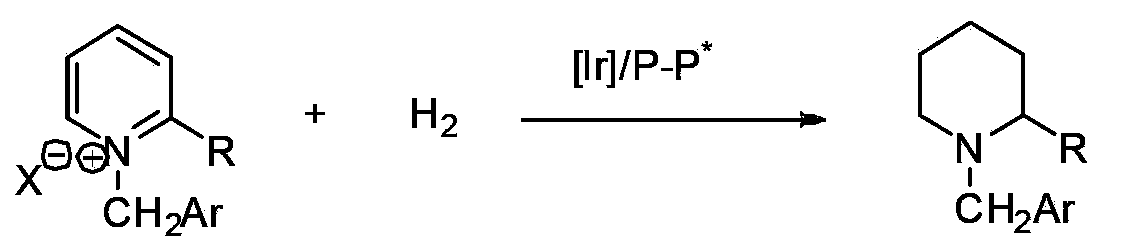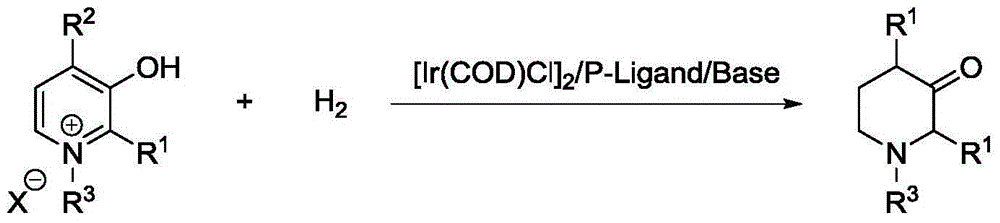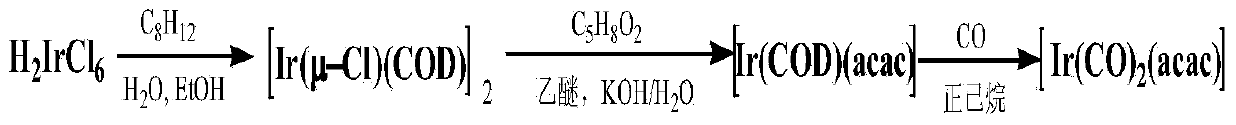Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "1,5-Cyclooctadiene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
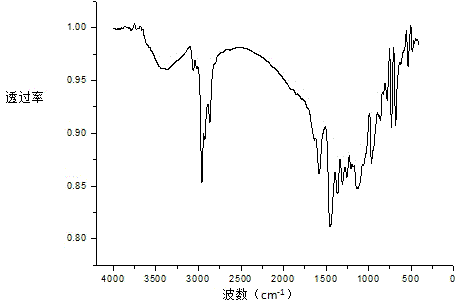
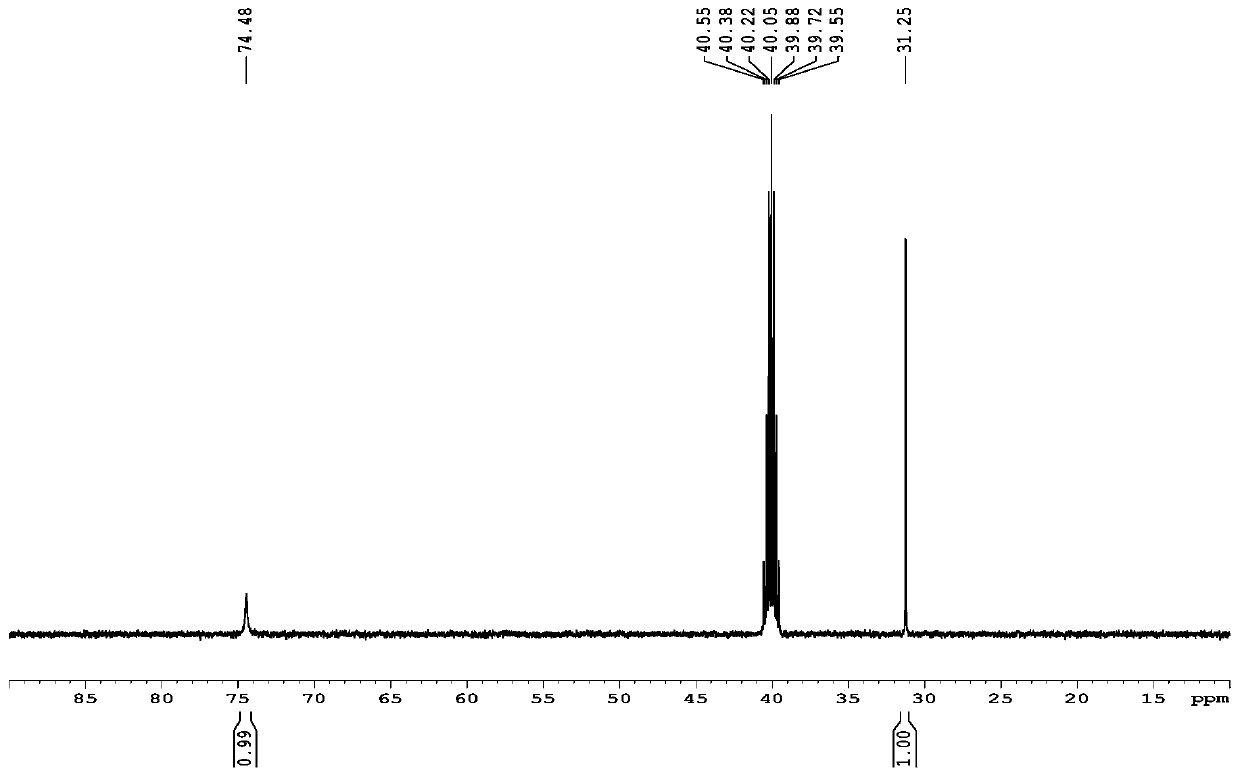
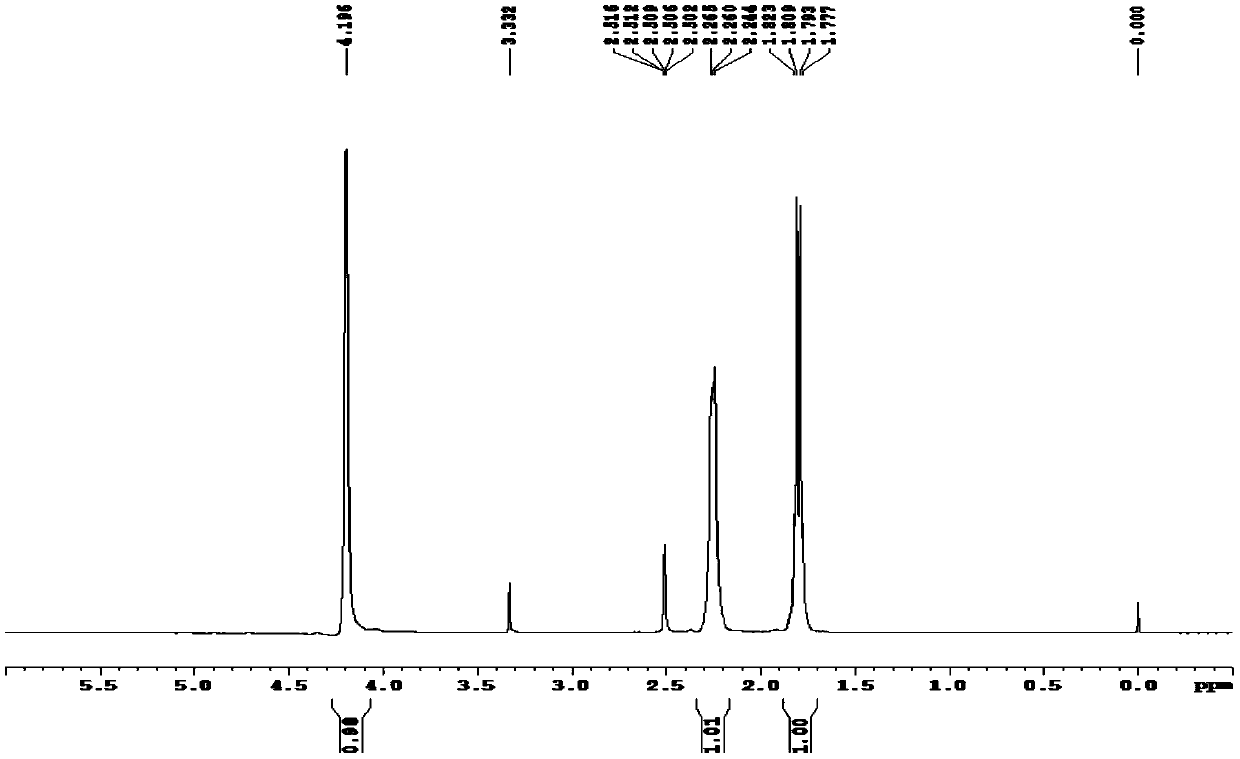
1,5-Cyclooctadiene is the organic compound with the chemical formula C₈H₁₂. Generally abbreviated COD, this diene is a useful precursor to other organic compounds and serves as a ligand in organometallic chemistry. It is a colorless liquid with a strong odor. 1,5-Cyclooctadiene can be prepared by dimerization of butadiene in the presence of a nickel catalyst, a coproduct being vinylcyclohexene. Approximately 10,000 tons were produced in 2005.
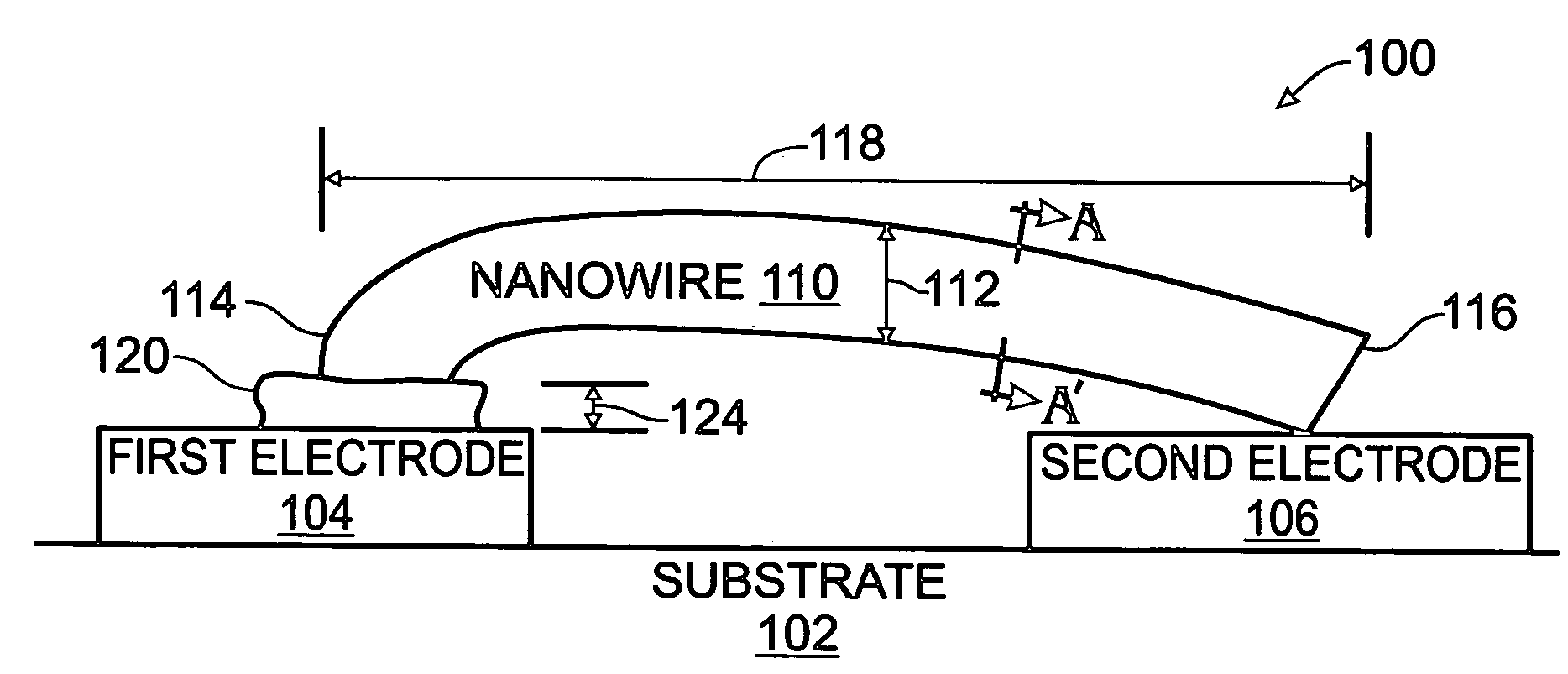
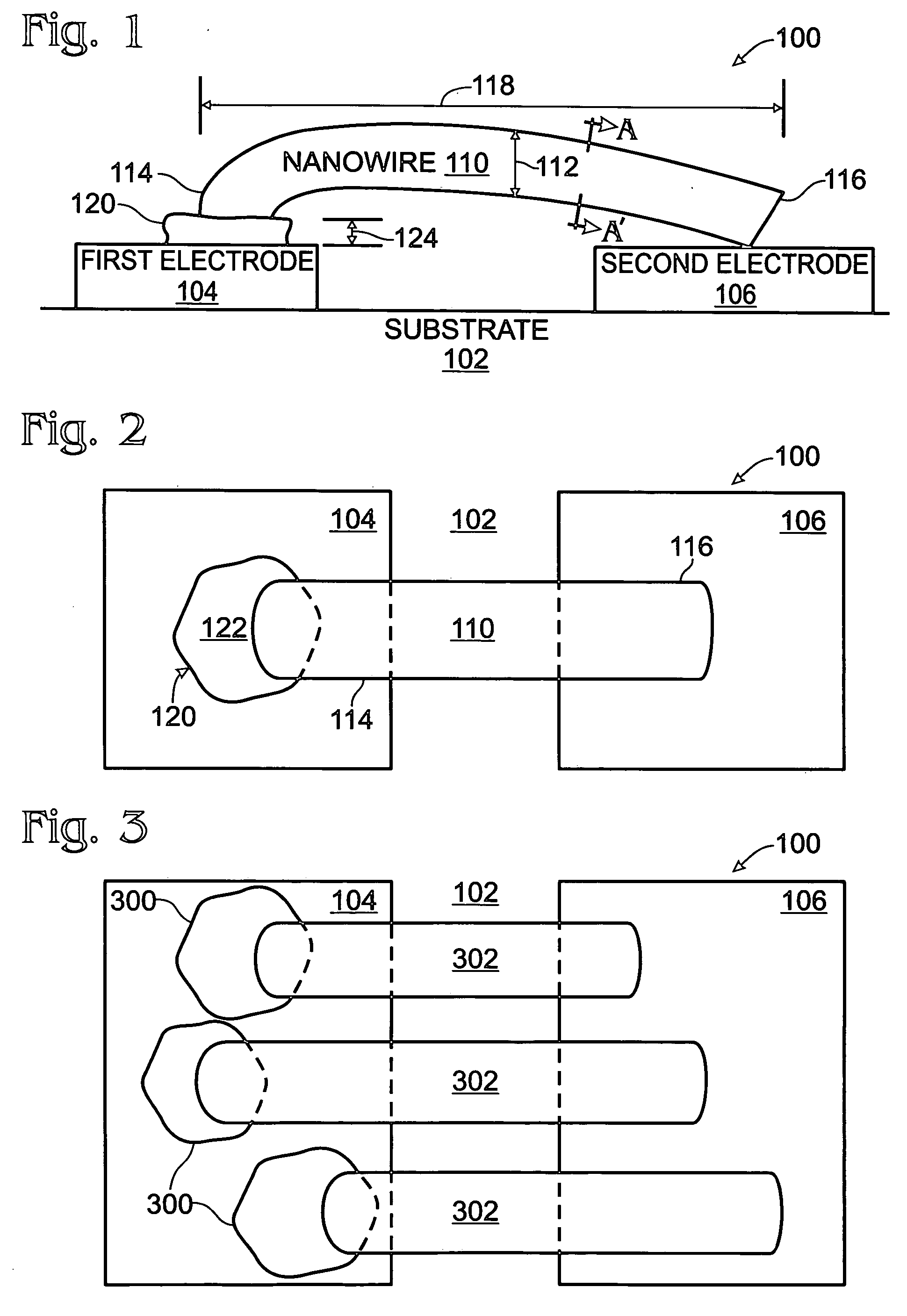
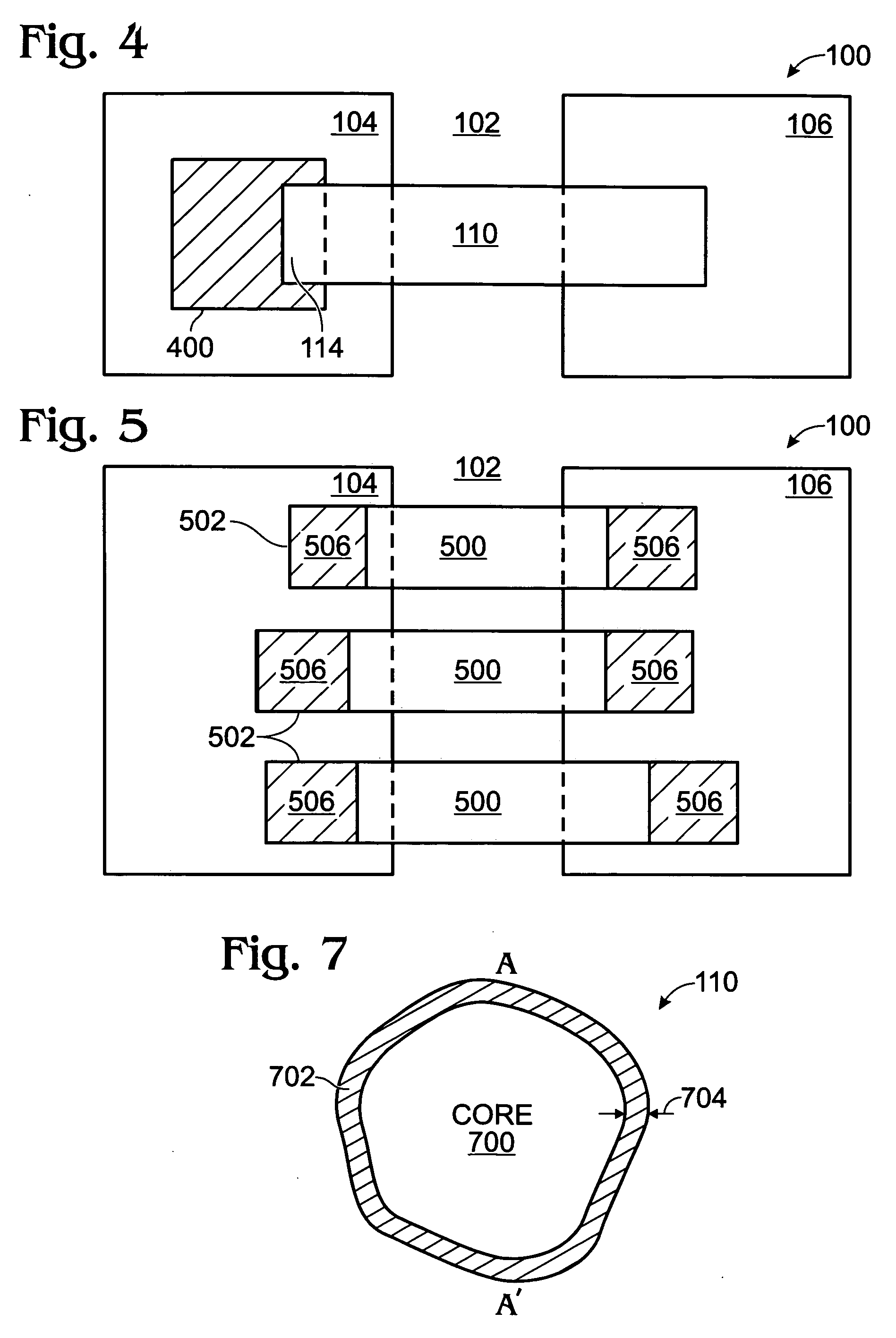
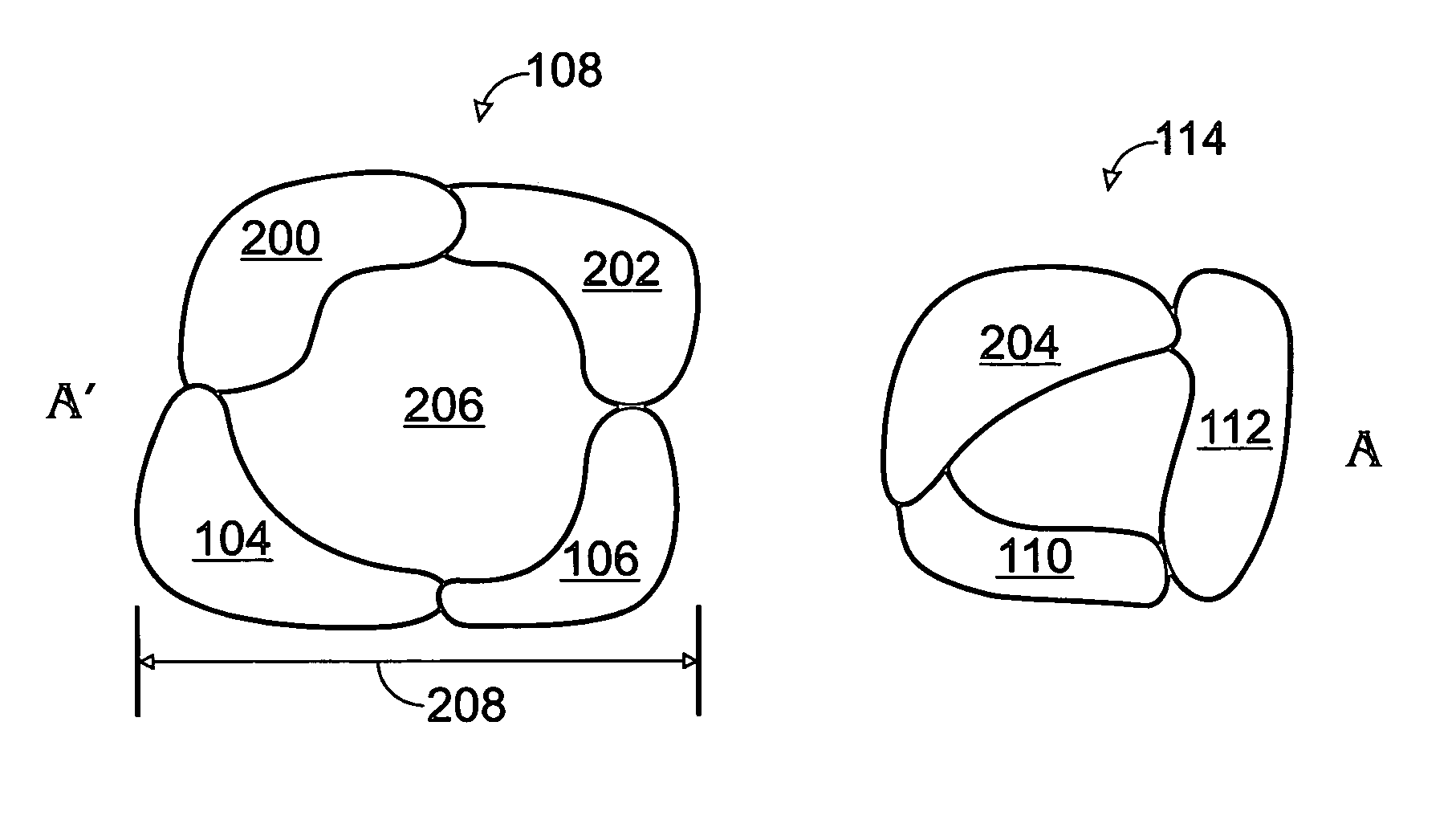
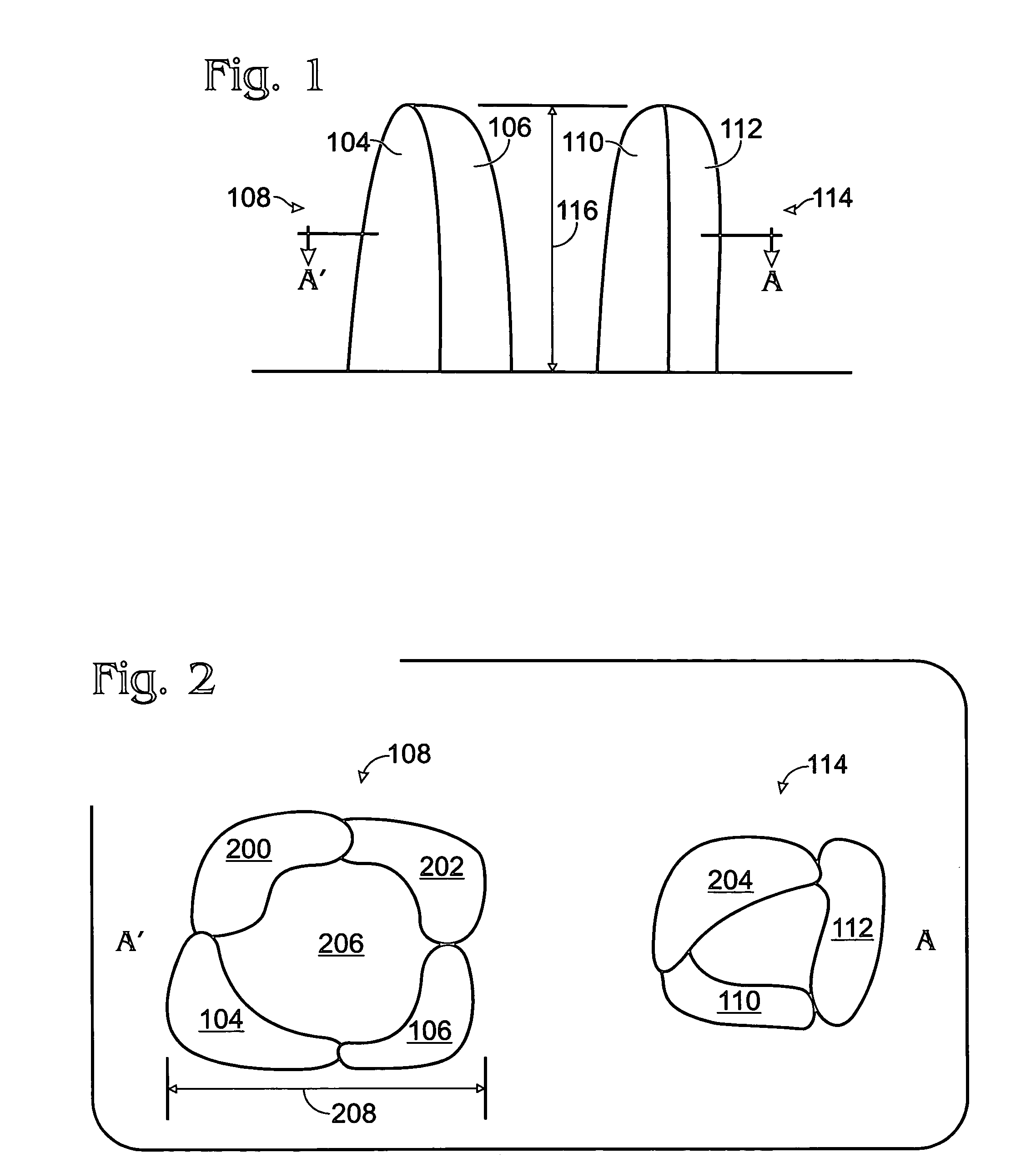
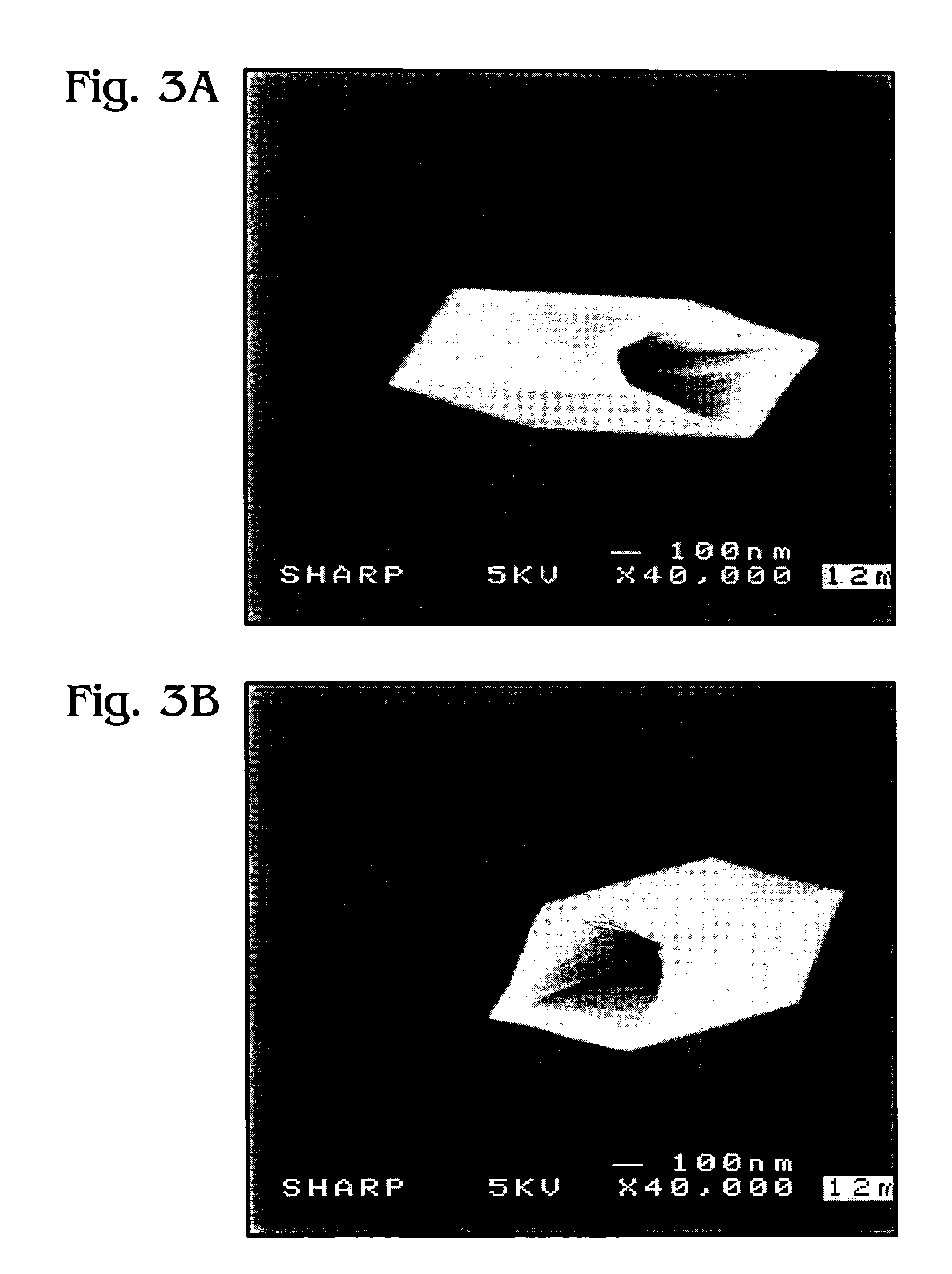
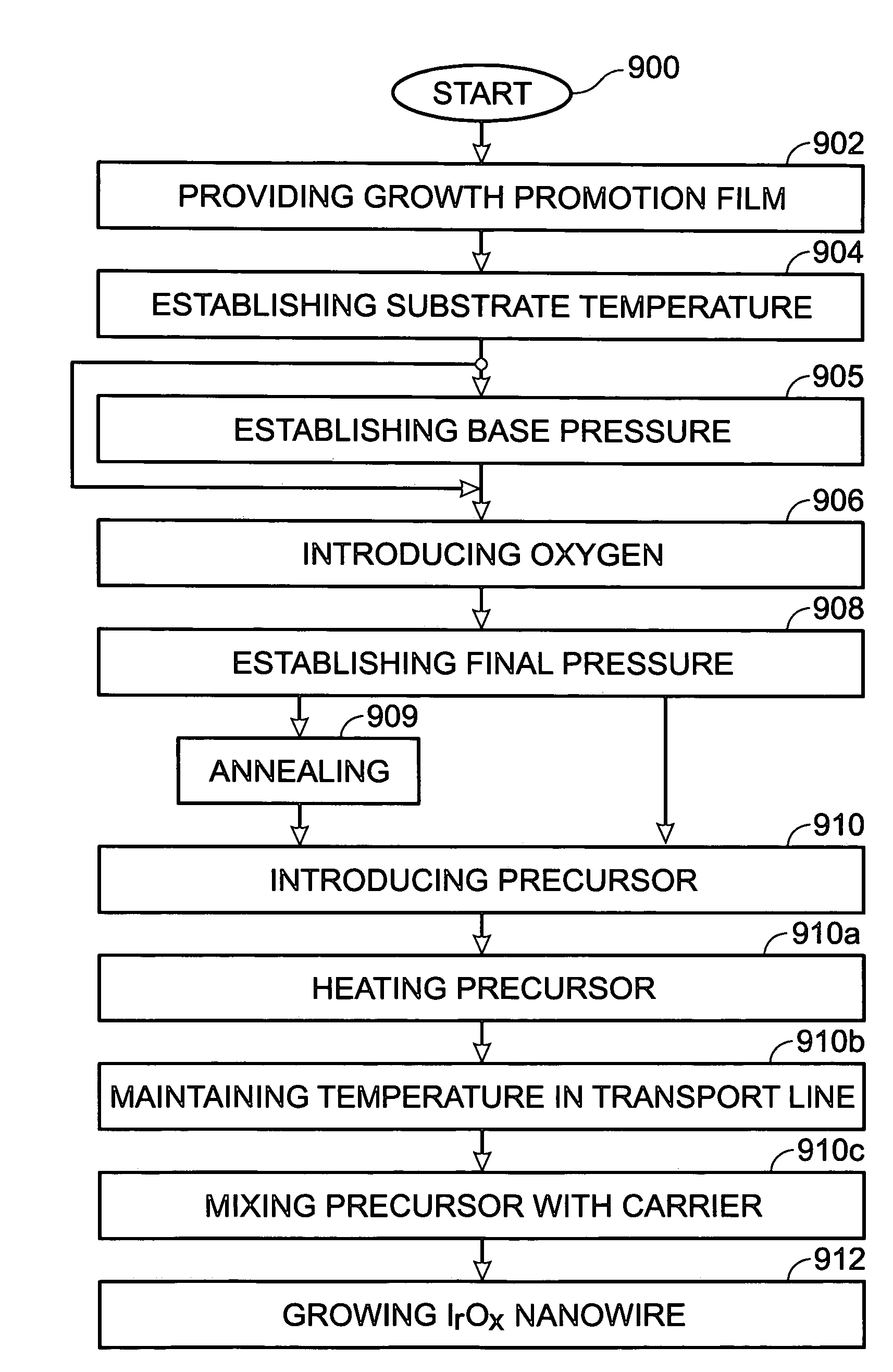
Iridium oxide nanowires and method for forming same
InactiveUS20060086314A1Good crystallinity and electrical propertyNanotechPolycrystalline material growthNanowireSingle crystal
Iridium oxide (IrOx) nanowires and a method forming the nanowires are provided. The method comprises: providing a growth promotion film with non-continuous surfaces, having a thickness in the range of 0.5 to 5 nanometers (nm), and made from a material such as Ti, Co, Ni, Au, Ta, polycrystalline silicon (poly-Si), SiGe, Pt, Ir, TiN, or TaN; establishing a substrate temperature in the range of 200 to 600 degrees C.; introducing oxygen as a precursor reaction gas; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx nanowires from the growth promotion film surfaces. The IrOx nanowires have a diameter in the range of 100 to 1000 Å, a length in the range of 1000 Å to 2 microns, an aspect ratio (length to width) of greater than 50:1. Further, the nanowires include single-crystal nanowire cores covered with an amorphous layer having a thickness of less than 10 Å.
Owner:SHARP KK
Iridium oxide nanotubes and method for forming same
ActiveUS7098144B2Material nanotechnologyPolycrystalline material growthOxygenChemical vapor deposition
