Method for preparing carbon nanoring and cyclic compound suitable for use as raw material thereof
一种环状化合物、碳纳米环的技术,应用在有机化合物的制备、碳化合物催化剂、从含氧有机化合物制烃等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
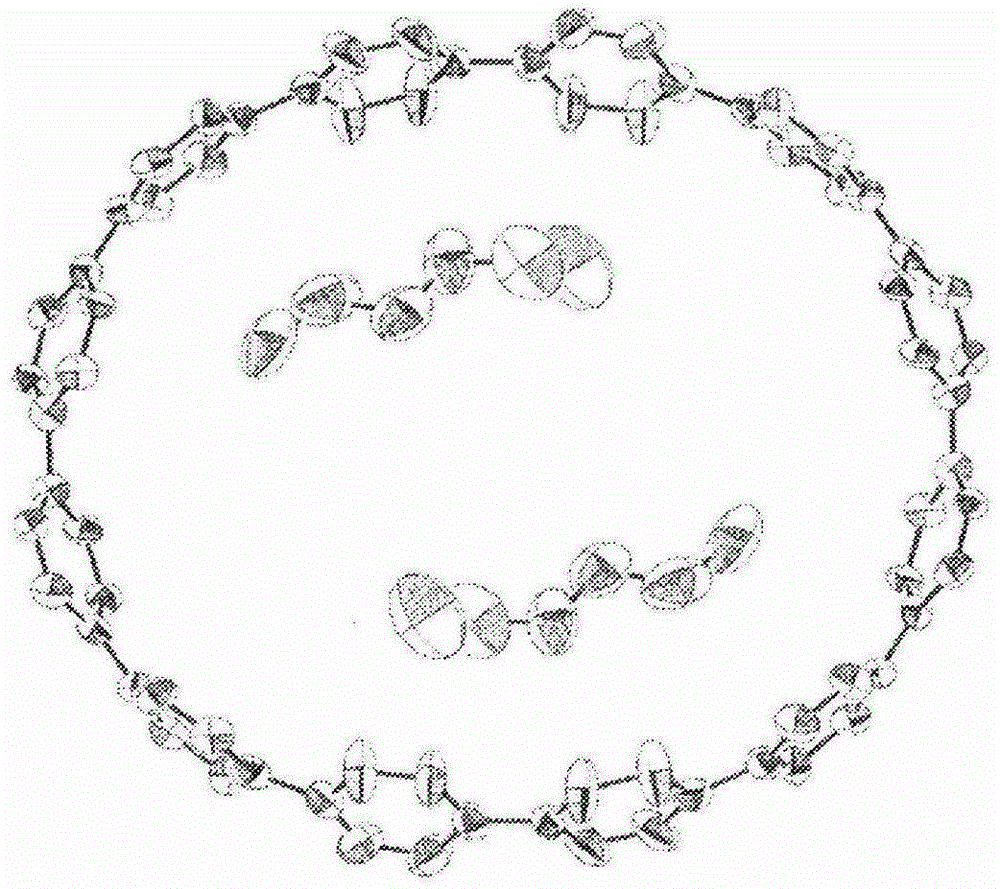
[0116] [1] Production method of cyclic compound (1)
[0117] The production method of the cyclic compound (1) of the present invention comprises a step (I): forming the compound (1) using the compound (3) in the presence of a nickel compound.
[0118] In this step (I), a plurality of compounds (3) of the same type are bonded (self-coupling) to form a cyclic compound (Z). The bonding between multiple and similar compounds (3) in the step (I) is a well-known bonding reaction called Yamamoto coupling. The compound (3) has two halogen atoms, and by using a nickel compound, the carbon atoms bonded to the halogen atoms can be bonded to each other, that is, the carbon atoms bonded to the halogen atoms in a compound (3), It is bonded to a carbon atom bonded to a halogen atom in another compound (3). Accordingly, the coupling reaction between the compounds (3) can be continuously carried out, and the carbon atoms can be bonded to each other to obtain the cyclic compound (1).
[0119...
Embodiment
[0326] Hereinafter, the present invention will be specifically described by way of examples, but the present invention is not limited by these examples. In addition, in the synthesis examples and examples, NMR measurement was performed with a nuclear magnetic resonance apparatus (A-400) (model name) manufactured by JEOL Corporation.
