Preparing method for di-tertiary butyl-4-dimethylamino phenylphosphine and bis(di-tertiary butyl-4-dimethylamino phenylphosphine) palladium chloride
A technology of dimethylaminophenylphosphine and di-tert-butylphosphine, which is applied in the field of preparing di-tert-butyl-4-dimethylaminophenylphosphine and palladium dichloride, and can solve the problem of high product prices and preparation Problems such as high cost and low product purity have achieved the effect of being suitable for large-scale industrial production, reducing preparation costs and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
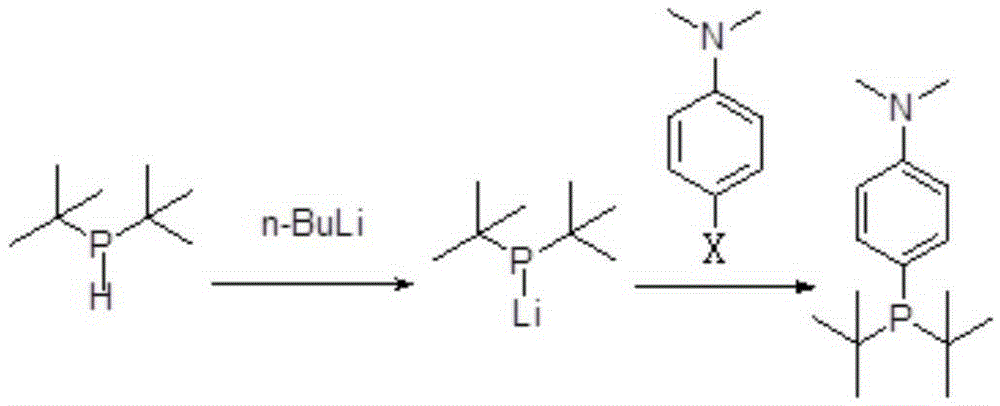
[0062] Preparation of di-tert-butyl-4-dimethylaminophenylphosphine
[0063] Replace the reaction bottle with a nitrogen atmosphere, add 32g of di-tert-butylphosphine and 200mL of toluene into a 1L reaction bottle, start magnetic stirring, add 96mL of 2.5M n-butyllithium dropwise at -10°C, and raise the temperature to 50°C for 12 hours , the temperature of the reaction solution was lowered to -10°C, 200mL of toluene solution of 40g N,N-dimethyl-p-bromoaniline was added dropwise, raised to 50°C and reacted for 12 hours, after which the reaction solution was lowered to room temperature, and dropped in an ice-water bath Add 10g of triethylamine aqueous solution 100mL, desolventize the upper organic phase under nitrogen protection, and distill under reduced pressure to collect 49.2g of di-tert-butyl-4-dimethylaminophenylphosphine at 120°C (15mmHg).
Embodiment 2
[0065] Preparation of bis(di-tert-butyl-4-dimethylaminophenylphosphine)palladium chloride
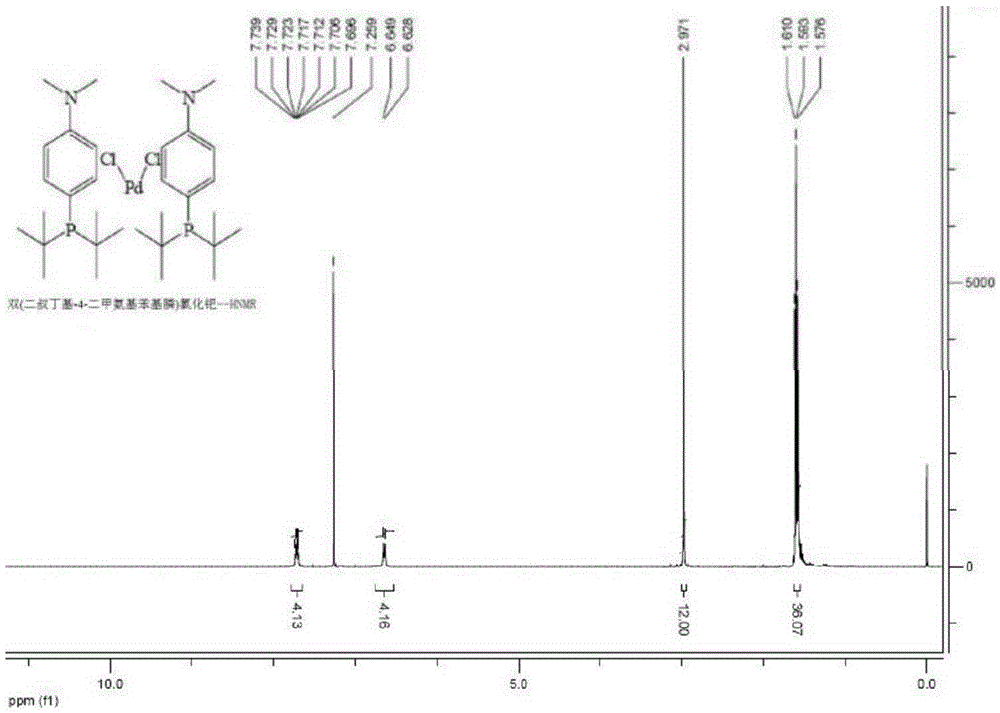
[0066] Add 10g (1,5-cyclooctadiene) palladium dichloride to a 500mL reaction, replace the reaction bottle with a nitrogen atmosphere, and add di-tert-butyl-4-dimethylaminophenylphosphine 19.6 prepared in Example 1 g and anhydrous tetrahydrofuran 200mL, stirred at room temperature for 16 hours, a solid was precipitated, filtered and dried to obtain a light yellow powder product bis(di-tert-butyl-4-dimethylaminophenylphosphine)palladium chloride 24.1g, the yield was 97% (The yield is calculated based on (1,5-cyclooctadiene) palladium dichloride). The purity of the product obtained by XY-1A intelligent elemental analyzer is 99.8%.
[0067] figure 1 and figure 2 Respectively the two (di-tert-butyl-4-dimethylaminophenylphosphine) palladium chloride prepared 1 H-NMR spectrum and 31 P-NMR spectrum, the characterization results are as follows: 400MHz- 1 H-NMR (CDCl 3 ): δ=1.57-1.61 (m, 3...
Embodiment 3
[0070] Replace the reaction bottle with a nitrogen atmosphere, add 31g of di-tert-butylphosphine and 200mL of xylene into a 1L reaction bottle, start magnetic stirring, add 90mL of 2.5M n-butyllithium dropwise at 0°C, and raise the temperature to 55°C to react 8 hour, the temperature of the reaction solution was lowered to -5°C, and 200 mL of a xylene solution of 40 g N,N-dimethyl-p-bromoaniline was added dropwise. 100 mL of 6 g of ammonium chloride aqueous solution was added dropwise, and the upper organic phase was desolvated under nitrogen protection, and distilled under reduced pressure to collect 48 g of di-tert-butyl-4-dimethylaminophenylphosphine at 120° C. (15 mmHg).
[0071] Add 10g (1,5-cyclooctadiene)palladium dichloride to another 500mL reaction, replace the reaction bottle with nitrogen atmosphere, add 19.6g di-tert-butyl-4-dimethylaminophenylphosphine and anhydrous Methyltetrahydrofuran 200mL, stirred at room temperature for 12 hours, solids were precipitated, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com