A method is provided for forming iridium oxide (IrOx) nanotubes. The method comprises: providing a substrate; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; introducing oxygen as a precursor reaction gas; establishing a final pressure in the range of 1 to 50 Torr; establishing a substrate, or chamber temperature in the range of 200 to 500 degrees C.; and using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx hollow nanotubes from the substrate surface. Typically, the (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor is initially heated in an ampule to a first temperature in the range of 60 to 90 degrees C., and the first temperature is maintained in the transport line introducing the precursor. The precursor may be mixed with an inert carrier gas such as Ar, or the oxygen precursor reaction gas may be used as the carrier.
Owner:SHARP KK
Iridium oxide nanowires and method for forming same
InactiveUS7255745B2Good crystallinity and electrical propertyNanotechPolycrystalline material growthIridiumNanowire
Iridium oxide (IrOx) nanowires and a method forming the nanowires are provided. The method comprises: providing a growth promotion film with non-continuous surfaces, having a thickness in the range of 0.5 to 5 nanometers (nm), and made from a material such as Ti, Co, Ni, Au, Ta, polycrystalline silicon (poly-Si), SiGe, Pt, Ir, TiN, or TaN; establishing a substrate temperature in the range of 200 to 600 degrees C.; introducing oxygen as a precursor reaction gas; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx nanowires from the growth promotion film surfaces. The IrOx nanowires have a diameter in the range of 100 to 1000 Å, a length in the range of 1000 Å to 2 microns, an aspect ratio (length to width) of greater than 50:1. Further, the nanowires include single-crystal nanowire cores covered with an amorphous layer having a thickness of less than 10 Å.
Owner:SHARP KK
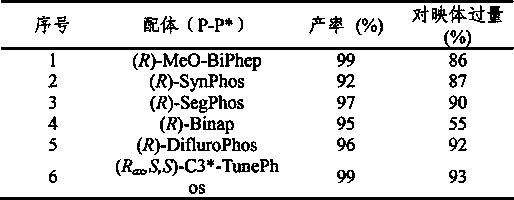
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
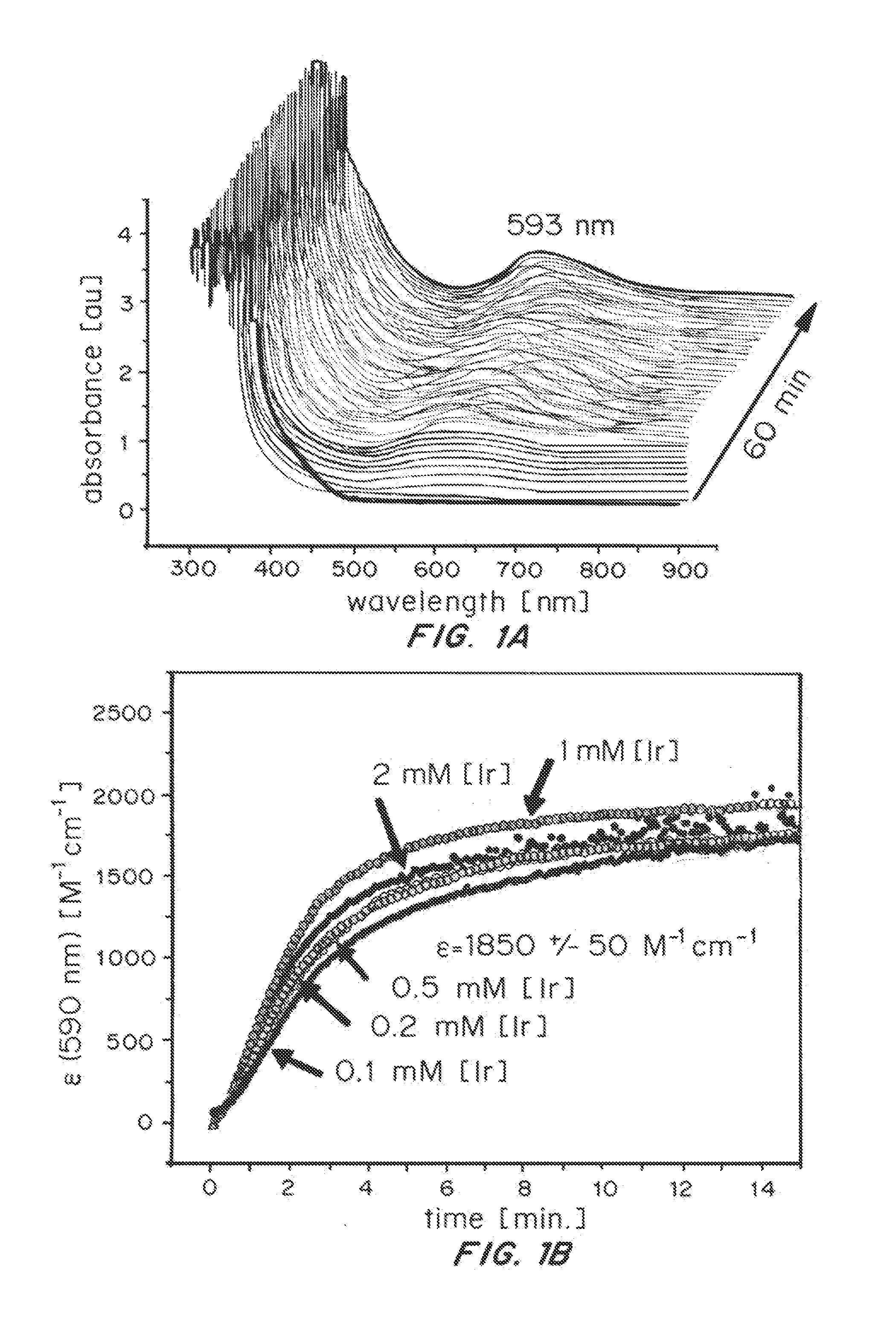
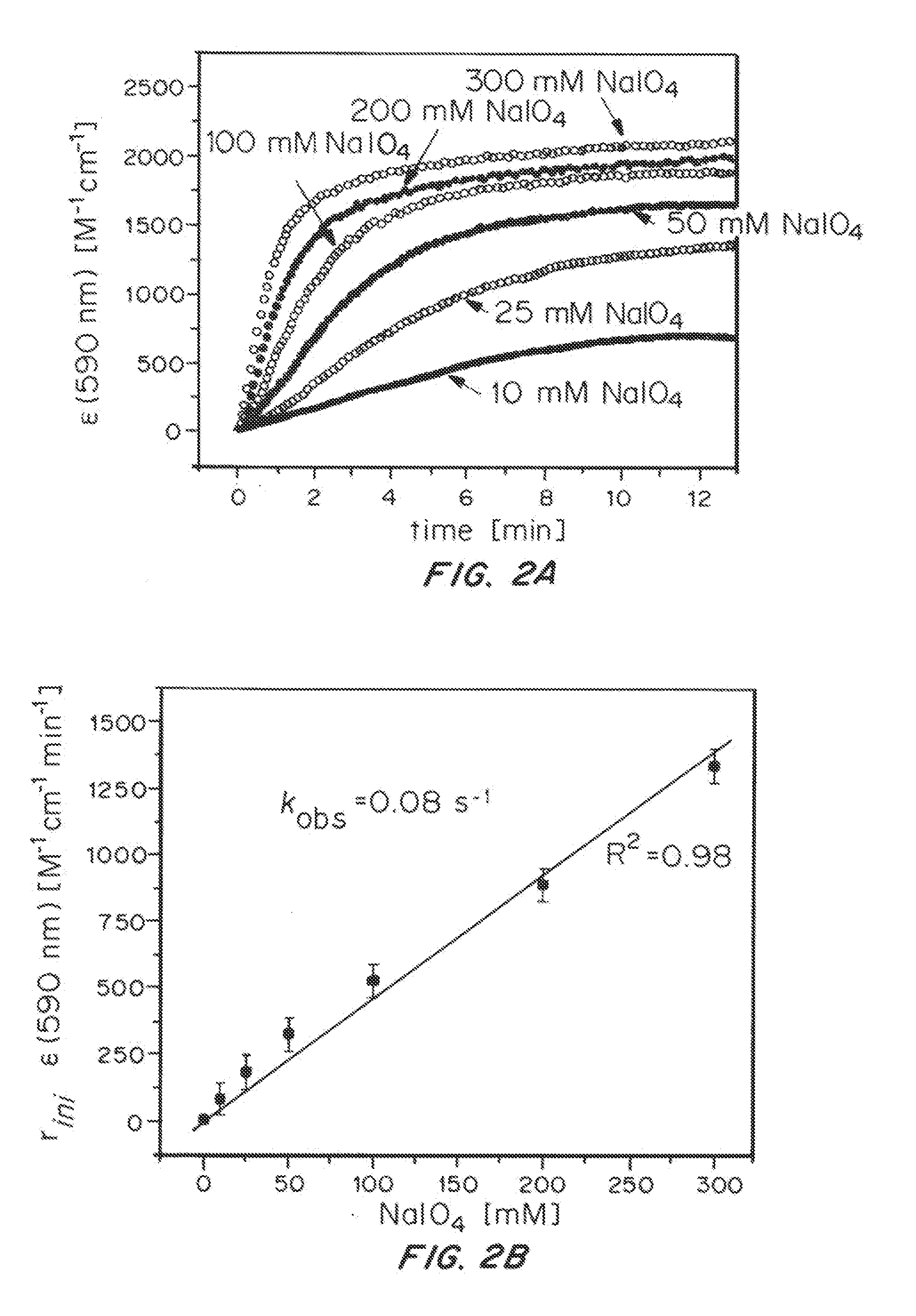
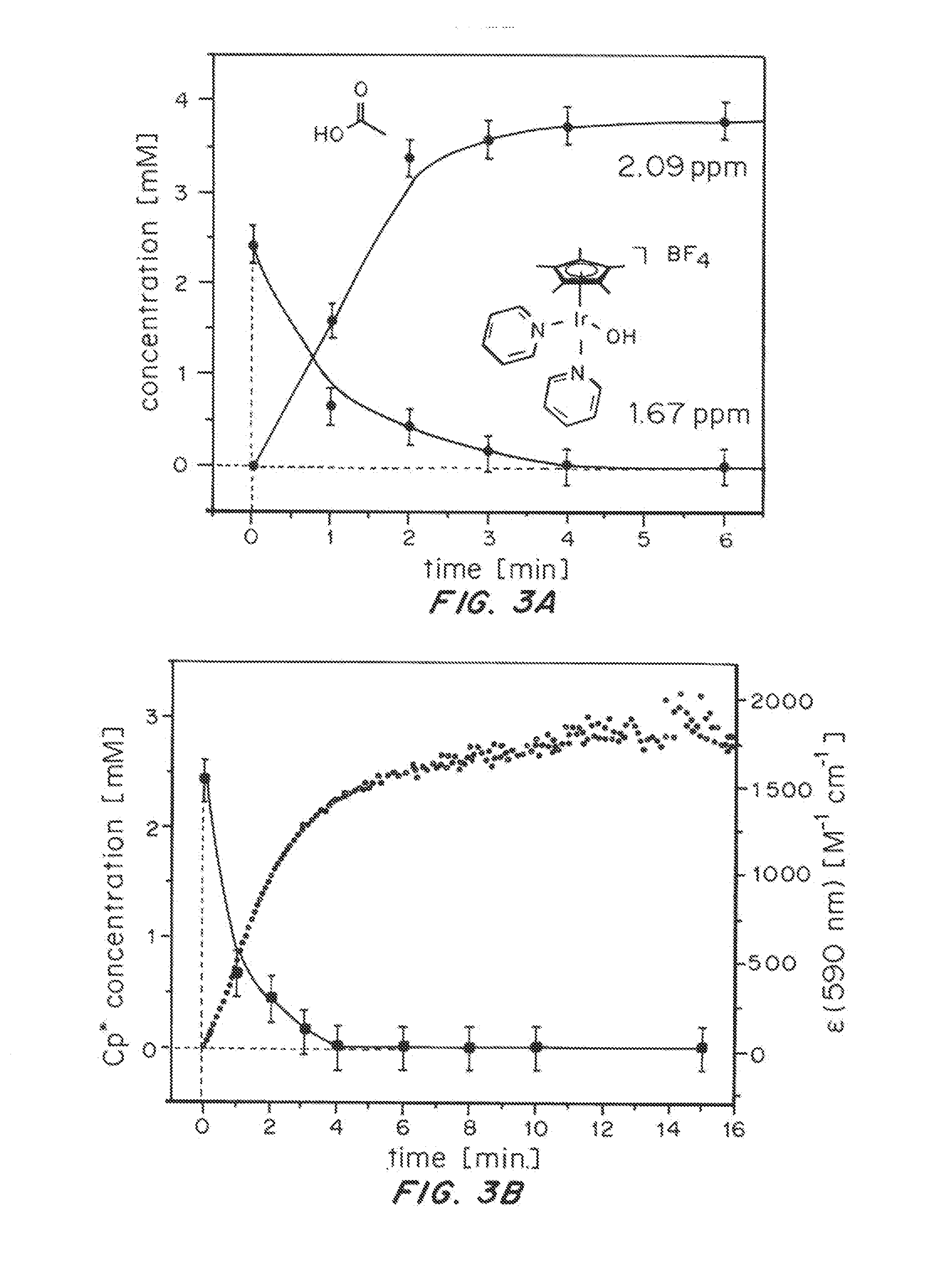
Iridium complexes for electrocatalysis
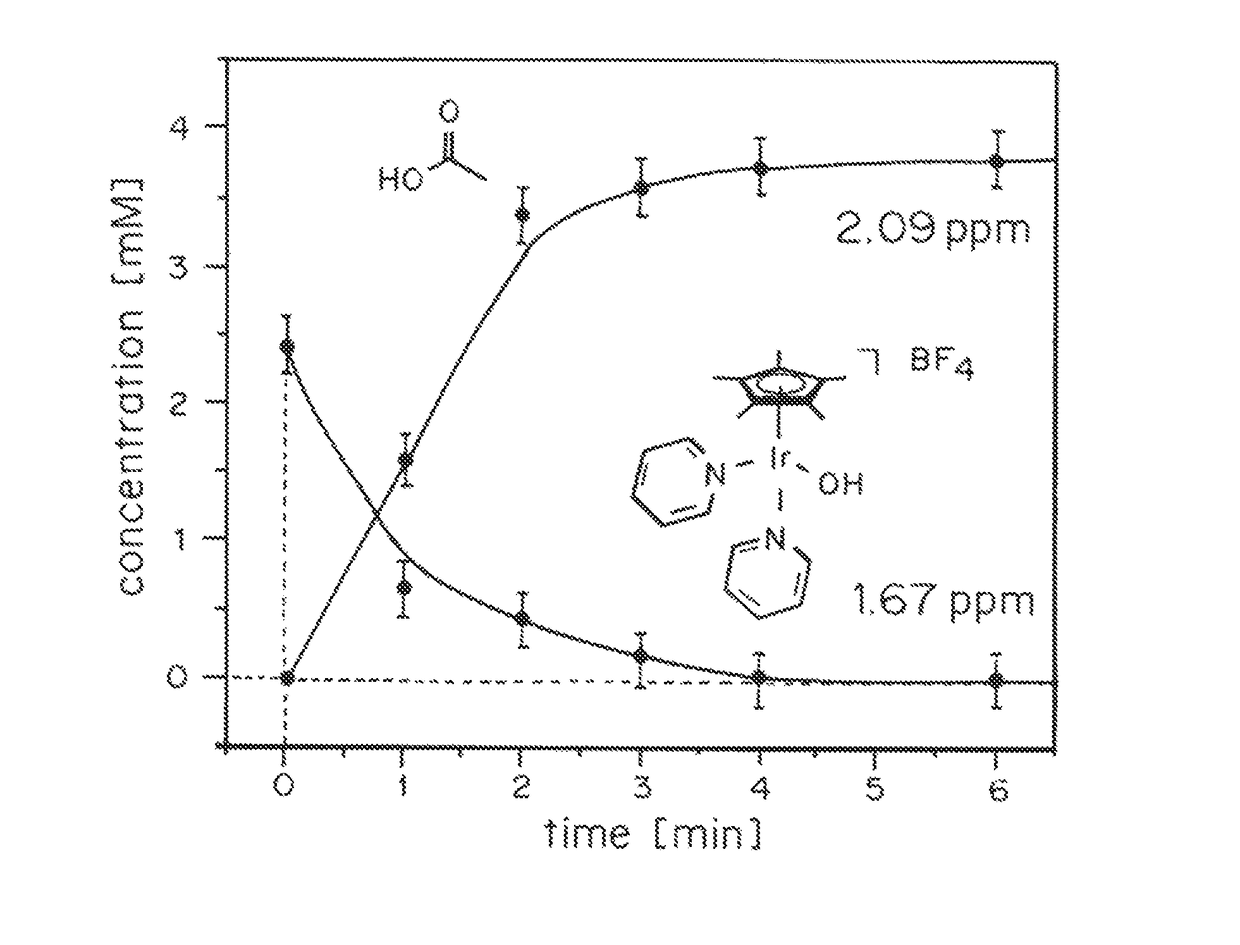
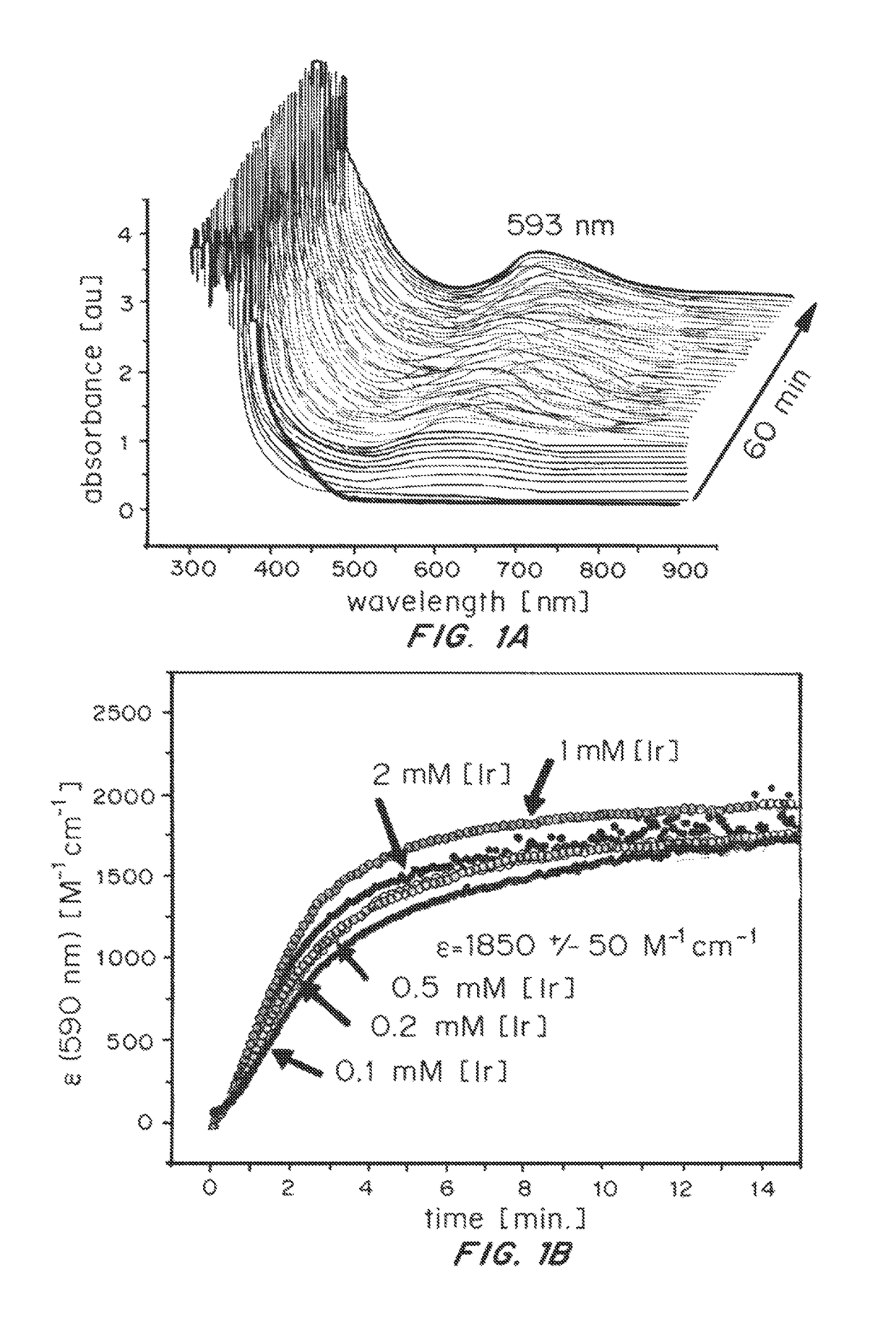
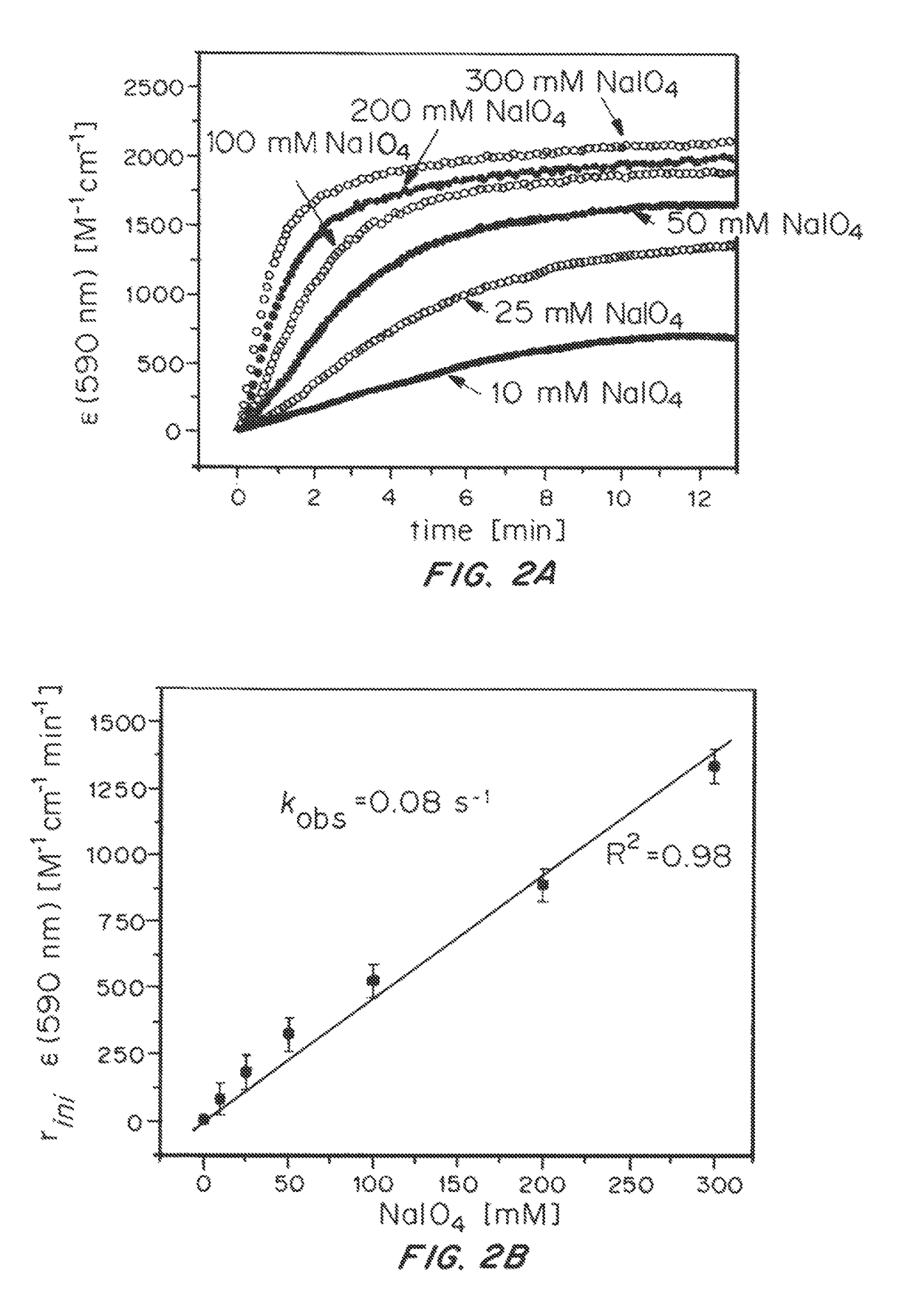
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
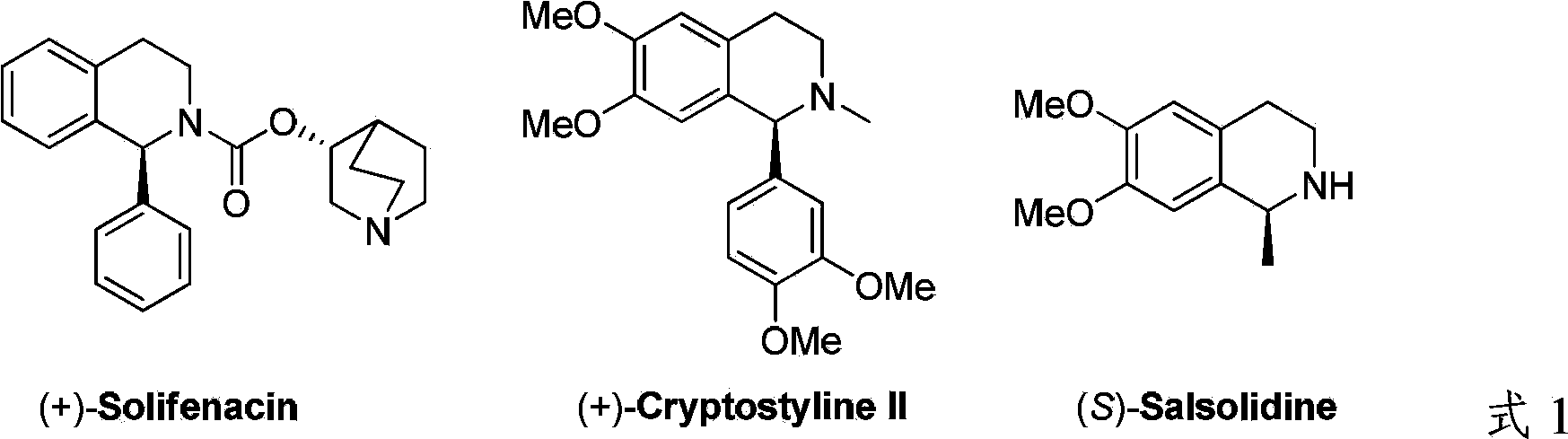
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Asymmetric hydrogenation synthetic method of chiral aromatic amine compound with high steric hindrance
InactiveCN103570484AThe hydrogenation reaction conditions are mildSmooth responseOrganic compound preparationCarboxylic acid amides preparationIridiumPotassium iodine
