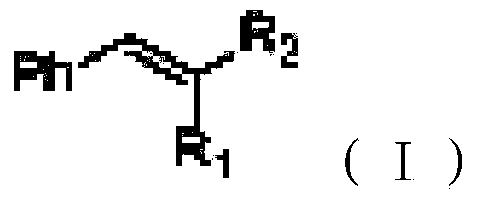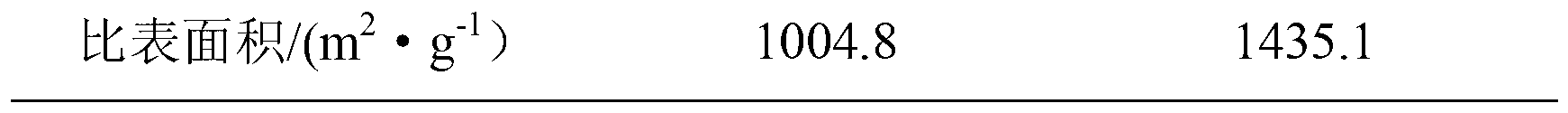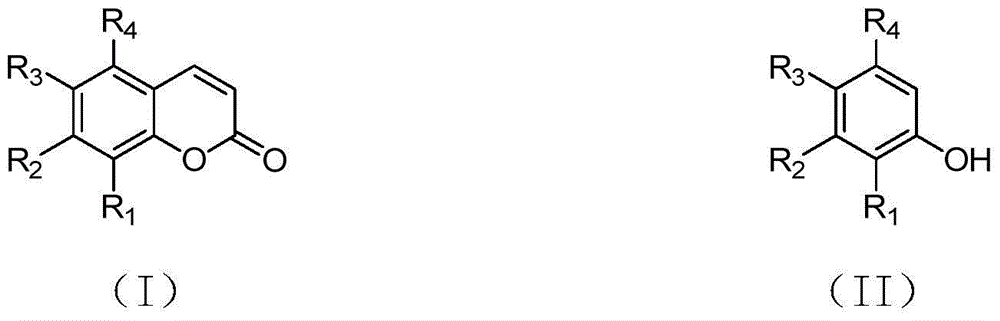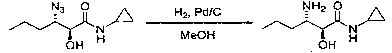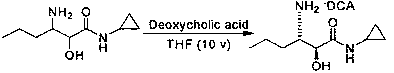Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about How to "Reaction conditions green" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for manufacturing catalyst used for synthesizing benzaldehyde
InactiveCN101108367AEasy to prepareEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationActive componentBenzaldehyde
A catalyst use for synthetizing benzaldehyde is provided, which is characterized in that the invention makes Co schiff base composition as the active component and silica gel as carrier. The silica gel is that: Co schiff base composition equals to 1g / 0.04 to 0.4mmol. The invention has simple preparation method and easy operation. In benzaldehyde synthesis reaction, the invention has the advantages of transforming benzaldehyde is higher, the produced benzaldehyde has good selectivity and good catalyst repeat use performance.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
