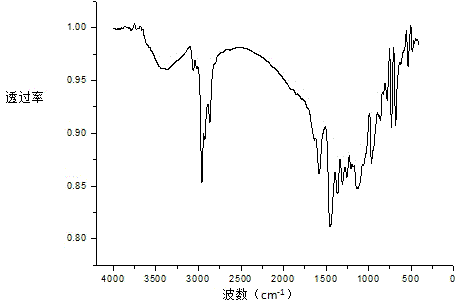
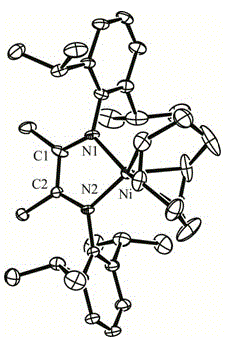
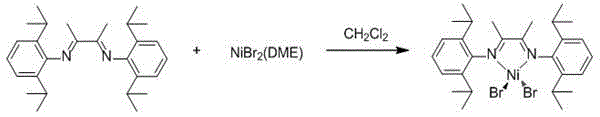Alpha-diimine nickel metal organic compound and preparation method thereof
An organic compound, nickel diimide technology, applied in nickel organic compounds, organic chemical methods, organic chemistry, etc., can solve the problems of catalytic principle and catalytic structure optimization research obstacles, catalytic reaction steps are not very clear, etc., to achieve atomic utilization The effect of high yield, high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Preparation of α-diimine nickel bromide complex
[0025] Under inert gas conditions, add NiBr 2 (DME) (1.543g, 5.0mmol) and ɑ-diimine ligand (2.100g, 5.2mmol), then add 30 mL of dichloromethane solution after dehydration, stir at room temperature for 12 hours, spin evaporate Part of the dichloromethane solution was washed with 20 mL of n-hexane and dried to obtain ɑ-diimine nickel bromide complex.
[0026] 2. Preparation of α-diimine mononuclear nickel metal organic compounds
[0027] Under anhydrous and oxygen-free conditions, 0.046 g (2.0 mmol) of metal Na was added to the diethyl ether (30 mL) turbid solution containing 0.622 g (1.0 mmol) of the α-diimine nickel bromide complex synthesized above, and stirred The solution turned green in 24 hours, and then 125 μL of 1,5-cyclooctadiene (1.0 mmol) was added to the solution, stirred at room temperature for 24 hours, the solution turned dark green, concentrated to about 6 mL, and crystallized at room temperature to o...
Embodiment 2
[0031] In step 2 of the preparation of ɑ-diimine mononuclear nickel metal organic compound in Example 1, under anhydrous and oxygen-free conditions, 0.046 g (2.0 mmol) of metal Na was added to the 0.622 g (1.0 mmol) In the diethyl ether (30 mL) turbid solution of ɑ-diimine nickel bromide complex, stir for 24 hours and the solution turns green, then add 125 μL 1,5-cyclooctadiene (1.0 mmol) to the solution, stir at room temperature After 36 hours, the solution turned dark green, concentrated to about 6mL, and stood to crystallize at -20°C to obtain dark green crystals, which were ɑ-diimine mononuclear nickel metal organic compounds. The yield was 0.435 g, and the yield was 76.2%, belonging to the monoclinic system, and its crystal structure and unit cell parameters are the same as in Example 1.
Embodiment 3
[0033] In step 2 of the preparation of ɑ-diimine mononuclear nickel metal organic compound in Example 1, under anhydrous and oxygen-free conditions, 0.046 g (2.0 mmol) of metal Na was added to the 0.622 g (1.0 mmol) ɑ-Diimine nickel bromide complex in n-hexane (30 mL) turbid solution, stirred for 24 hours, the solution turned green, then added 125 μL 1,5-cyclooctadiene (1.0mmol) to the solution, room temperature Stirred for 36 hours, the solution turned dark green, concentrated to about 6mL, and stood to crystallize at room temperature to obtain dark green crystals, which were ɑ-diimine mononuclear nickel organometallic compounds, with a yield of 0.459 g and a yield of 80.3 %, belongs to the monoclinic system, and its crystal structure and unit cell parameters are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com