The invention relates to an asymmetric hydrogenation synthetic method of a chiral aromatic amine compound of iridium-catalyzed imine with high steric hindrance. The catalyst adopted is a catalyst generated by in-situ reaction of a 1, 5-cyclooctadiene iridium chloride dimer and a chiral phosphine-phosphoramidite ligand. The reaction can be carried out under the following condition: the additives comprise iodine, potassium iodide and tetrabutyl ammonium iodide; the temperature is 0-200 DEG C; the solvent is dichloromethane, 1, 2-dichloroethane to the like; the pressure is 10-100 barometric pressures; the time is 12-48 hours; and the proportion of a primer and the catalyst can reach 100000 / 1. The method provided by the invention has the characteristics of mild reaction condition, high activity, high stereoselectivity, wide application range of the primer and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Alpha-diimine nickel metal organic compound and preparation method thereof
InactiveCN104892681AHigh yieldReaction raw materials are cheap and easy to obtainOrganic chemistry methodsNickel organic compoundsDiethyl etherOrganic compound
The invention relates to an alpha-diimine nickel metal organic compound and a preparation method thereof. The preparation method comprises the following steps: adding NiBr2 (DME) and alpha-diimine into a dichloromethane solution for reaction under the protection of an inert atmosphere, reducing an obtained product in a diethyl ether or n-hexane solution by using metallic sodium, and then reacting with 1,5-cyclooctadiene to obtain a mononuclear nickel metal organic compound of alpha-diimine. The preparation method provided by the invention is simple, is high in product yield and good in purity and can be used as an olefin polymerization catalyst.
Owner:NORTHWEST UNIV(CN)
Method for synthesis of chiral piperidine derivative through iridium-catalyzed asymmetric hydrogenation of pyridine
ActiveCN103387533AHigh reactivityHigh enantioselectivityAsymmetric synthesesDiphosphinesIridium chloride
A method for synthesis of chiral piperidine derivatives through iridium-catalyzed asymmetric hydrogenation of pyridine employs a catalytic system of chiral diphosphine complex of iridium. The reaction can be carried out under the following conditions: temperature of 25-60 DEG C; a mixed solvent of toluene / dichloromethane (V / V=1:1); pressure of 13-50 barometric pressure; a ratio of substrate and catalyst of 50 / 1; and a catalyst of a complex of (1,5-cyclo-octadiene) iridium chloride dimer and diphosphine ligand. Hydrogenation of pyridine salt can obtain a corresponding chiral 2-substituted piperidine derivative, whose enantiomeric excess can reach 93%. The invention has advantages of simple and practical operation, easily available raw materials, high enantioselectivity, and good yield; in addition, the reaction has green atom economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Adhesive monomer and method for improving adhesion strength between dental zirconia and resin
