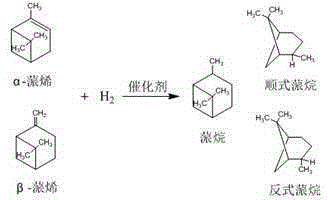
Preparation method of high-selectivity pinane
A high-selectivity, pinane technology, used in the chemical industry, can solve the problems of harsh operating conditions, poor enantioselectivity, and large safety hazards, and achieve the effects of simple preparation process, mild reaction conditions, and low equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of highly selective pinane described in the present embodiment specifically comprises the following steps:
[0026] (1) Take turpentine and put it in the reaction kettle, then add [Rh(COD)Cl] according to the mass of the catalyst as 1% of the mass of the raw material 2 Catalyst, sealed reactor.
[0027] (2) Use N at 0.3MPa 2 Replace 3 times, and then use H 2 Replace 3 times at 0.3MPa, adjust the hydrogen pressure in the reactor to 3MPa, maintain the pressure and check for leaks, control the rotation speed at 600r / min, and react at 40°C for 3h.
[0028] (3) After the reaction is over, stop heating and stirring, and open the reactor after pressure relief to separate the catalyst from the reaction product.
[0029] The reaction product was detected and analyzed by gas chromatography, the conversion rate of pinene was 98.13%, and the enantioselectivity of cis-pinane was 98.09%.
[0030] [Rh(COD)Cl] described in this embodiment 2 Catalyst is prepa...
Embodiment 2
[0036] (1) Take turpentine and put it in the reaction kettle, then add [Rh(COD)Cl] according to the mass of the catalyst as 5% of the mass of the raw material 2 Catalyst, sealed reactor.
[0037] (2) Use N at 0.5MPa 2 Replaced twice, then H 2 Replace twice at 0.5MPa, adjust the hydrogen pressure in the reactor to 3MPa, maintain the pressure and check for leaks, control the rotation speed at 600r / min, and react at 70°C for 3h.
[0038] (3) After the reaction is over, stop heating and stirring, and open the reactor after pressure relief to separate the catalyst from the reaction product.
[0039] The reaction product was detected and analyzed by gas chromatography, the conversion rate of pinene was 92.01%, and the enantioselectivity of cis-pinane was 96.00%.
[0040] [Rh(COD)Cl] described in this embodiment 2 Catalyst is prepared by following method, specifically comprises the following steps:
[0041] (1) Add RhCl at 0.033g / mL 3 ·3H 2 O is heated and dissolved in absolut...
Embodiment 3
[0046] (1) Take turpentine and put it in the reaction kettle, then add [Rh(COD)Cl] according to the mass of the catalyst as 3% of the mass of the raw material 2 Catalyst, sealed reactor.
[0047] (2) Use N at 0.1MPa 2 Replace 3 times, and then use H 2Replace 3 times at 0.1MPa, adjust the hydrogen pressure in the reactor to 2MPa, maintain the pressure and check for leaks, control the speed at 600r / min, and react at 40°C for 3h.
[0048] (3) After the reaction is over, stop heating and stirring, and open the reactor after pressure relief to separate the catalyst from the reaction product.
[0049] The reaction product was detected and analyzed by gas chromatography, the conversion rate of pinene was 96.98%, and the enantioselectivity of cis-pinane was 98.05%.
[0050] [Rh(COD)Cl] described in this embodiment 2 Catalyst is prepared by following method, specifically comprises the following steps:
[0051] (1) Add RhCl at 0.033g / mL 3 ·3H 2 O is heated and dissolved in absolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com