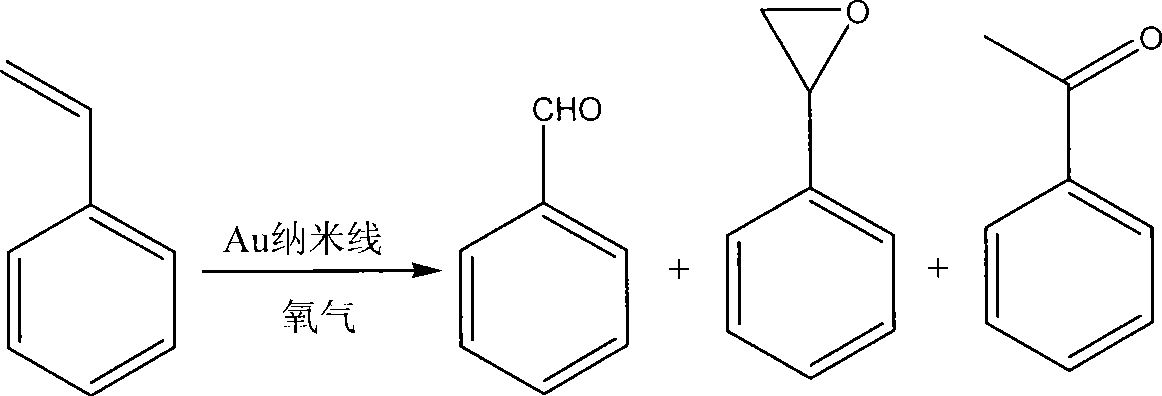
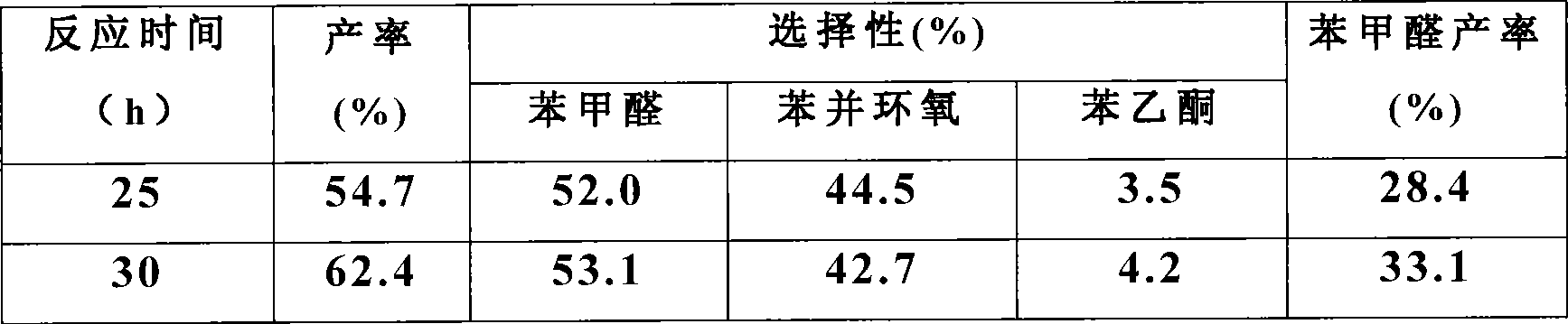
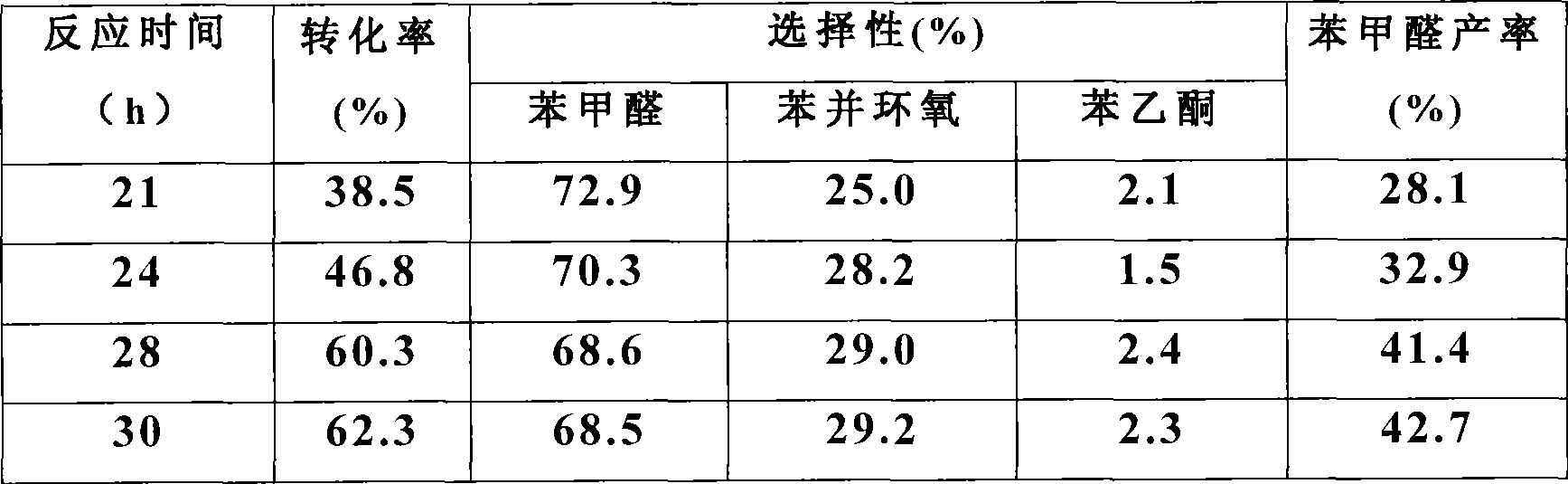
Method for preparing benzaldehyde through styrene catalytic oxidation
InactiveCN101531576AHigh yieldImprove conversion rateOrganic compound preparationEssential-oils/perfumesBenzaldehydeCatalytic oxidation
The invention discloses a method for preparing benzaldehyde through styrene catalytic oxidation, which comprises the following steps: using styrene as a reactant and a gold nano-wire of which the diameter is less than or equal to 5 nanometers as a catalyst, reacting at a temperature of between 80 and 100 DEGC for 20 to 48 hours in a normal pressure oxygen atmosphere in the presence of a solvent, and cooling and separating the mixture after the reaction to obtain the benzaldehyde, wherein the solvent is methylbenzene. The method has the advantages of higher conversion rate, higher benzaldehyde yield, and milder and greener reaction conditions.