InactiveCN103601753AHigh bonding strengthGood biocompatibilityImpression capsDentistry preparationsPolymer sciencePhosphate
The invention discloses an adhesive monomer and a method for improving adhesion strength between dental zirconia and resin. The adhesive monomer is prepared from 1, 5-cyclooctadiene by performing epoxidation, ring-opening reaction, redox, exterification and the like, the main chain of the adhesive monomer is a n-octane carbon chain with one end of acrylic ester and the other end of phosphate ester, the forth position and the fifth position on the carbon chain are respectively connected with a R group which represents hydroxyl, sulfydryl or carboxyl, and the adhesive monomer has the structural formula shown in the description. The adhesive monomer helps to improve the adhesion strength between dental zirconia ceramic and resin, has the same improving effect with common 10-MDP-containing zirconia primer coating products in current market, has relatively good biological compatibility, is qualified in cytotoxicity tests, and has important application value during tooth repairing processing.
Owner:NANJING UNIV +1
Method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation
A method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation is provided, wherein a used catalyst is a triphenylphosphine complex of iridium. A reaction can be carried out under the following conditions: the temperature is 40-60 DEG C; the solvent is 1,2-dichloroethane; the pressure is 20-50 atmospheric pressures; the ratio of a substrate to a catalyst is 100 to 1; and the catalyst is a complex of chloro(1,5-cyclooctadiene)iridium(I) dimer and triphenylphosphine. Hydrogenation of 3-hydroxypyridine benzyl bromide salt can obtain the 3-piperidone derivatives with excellent chemoselectivity, the highest yield can reach 97%, and the chemical selectivity of ketones and alcohols is greater than 20:1. The method has the advantages of simple and convenient operation, easily obtained raw materials, high chemoselectivity, and good yield, and provides an atom economic and environment-friendly route for synthesis of a series of 3-piperidone derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of high-selectivity pinane
InactiveCN105330505ASimple preparation processReduce energy consumptionHydrocarbon by hydrogenationOrganic-compounds/hydrides/coordination-complexes catalystsChemical industryFiltration
