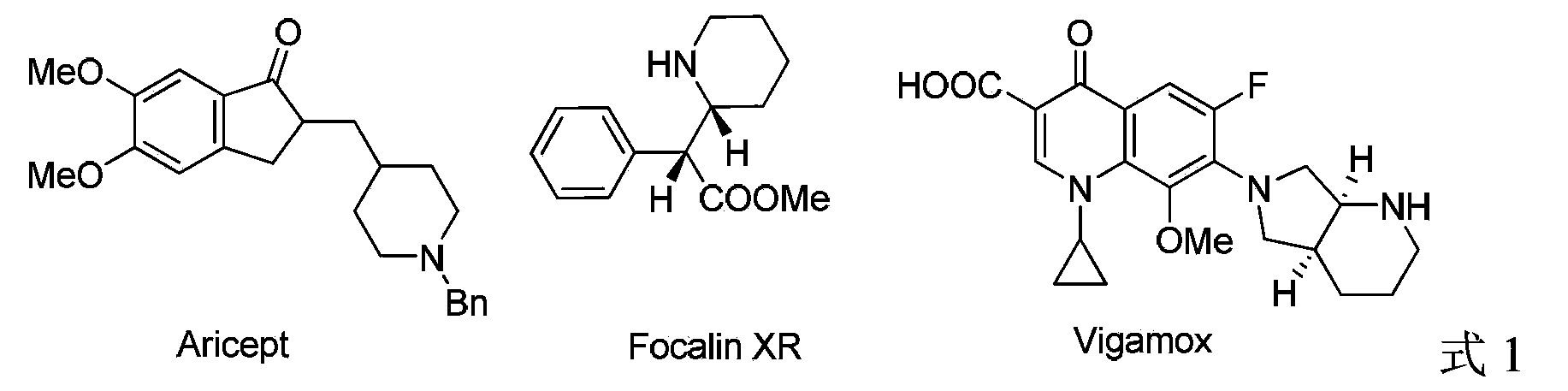
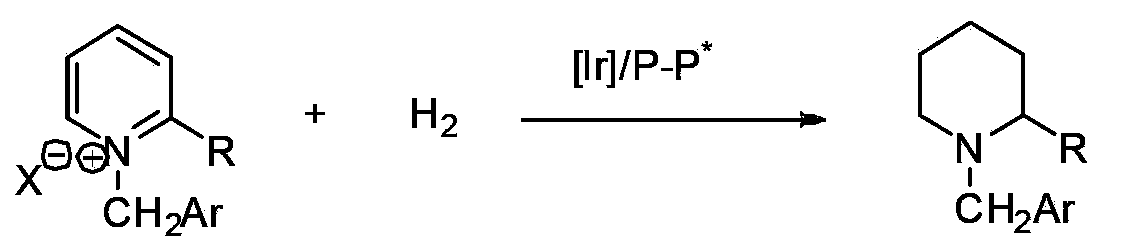Method for synthesis of chiral piperidine derivative through iridium-catalyzed asymmetric hydrogenation of pyridine
A technology that catalyzes pyridine and asymmetry is applied in asymmetric synthesis, organic chemistry methods, chemical instruments and methods, etc. It can solve problems such as low ee value and 2-aryl substituents that have not been reported, and achieve convenient separation, Simple and practical reaction operation, high reactivity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: condition optimization
[0033] In a nitrogen-filled glove box, add (1,5-cyclooctadiene) iridium chloride dimer (0.0025 mmol, 1.7 mg) and chiral ligand (0.0055 mmol) to the reaction vial Add 1mL mixed solvent toluene / dichloromethane (v / v=1:1), stir at room temperature for 10-30 minutes, then transfer the prepared catalyst to another N-benzylpyridinium salt (0.25mL mol) into the reaction flask, add 2mL solvent mixture solvent toluene / dichloromethane (v / v=1:1). Put the reaction bottle into a stainless steel autoclave, feed hydrogen gas at 600psi, and react at room temperature for 20-24 hours. Slowly release hydrogen, and add saturated Na to the system 2 CO 3 The aqueous solution was stirred for 10 min, then extracted three times with dichloromethane, the organic phases were combined and dried, and the solvent was removed by a rotary evaporator, followed by direct column chromatography (the volume ratio of eluent sherwood oil and ethyl acetate was 10:1-5:1...
Embodiment 2
[0038] Example 2: Synthesis of various chiral piperidine derivatives by iridium-catalyzed asymmetric pyridine hydrogenation
[0039]In a nitrogen-filled glove box, (1,5-cyclooctadiene) iridium chloride dimer (0.0025 mmol, 1.7 mg) and chiral ligand (R)-SynPhos (0.0055 mmol ) into the reaction bottle of 1mL mixed solvent toluene / dichloromethane (v / v=1:1), stirred at room temperature for 10-30 minutes, and then transferred the prepared catalyst to another containing raw material pyridinium salt (0.25 millimoles), add 3mL solvent mixture solvent toluene / dichloromethane (v / v=1:1). Put the reaction bottle into a stainless steel autoclave, feed hydrogen gas at 600psi, and react at room temperature for 20-24 hours. Slowly release hydrogen, and add saturated Na to the system 2 CO 3 The aqueous solution was stirred for 10 min, then extracted three times with dichloromethane, the organic phases were combined and dried, and after removing the solvent with a rotary evaporator, direct co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com