Owner:SUZHOU UNIV
Green conjugated double bond reduction method
ActiveCN103467223AAchieve restorationReaction conditions greenOrganic reductionCarboxylic acid nitrile preparationDistillationOrganic layer
The invention relates to a green conjugated double bond reduction method capable of taking water as a solvent and dihydropyridine ester as a hydrogen source, and selectively reducing the conjugated double bond with strong electron-withdrawing groups. By adopting the method, conjugated carbon-carbon double bonds with strong electron-withdrawing groups are selectively reduced by taking water as the solvent and the dihydropyridine ester as the hydrogen source under the condition that no catalyst is needed. The method comprises the following steps: (1) adding a compound of conjugated carbon-carbon double bonds with strong electron-withdrawing groups and the dihydropyridine ester to water according to the molar ratio of (1:1) to (1:3), heating and warming to 60-100 DEG C; stirring and reacting for 5-24 hours at the temperature; (2) adding an organic solvent to the solution obtained in the step (1) to extract for at least three times according to the volume ratio of 2:5; (3) merging, drying and carrying out reduced pressure distillation on an organic layer of the product obtained in the step (2), and then carrying out column chromatography, so as to obtain the reduced product. The green conjugated double bond reduction method has the advantages of being non-toxic, mild in reaction condition, free of adding of a catalyst, high in chemical selectivity and the like.
Owner:无锡富泽药业有限公司
Nickel/copper catalyst and preparation method thereof, and method for directly preparing 1,2-hexanediol from cellulosan by using nickel/copper catalyst
InactiveCN103055870AHigh catalytic activityLarge specific surface areaOrganic compound preparationHydroxy compound preparationCarrying capacityCopper
The invention relates to a supported nickel / copper catalyst and a preparation method thereof, and a method for preparing 1,2-hexanediol from cellulosan by using the supported nickel / copper catalyst. The weight ratio of nickel to copper in the catalyst is between 1:10 and 10:1. Based on the total weight of the catalyst, the carrying capacity of the nickel is 0.05 to 40 percent, and the carrying capacity of the copper is 0.05 to 50 percent; meanwhile, the