The invention relates to a preparation method of high-selectivity pinane, and belongs to the field of chemical industry. The method comprises the technological steps: heating RhCl3.3H2O to be dissolved in an ethanol solution, adding 1,5-cyclooctadiene (COD) according to the molar ratio of 1,5-cyclooctadiene to rhodium of 3 to 7, backflowing for a certain period of time, then carrying out suction filtration on the mixture, then washing with anhydrous ethanol, and drying to obtain a rhodium complex [Rh(COD)Cl]2; and adding the prepared rhodium complex [Rh(COD)Cl]2 in a high pressure reaction kettle with the amount 1-7% of the mass of turpentine oil, covering a lid, sealing, respectively replacing with nitrogen gas and hydrogen gas, carrying out pressure maintaining and leakage detection, adjusting the reaction kettle pressure to be 1-5 MPa, and carrying out a reaction for 1-5 h at the reaction temperature of 30-70 DEG C. The method has the advantages of mild reaction condition, simple technological process, low energy consumption, high alpha-pinene conversion rate, and high selectivity of the product cis-pinane.
Owner:KUNMING UNIV OF SCI & TECH
Iridium complexes for electrocatalysis
ActiveUS9790605B2Improve stabilityConvenient bindingIndium organic compoundsCell electrodesIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
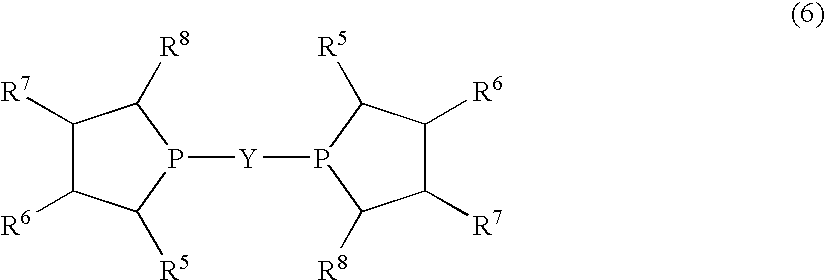
Method for producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid
InactiveUS7772426B2Efficient productionHigh enantiomeric excessOrganic compound preparationGroup 5/15 element organic compoundsHydrogen atomAsymmetric hydrogenation
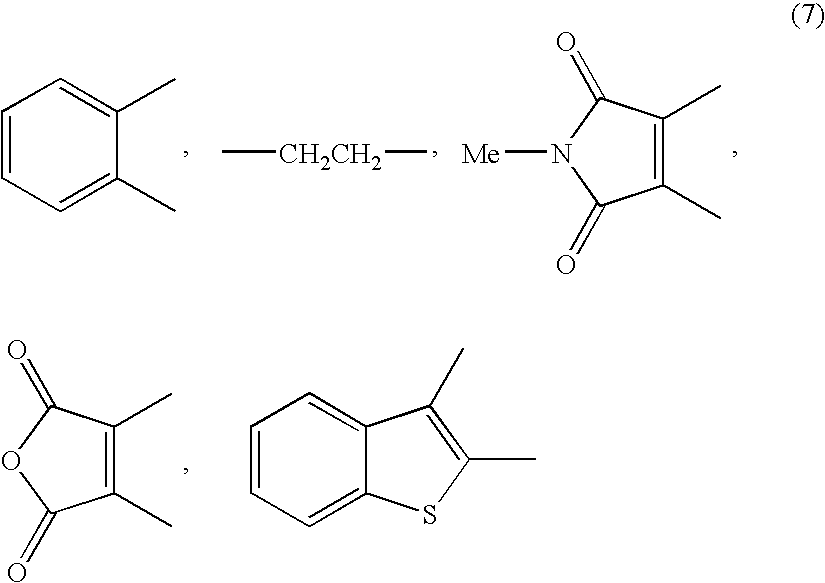
Disclosed is a method for efficiently and highly selectively producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid, which is useful as a herbicide, through a catalytic asymmetric synthesis reaction. Specifically disclosed is a method for producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid which is characterized in that a dehydroamino acid is subjected to an asymmetric hydrogenation by using a rhodium catalyst represented by the formula (2) below and having an optically active cyclic phosphine ligand, and then the resulting product is subjected to hydrolysis:[Rh(R4)(L)]X (2)[where R4 represents 1,5-cyclooctadien or norbornadien; L represents a substance represented by the following formula (6):(wherein R5 and R8 respectively represent a C1-4 alkyl group; R6 and R7 respectively represent hydrogen atom or hydroxyl group; and Y represents a group selected from groups represented by the following formula (7):(where Me represents methyl group)).].
