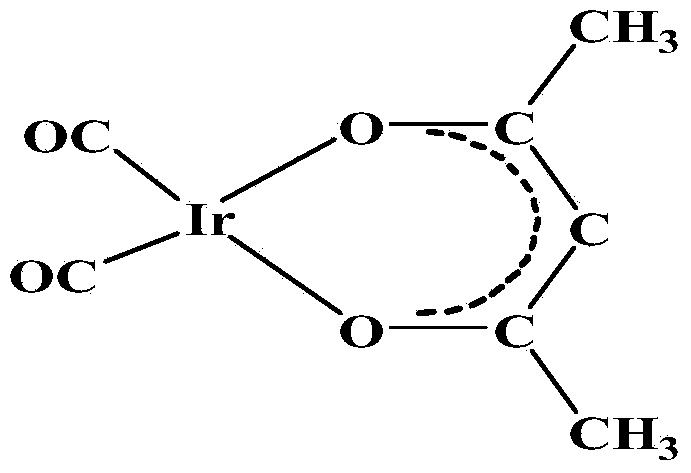
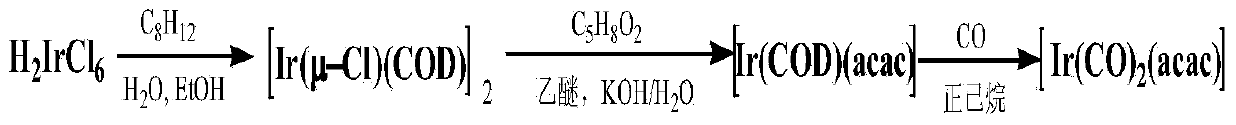Method for preparing dicarbonyl iridium acetylacetonate (I)
A technology of acetone dicarbonyl iridium and acetylacetone, which is applied in the field of preparation of metal organic iridium compound acetylacetonate dicarbonyl iridium, can solve the problems of high cost and low production yield, and achieve the effect of short reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: IrCl 3 ·3H 2 O (2.0g, 2.84mmol) and 30mL of secondary deionized water, 80mL of ethanol and 20mL of 1,5-cyclooctadiene were placed in a 250mL round-bottom flask, under the control of standard Schlenk vacuum line technology, stirred and heated to reflux for 5h . The reaction was stopped, the reaction solution was concentrated, and most of the solvent was removed, and a solid was precipitated, filtered, and the filter cake was washed with distilled water and methanol. After vacuum drying for 5 hours, 1.81 g was obtained with a yield of 95.2%.
[0022] [Ir(COD)(μ-Cl)] 2 (1.0 g, 1.49 mmol) and 150 mL of tetrahydrofuran (THF) were placed in a 250 mL round bottom flask, and the system was under an argon atmosphere under standard Schlenk vacuum line technology control. Then, CO gas was introduced for 3 minutes, and the solution changed from yellow to blue-black. Under argon atmosphere, quickly add NaHCO 3 (10 g, 119.04 mmol) and 1.5 mL acetylacetone (Hacac). ...
Embodiment 2
[0026] Example 2: IrCl 3 ·3H 2 O (10.0g, 14.2mmol) and 150mL of secondary deionized water, 400mL of ethanol and 100mL of 1,5-cyclooctadiene were placed in a 1000mL round-bottomed flask, under the control of standard Schlenk vacuum line technology, stirred and heated to reflux for 5h . The reaction was stopped, the reaction solution was concentrated, and most of the solvent was removed, and a solid was precipitated, filtered, and the filter cake was washed with distilled water and methanol. After vacuum drying for 5 hours, 9.1 g was obtained with a yield of 95.8%.
[0027] [Ir(COD)(μ-Cl)] 2 (5.0 g, 7.45 mmol) and 750 mL of tetrahydrofuran (THF) were placed in a 1000 mL round bottom flask, and the system was under an argon atmosphere under standard Schlenk vacuum line technology control. Then, CO gas was introduced for 3 minutes, and the solution changed from yellow to blue-black. Under argon atmosphere, quickly add NaHCO 3 (50 g, 595.2 mmol) and 7.5 mL acetylacetone (Haca...
Embodiment 3
[0029] Embodiment 3: the IrCl 3 ·3H 2O (20.0g, 28.4mmol) and 300mL of secondary deionized water, 800mL of ethanol and 200mL of 1,5-cyclooctadiene were placed in a 2000mL round-bottomed flask, under the control of standard Schlenk vacuum line technology, stirred and heated to reflux for 5h . The reaction was stopped, the reaction solution was concentrated, and most of the solvent was removed, and a solid was precipitated, filtered, and the filter cake was washed with distilled water and methanol. After vacuum drying for 5 hours, 18.2 g was obtained with a yield of 95.8%.
[0030] [Ir(COD)(μ-Cl)] 2 (15.0 g, 22.35 mmol) and 2250 mL of tetrahydrofuran (THF) were placed in a 3000 mL round bottom flask, and the system was under an argon atmosphere under standard Schlenk vacuum line technology control. Then, CO gas was introduced for 3 minutes, and the solution changed from yellow to blue-black. Under argon atmosphere, quickly add NaHCO 3 (150 g, 1785.6 mmol) and 22.5 mL acetyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com