preparation process for the catalyst is simple; and the method for preparing 1,2-hexanediol from cellulosan by using the catalyst can be used for highly selectively preparing 1,2-hexanediol, and compared with conventional hexylene routes, the method has significant advantages of reproducible raw materials, environment-friendly reaction process and the like.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI +1
Gold nanocluster using transferrin as template, and preparation method and application thereof
ActiveCN109705841AGood compatibilityReduce the ratioMaterial nanotechnologyNanoopticsCancer cellLysosome
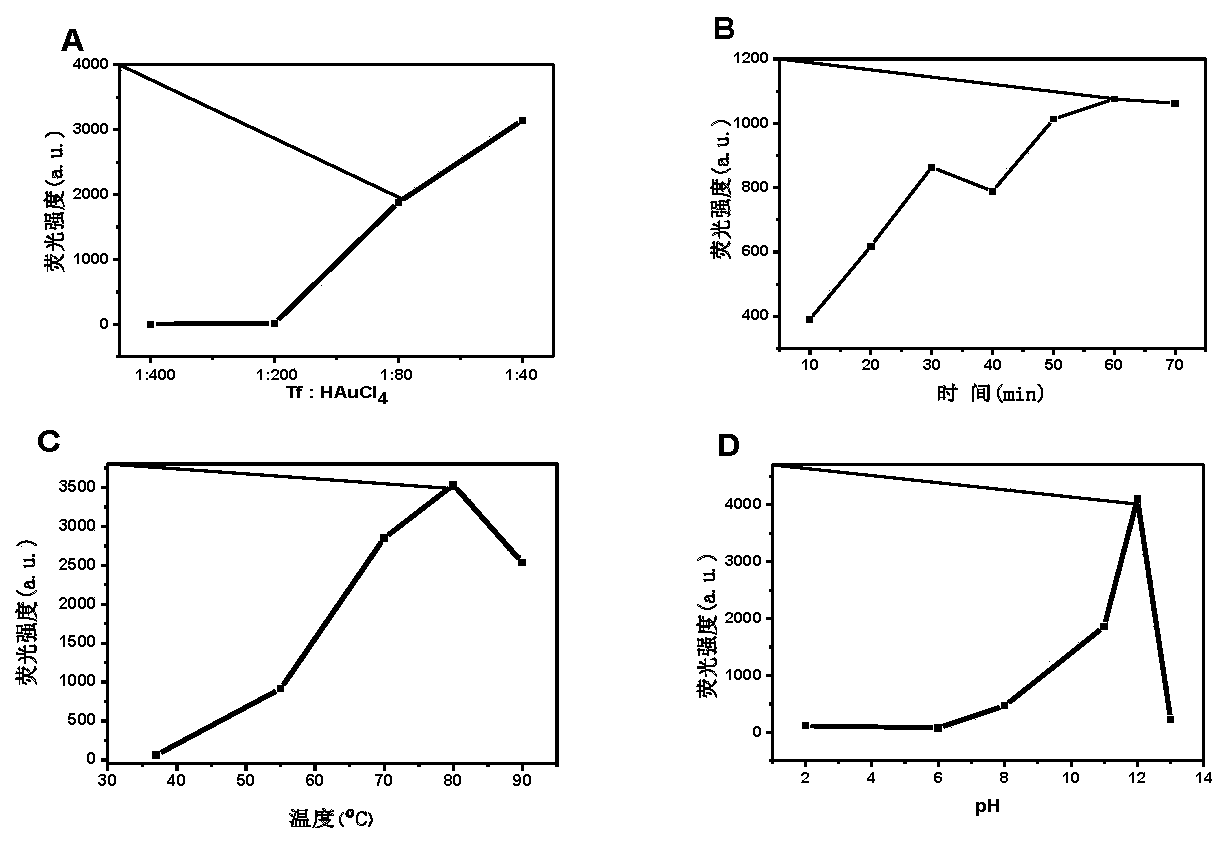
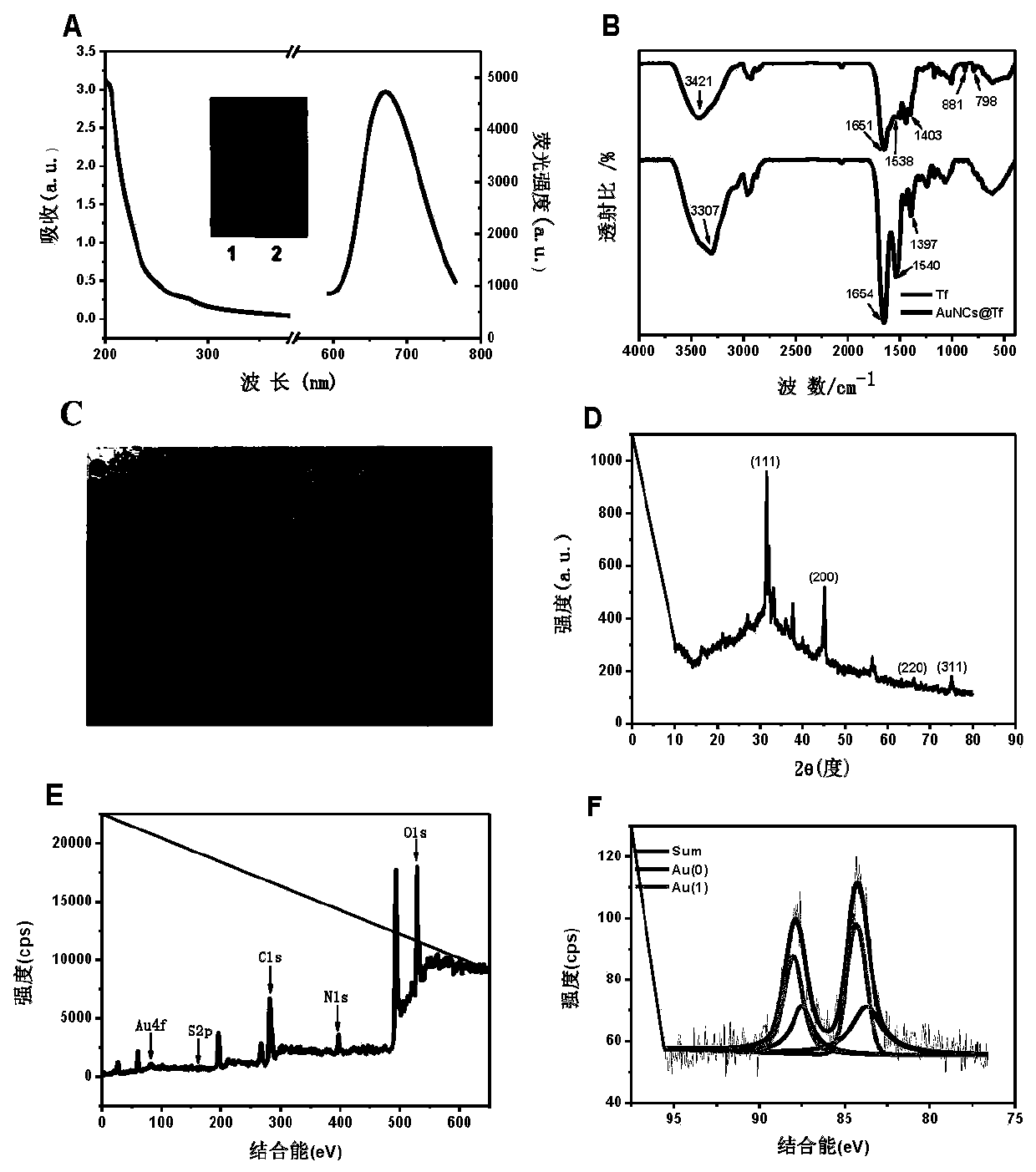
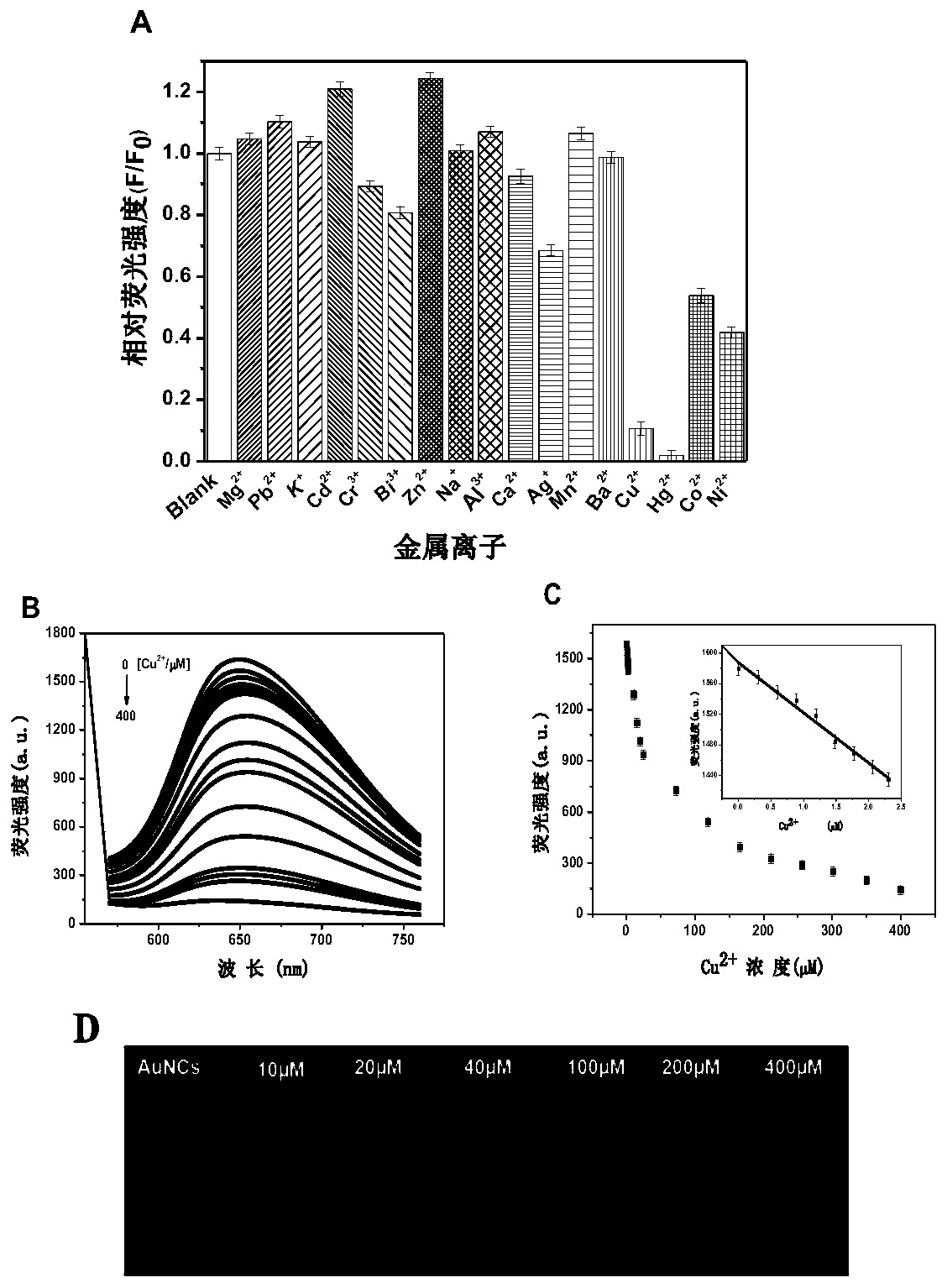
The invention provides a gold nanocluster using transferrin as template, and a preparation method and application thereof. The preparation method of the nanoclusters comprises the following steps: respectively configuring aqueous solutions of HAuCl4 and Tf, mixing them according to the molar ratio of 20-60:1, stirring for 2-5 minutes at room temperature, adjusting the pH of the mixed solution to 12 with NaOH solution, placing the solution in a