Owner:MEIJI SEIKA KAISHA LTD
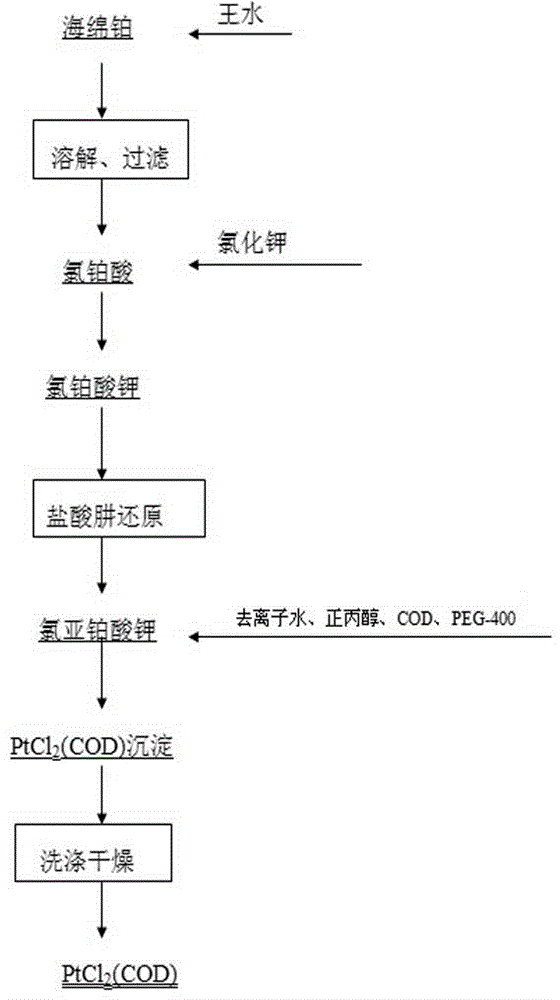
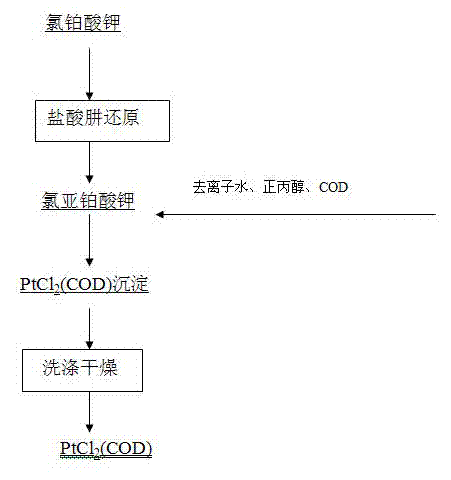
Preparation method of dichloro(1,5-cyclooctadiene) platinum (II)
InactiveCN104926884AReduce dosageShorten the timeGroup 8/9/10/18 element organic compoundsRotary evaporatorPotassium tetrachloroplatinate
The invention relates to a preparation method of dichloro(1,5-cyclooctadiene) platinum (II). The preparation method comprises the following steps: reacting potassium tetrachloroplatinate and ligand 1,5-cyclooctadiene (COD) with a n-propyl alcohol mixed solution in water by adding a phase transfer catalyst PEG-400 to generate the milk white precipitate dichloro(1,5-cyclooctadiene) platinum (II), wherein the mole ratio of the potassium tetrachloroplatinate to the ligand 1,5-cyclooctadiene (COD) is 1: 6.4, the volume ratio of n-propyl alcohol to deionized water is 1: 0.1, and the volume ratio of PEG-400 to COD is 1.0: 1; maintaining the reaction for 4h at the reaction temperature of 50 DEG C, performing suction filtration on the milk white precipitate, washing with absolute ethyl alcohol and water, and drying to obtain the reaction product dichloro(1,5-cyclooctadiene) platinum (II). The product yield is 90% to 95%. A solvent and platinum are recycled respectively after the treatment of a filter liquor through a rotary evaporator.
Owner:JINCHUAN GROUP LIMITED
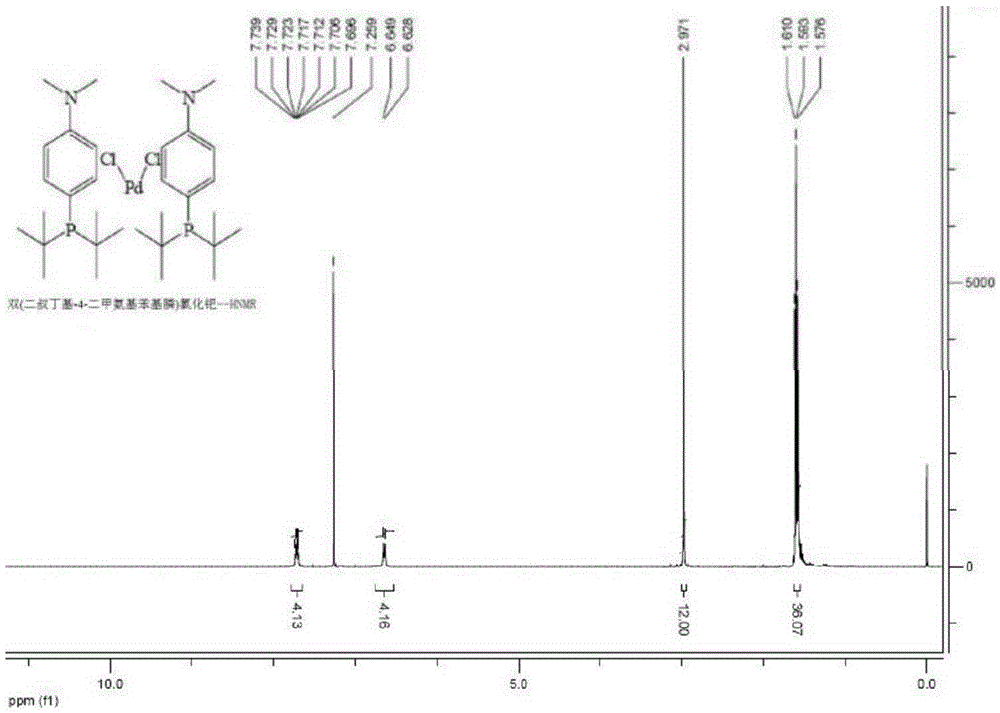
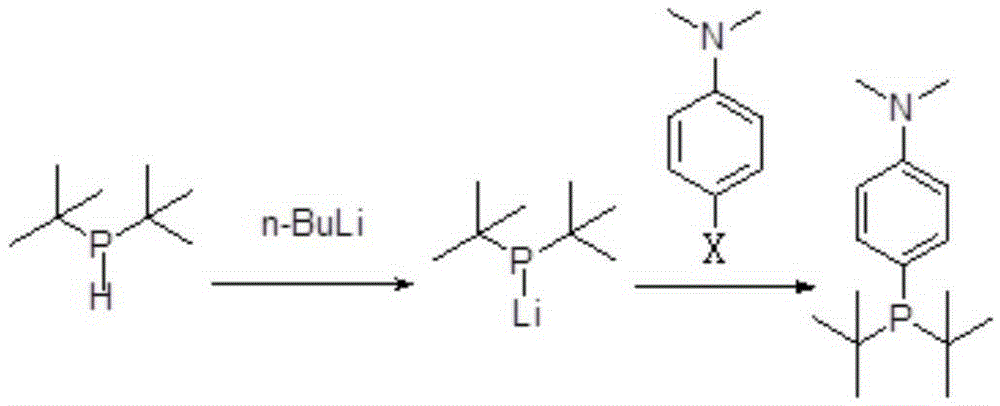
Preparing method for di-tertiary butyl-4-dimethylamino phenylphosphine and bis(di-tertiary butyl-4-dimethylamino phenylphosphine) palladium chloride
ActiveCN105237568AReduce manufacturing costReduce lossGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsAcetonitrilePhenylphosphine
The invention provides a preparing method for di-tertiary butyl-4-dimethylamino phenylphosphine. Di-tertiary butylphosphine is subjected to low-temperature lithiation under shielding of inert gas and then reacts with N,N-dimethyl p-chloroaniline, and di-tertiary butyl-4-dimethylamino phenylphosphine is obtained. According to the preparing method, di-tertiary butyl-4-dimethylamino phenylphosphine is purified after a quenching reaction. The invention further provides a preparing method for bis(di-tertiary butyl-4-dimethylamino phenylphosphine) palladium chloride. Di-tertiary butyl-4-dimethylamino phenylphosphine obtained through the preparing method is further subjected to complexation with (1,5-cyclooctadiene) palladium dichloride or bispalladium dichloride. A di-tertiary butyl-4-dimethylamino phenylphosphine ligand is prepared through the novel method, purified and then subjected to palladium complexation, and the preparing yield is greatly increased; meanwhile, the loss of palladium is reduced, the preparing cost is greatly reduced, and the obtained products are high in purity and good in quality.
Owner:BEIJING GREENCHEM TECH
Metallisation
InactiveUS20050227181A1Improve conductivityConductive layers on insulating-supportsMolten spray coatingElectrical conductionMetal
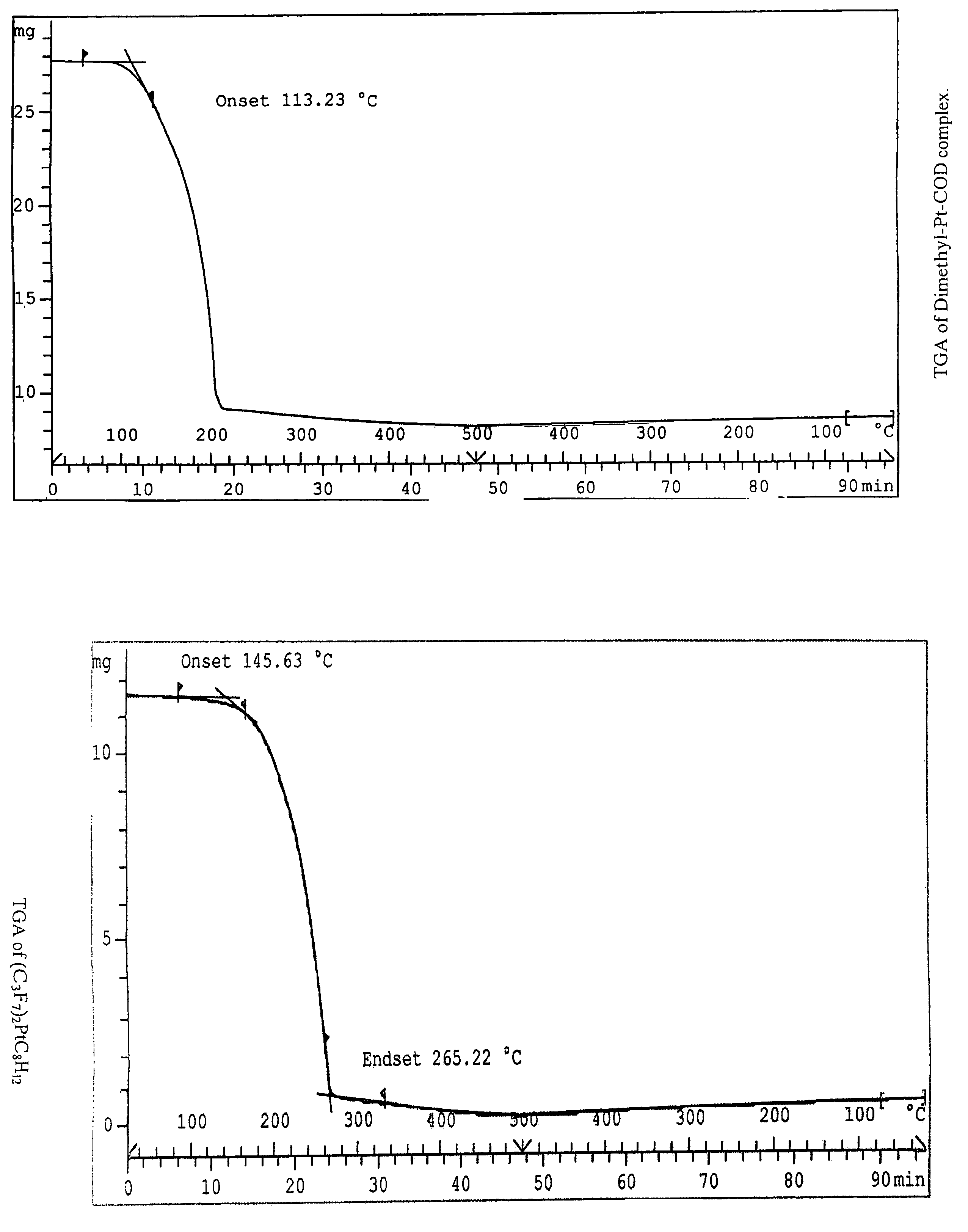
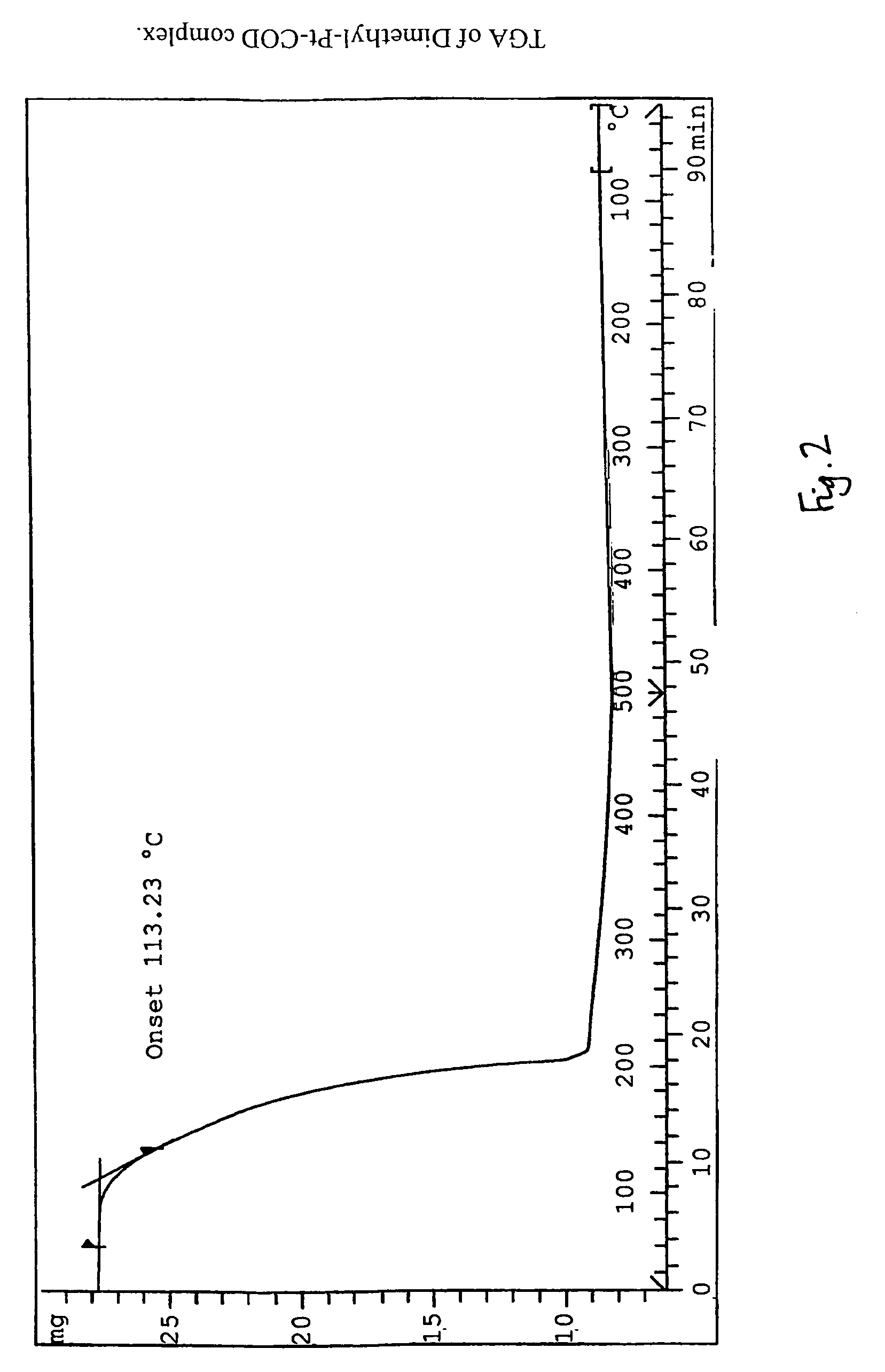
This invention relates to photosensitive organometallic compounds which are used in the production of metal deposits. In particular, this invention relates to photosensitive organometallic compounds such as bis-(perfluoropropyl)-1,5-cyclooctadiene platinum (II) (i.e. (C3F7)2PtC8H12) which on exposure to UV radiation and then a reduction process forms a platinum metal deposit such as a substantially continuous thin ‘sheet-like’ film or a substantially narrow line which is capable of electrical conduction.