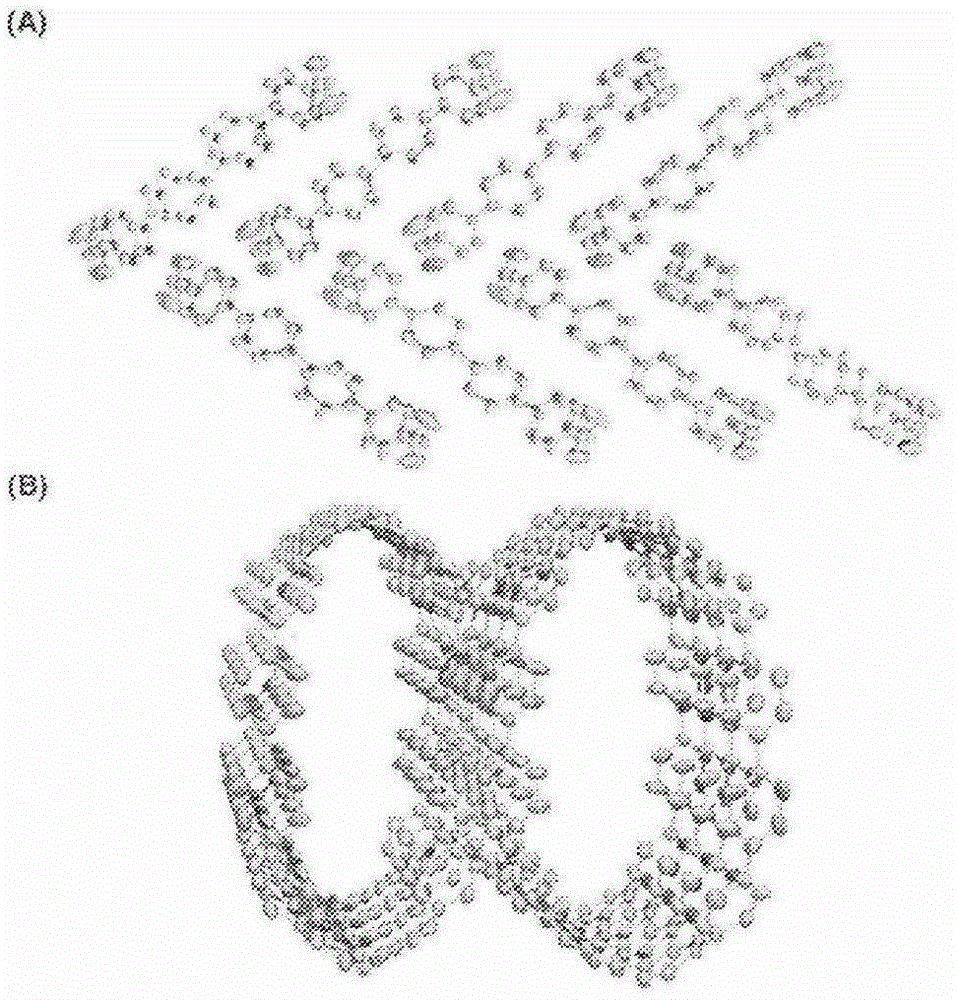
[0327] In this example, carbon nanorings composed of cycloparaphenylene were prepared. First, after preparing cis-1,4-bis(4-iodophenyl)-1,4-cyclohexanediol (Synthesis Example 1~3), the cis-1,4-bis(4-iodophenyl) )-1,4-cyclohexanediol to prepare cis-1,4-bis(4-iodophenyl)-1,4-bis(methoxymethyl ether)cyclohexane (synthesis example 4). Next, carbon nanorings were prepared using this cis-1,4-bis(4-iodophenyl)-1,4-bis(methoxymethyl ether)cyclohexane (Examples 1 to 3). Furthermore, the cycloparaphenylene compounds obtained in Examples 1 to 3 were crystallized and analyzed for their structures. In addition, in Examples 4 to 5, a cyclo-paraphenylene com...
Synthetic example 1
[0328] Synthesis Example 1: cis-1,4-bis(4-iodophenyl)-1,4-cyclohexanediol (compound (3a-1a)) Synthesis of (one)
[0329] Add 1,4-diiodobenzene (49.5 g, 150 mmol) and anhydrous tetrahydrofuran (300 cm 3 ), after the solution was obtained, it was cooled to -78°C. Then, slowly add to this solution (adding speed 3cm 3 / min) hexane solution of n-butyllithium (93.8cm 3 , 1.6M, 150mmol), after the addition, keep the temperature (-78°C) and stir for 1 hour. Then, while stirring the reaction solution, 1,4-cyclohexadiene (5.68 g, 50 mmol) in anhydrous tetrahydrofuran (160 cm 3 ) solution, reacted at -78°C for 1 hour, and further reacted at room temperature (25°C) for 2 hours. After the reaction, distilled water (100 cm 3 ) and ethyl acetate (500cm 3 ), and put the mixture into a separatory funnel. By the extraction operation using this separatory funnel, two layers consisting of an ethyl acetate layer (i) and a water layer were separated. After recovering the ethyl acetate l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com