Owner:CEIMIG
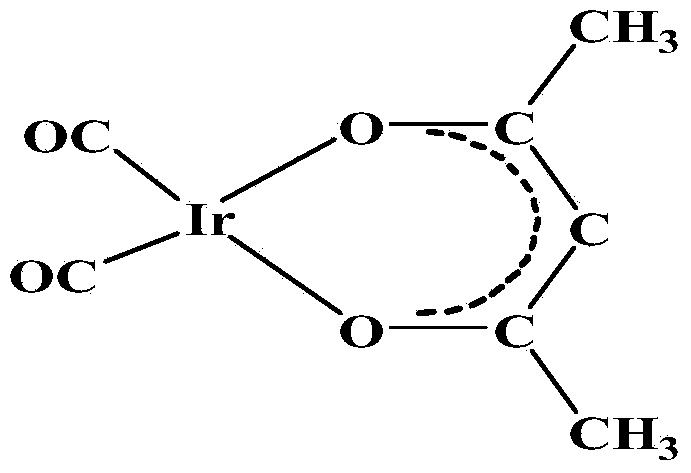
Method for preparing dicarbonyl iridium acetylacetonate (I)
InactiveCN104230999AShort reaction timeHigh yieldGroup 8/9/10/18 element organic compoundsIridium chlorideHomogeneous catalysis
The invention discloses a method for preparing dicarbonyl iridium acetylacetonate (I). Dicarbonyl iridium acetylacetonate (I) is a precursor compound for preparing an iridium coating by MOCVD (metal-organic chemical vapor deposition), and is also a common carbonyl addition homogeneous catalyst. The method mainly comprises the following steps: (1) reacting iridous chloride hydrate and 1,5-cyclooctadiene to generate an intermediate (1,5-cyclooctadiene) iridium chloride (I) dimer; and (2) reacting the intermediate and carbon monoxide to introduce carbonyl group, reacting with acetylacetone to obtain the dicarbonyl iridium acetylacetonate (I). The preparation method has the advantages of simple technical process, high yield and high product purity, and is suitable for mass preparation of [Ir(CO)2(acac)].
Owner:KUNMING INST OF PRECIOUS METALS
Method for preparing dichloro(1,5-cyclooctadiene)platinum(II)
InactiveCN102127117AReduce wasteAvoid damageGroup 8/9/10/18 element organic compoundsPotassium tetrachloroplatinateFiltration
The invention provides a method for preparing dichloro(1,5-cyclooctadiene)platinum(II) and relates to a method for preparing a platinum group metal compound which is used in an addition reaction of silicon and hydrogen for preparing an organic silicon compound and takes 1,5-cyclooctadiene as the ligand. The method is characterized in that a preparation process comprises reacting potassium chloroplatinite with 1,5-cyclooctadiene (COD) serving as the ligand in mixed solvent of water and normal propyl alcohol to obtain dichloro(1,5-cyclooctadiene)platinum(II) milky precipitate and obtaining the reaction product dichloro(1,5-cyclooctadiene)platinum(II) by suction filtration, washing with absolute ethanol and water and drying, wherein the temperature is controlled to be 60 to 90 DEG C in a reaction process, and the reaction time is 3 to 4 hours. The key technical point of the preparation method provided by the invention is to raise the reaction temperature to 60 to 90 DEG C and reduce the reaction time to 3 to 4 hours from 48 hours reported in documents. Thus, the reaction time is reduced effectively, and raw material waste and environmental damage are reduced.
Owner:JINCHUAN GROUP LIMITED
Preparation method of 1,5-cyclooctadiene
InactiveCN108002970AReduce generationHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsHydrocarbon solventsTower
The invention discloses a preparation method of 1,5-cyclooctadiene. In water-free inert environment in a pressure reaction kettle, under the conditions of the temperature being 80 to 120 DEG C and thesurface pressure being 0.10 to 0.60MPa, 1,3-butadiene is added into an inert hydrocarbon solvent dissolved with Ni-Al-P system catalysts for many times; a reaction of performing 1,3-butadiene cyclizing dimerization to synthesize 1,5-cyclooctadiene is performed; after the reaction is completed, synthesis liquid is transferred into a rectifying tower to be subjected to negative pressure refining toobtain a finished product of 1,5-cyclooctadiene. Generally, the preparation method has the advantages that the reaction conditions are mild; the operation is simple and convenient; the raw material conversion rate is high; the reaction selectivity is high; the operation links can be effectively reduced; the synthesis efficiency is improved; the yield is high; the pollution is small; the product quality is high; the use value is very high.
Owner:濮阳盛华德化工有限公司
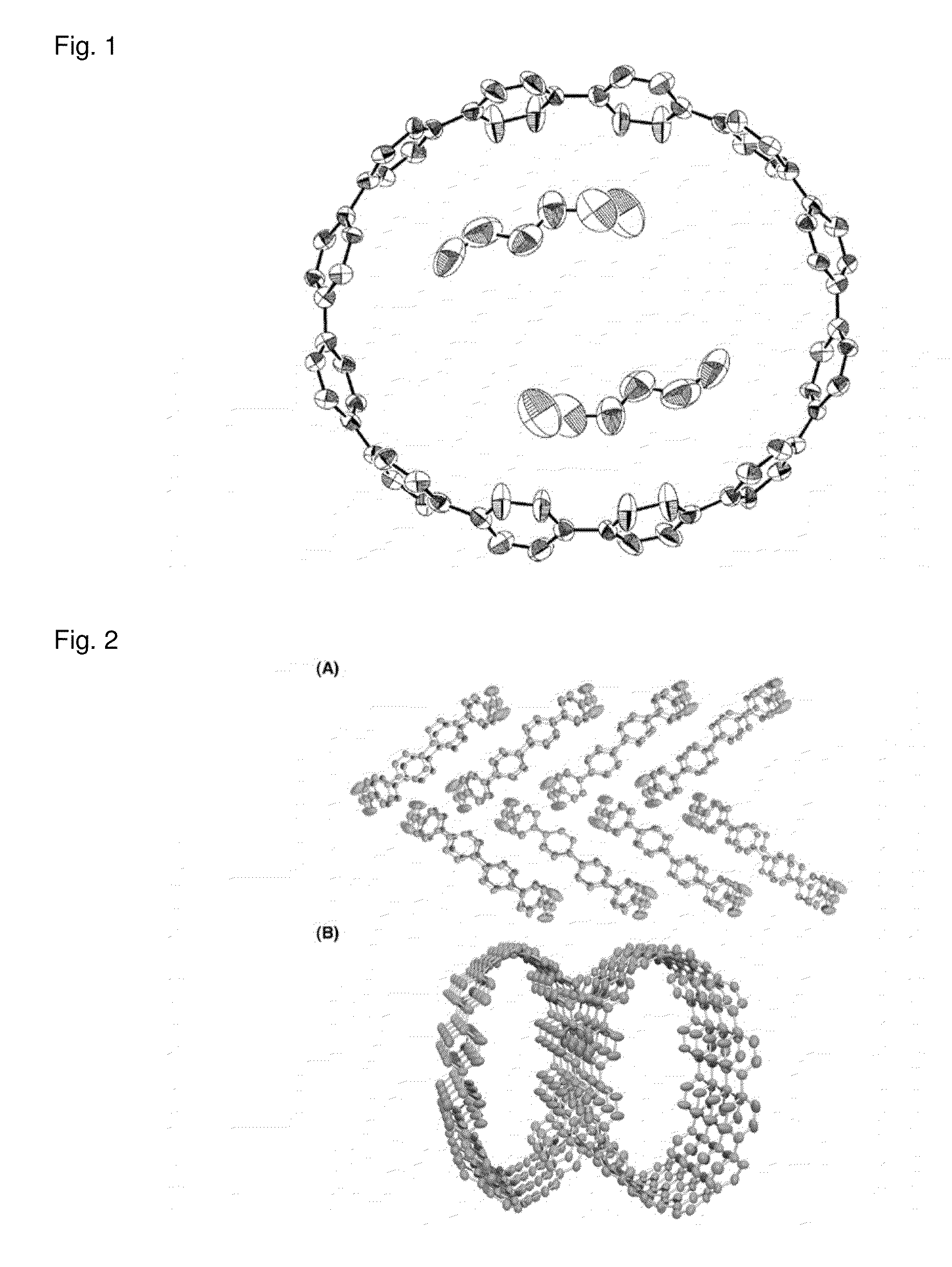
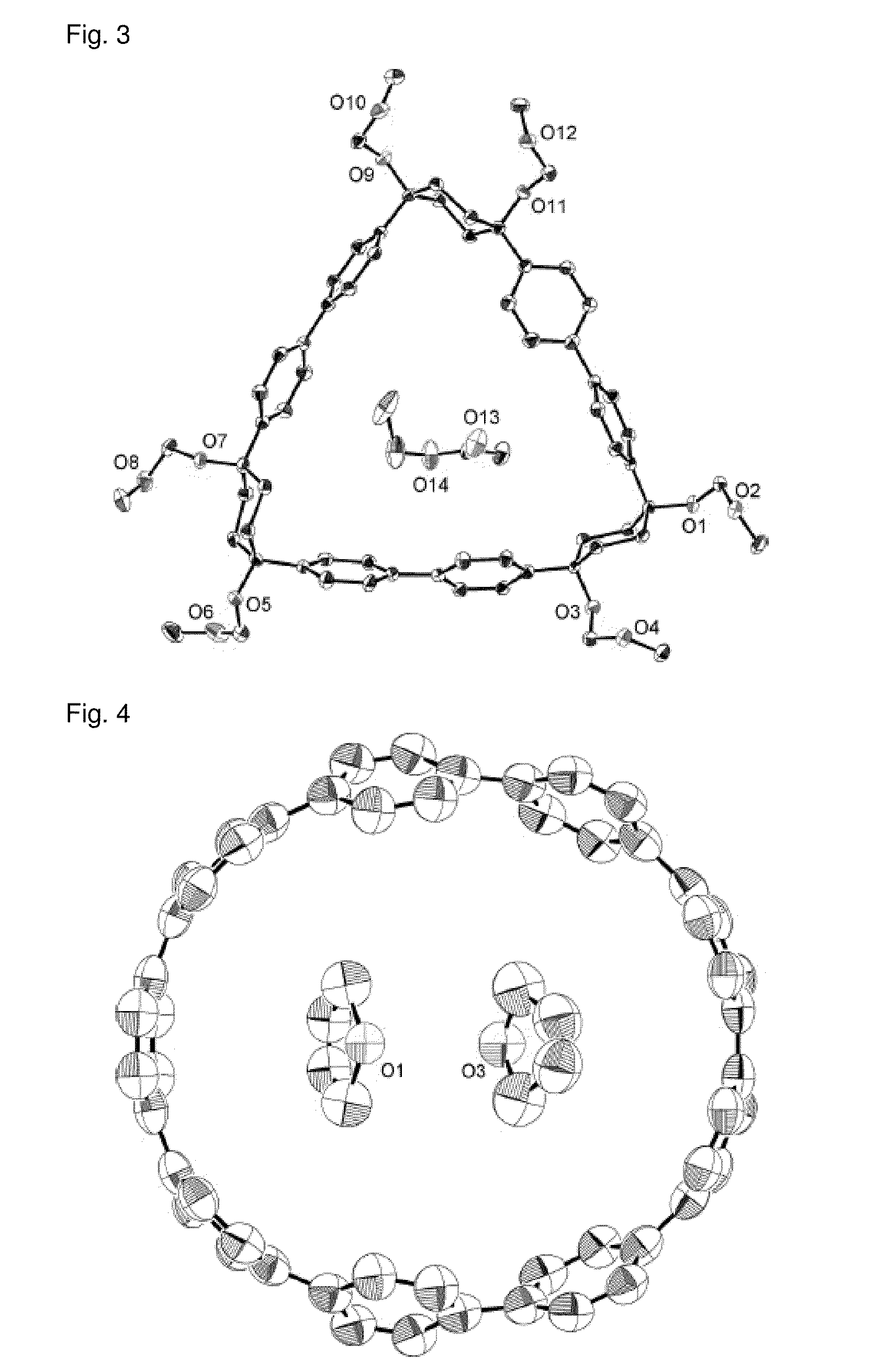
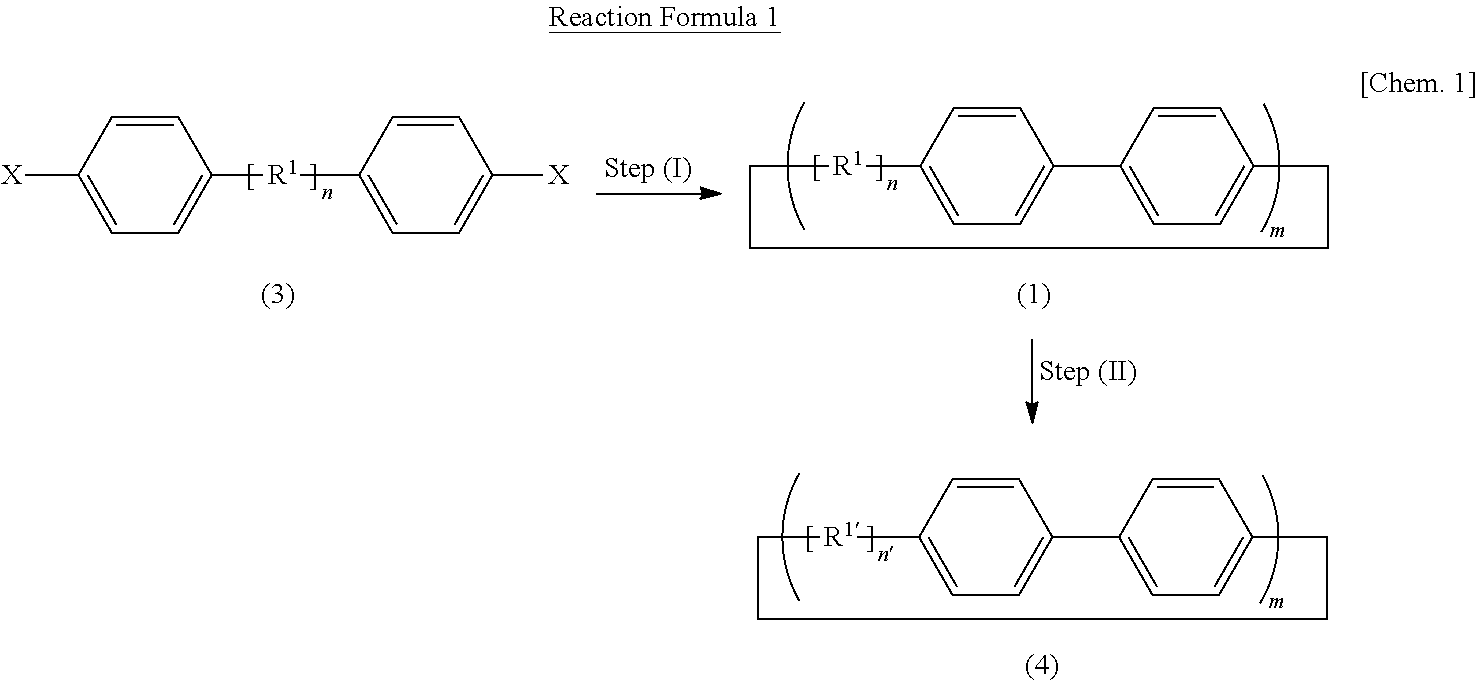
Carbon nanoring and method for producing a ring-shaped compound suitable as a starting material for production of the same
InactiveUS20130324768A1Short production processLow production costHydrocarbon by dehydrogenationOrganic compound preparationBenzeneHalogen
The present invention produces Cyclic Compound (1) in which organic ring groups including cyclohexane rings and benzene rings are continuously bonded, using a compound having at least one cyclohexane ring and benzene rings with halogen atoms at the two terminuses, in the presence of a nickel compound (bis(1,5-cyclooctadiene)nickel, etc.). Thereafter, by converting the cyclohexane rings in Cyclic Compound (1) into benzene rings, a desired carbon nanoring can be obtained. Thereby, the present invention efficiently produces a carbon nanoring made of a compound having a cyclic structure in which a desired number of organic ring groups are continuously bonded, with a short production process.
Owner:NAGOYA UNIVERSITY
Carbon nanoring and method for producing a ring-shaped compound suitable as a starting material for production of the same
InactiveCN102822123AShort processEfficient preparationOrganic compound preparationCatalystsHalogenChemical compound
Owner:NAGOYA UNIVERSITY
Metallisation
InactiveUS7410900B2Conductive layers on insulating-supportsMolten spray coating1,5-CyclooctadieneMetal
This invention relates to photosensitive organometallic compounds which are used in the production of metal deposits. In particular, this invention relates to photosensitive organometallic compounds such as bis-(perfluoropropyl)-1,5-cyclooctadiene platinum (II) (i.e. (C3F7)2PtC8H12) which on exposure to UV radiation and then a reduction process forms a platinum metal deposit such as a substantially continuous thin ‘sheet-like’ film or a substantially narrow line which is capable of electrical conduction.
Owner:CEIMIG
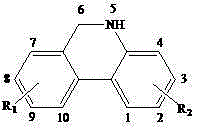
5,6-dihydrophenanthine compound and preparation method thereof
The invention relates to a 5,6-dihydrophenanthridine compound and a preparation method thereof. The formula of the compound is shown in the specification. The preparation method of the compound comprises the following steps of adding o-bromoarylamine and aryl methanol (or o-bromo aryl methanol and aryl amine), [Ir (cod) Cl] 2 (cod is equal to 1,5-cyclooctadiene), a palladium salt, a silver salt, tricyclohexyl phosphine and the base into a organic solvent, heating for reflux under the protection of N2, after the reaction is completed, filtering, evaporating to dryness, and recrystallizing to obtain the 5,6-dihydrophenanthridine compound. The 5,6-dihydrophenanthridine compound is synthesized in one step by using the method and the method has the advantages of mild reaction conditions, high yield, cost-effective reaction and wide application prospects.
Owner:LUOYANG NORMAL UNIV
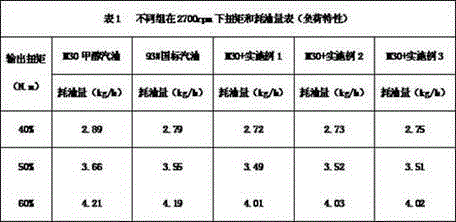
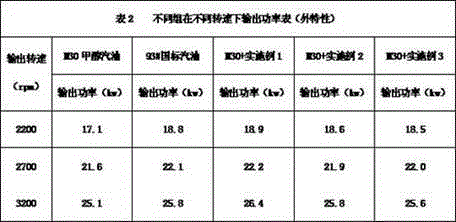
A kind of methanol gasoline power accelerator
InactiveCN104263434BNo pollution in the processSignificant power enhancement effectLiquid carbonaceous fuelsN-Propyl alcoholFatty amine
The invention provides a methanol gasoline power promoter. The methanol gasoline power promoter consists of isooctanol, diethoxyethylamine, 1,5-cyclooctadiene, 2-acetylbenzofuran, propargyl alcohol, n-propyl alcohol, zinc naphthenate, dinonylnaphthalene sulfonic acid, di(polyoxyethylene) fatty amine and pentamethyldiethylenetriamine. A preparing method of the methanol gasoline power promoter comprises the following steps: a, adding the isooctanol, the diethoxyethylamine, the 1, 5-cyclooctadiene and the 2-acetylbenzofuran into a reactor, and carrying out airtight ultrasonic agitation reaction at a temperature of 60-62 DEG C for 40-50 minutes; b, mixing the n-propyl alcohol, the zinc naphthenate and the dinonylnaphthalene sulfonic acid, and agitating for 15-25 minutes; c, sequentially adding the propargyl alcohol, a mixture in the step b, the di(polyoxyethylene) fatty amine and the pentamethyldiethylenetriamine into a mixture in the step a, agitating at a temperature of 33-35 DEG C for 20-25 minutes and cooling to obtain the methanol gasoline power promoter.
Owner:上海给力石油化工有限公司
A kind of preparation method of 1,5-cyclooctadiene iridium chloride dimer
ActiveCN106220688BHigh yieldReduce processing lossOrganic-compounds/hydrides/coordination-complexes catalystsMetallocenesIridiumOxygen
The invention relates to the field of noble metal catalyst synthesis, and discloses a method for preparing chloro(1,5-cyclooctadiene)iridium(I) dimer. The method includes the step that under the oxygen-free condition, an iridium-containing and chlorine-containing compound, aldehyde, 1,5-cyclooctadiene and solvent are subjected to a contact reaction. Traditionally used reactants (alcohol) are abandoned, and new reactants (aldehyde) are used, so the yield of chloro(1,5-cyclooctadiene)iridium(I) dimer can be as high as 75% in short reaction time. Consumption of water and 1,5-cyclooctadiene is low, waste liquid treatment loss is greatly reduced, and production cost is reduced.
Owner:NUTRICHEM LAB CO LTD
Methanol gasoline power promoter
InactiveCN104263434ANo pollution in the processSignificant power enhancement effectLiquid carbonaceous fuelsN-Propyl alcoholFatty amine
The invention provides a methanol gasoline power promoter. The methanol gasoline power promoter consists of isooctanol, diethoxyethylamine, 1,5-cyclooctadiene, 2-acetylbenzofuran, propargyl alcohol, n-propyl alcohol, zinc naphthenate, dinonylnaphthalene sulfonic acid, di(polyoxyethylene) fatty amine and pentamethyldiethylenetriamine. A preparing method of the methanol gasoline power promoter comprises the following steps: a, adding the isooctanol, the diethoxyethylamine, the 1, 5-cyclooctadiene and the 2-acetylbenzofuran into a reactor, and carrying out airtight ultrasonic agitation reaction at a temperature of 60-62 DEG C for 40-50 minutes; b, mixing the n-propyl alcohol, the zinc naphthenate and the dinonylnaphthalene sulfonic acid, and agitating for 15-25 minutes; c, sequentially adding the propargyl alcohol, a mixture in the step b, the di(polyoxyethylene) fatty amine and the pentamethyldiethylenetriamine into a mixture in the step a, agitating at a temperature of 33-35 DEG C for 20-25 minutes and cooling to obtain the methanol gasoline power promoter.
Owner:上海给力石油化工有限公司
Method for preparing carbon nanoring and cyclic compound suitable for use as raw material thereof
InactiveCN102822123BShort processEfficient preparationOrganic compound preparationCatalystsBenzeneHalogen
In the present invention, in the presence of a nickel compound (bis(1,5-cyclooctadiene)nickel, etc.), a compound containing at least one cyclohexane ring portion with benzene rings to which halogen atoms are attached at both ends is used , a cyclic compound (1) in which organic ring groups including a cyclohexane ring and a benzene ring are continuously bonded can be formed. Then, the desired carbon nanoring can be obtained by converting the cyclohexane ring part of the cyclic compound (1) into a benzene ring part. According to this, a carbon nanoring composed of a cyclic structure compound in which a desired number of organic ring groups are continuously bonded can be efficiently produced in a short process.
Owner:NAGOYA UNIVERSITY
A kind of recovery regeneration method of 1,5-cyclooctadiene iridium chloride dimer
ActiveCN104140450BHigh purityIncrease profitOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingAlcoholPresent method
The invention discloses a 1,5-cyclooctadiene iridium chloride dimer [(COD)IrCl] 2 The recovery and regeneration method: the inactivated or partially inactivated [(COD)IrCl] 2 Convert in hydrochloric acid to obtain iridium chloride mixture, react iridium chloride mixture and 1,5-cyclooctadiene in alcohol-water system to regain activity [(COD)IrCl] 2 compound. The recovery and regeneration method of 1,5-cyclooctadiene iridium chloride dimer disclosed by the invention can improve the utilization rate of precious metal iridium and effectively reduce production cost. The method has simple operation process, high recovery rate and mild conditions. The invention is suitable for the recovery of precious metal iridium catalysts.
Owner:XIAN MODERN CHEM RES INST
Method for recycling [(COD)IrCl]2
ActiveCN104140450AHigh purityIncrease profitOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingAlcoholCyclooctadiene iridium chloride dimer
The invention discloses a method for recycling [(COD)IrCl]2. By the adoption of the method for recycling [(COD)IrCl]2, inactive [(COD)IrCl]2 or partially inactive [(COD)IrCl]2 is converted in hydrochloric acid, so that an iridium chloride mixture is obtained, the iridium chloride mixture and 1,5-cyclooctadiene react in an alcohol-water system, so that an active [(COD)IrCl]2 compound is obtained again. By the adoption of the method for recycling [(COD)IrCl]2, the utilization rate of the noble metal iridium can be increased, and the production cost can be effectively reduced; in addition, the operation process is simple, the recycling rate is high, and conditions are gentle. The method for recycling [(COD)IrCl]2 is suitable for recycling of iridium catalysis, wherein iridium is noble metal.
Owner:XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com