microwave reactor and sealing, reacting at 70-90 DEG C for at least 50 minitues to obtain AuNCs@Tf emitting intense red fluorescence. The nanoclusters of the invention can be used for detecting copper ions, and can be applied to a copper ion detection test paper to visualize the detection of copper ions. The nanoclusters of the invention can also beused for detecting glutathione, and the cell experiment shows that the nanoclusters can target lysosomes to recognize cancer cells. The nanoclusters of the invention can also be used for encrypting and decrypting fingerprint information.
Owner:SHANXI UNIV
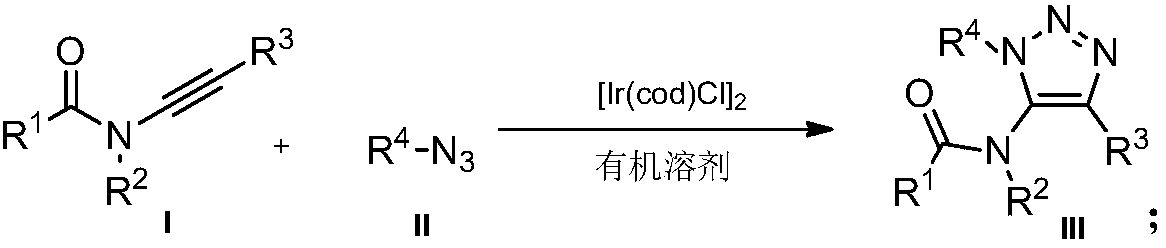
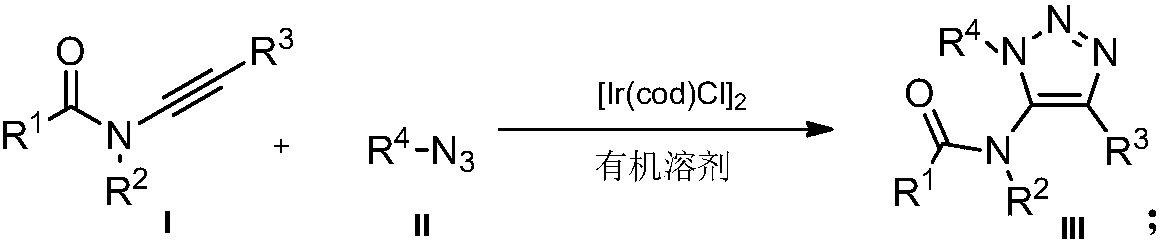
Preparation method of novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole
InactiveCN107721984AMild reaction conditionsReaction conditions greenOrganic chemistryOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis, and relates to a preparation method of novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole. The novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole is prepared by catalyzing a ynamine compound and nitrine in an organic solvent and under the action of a catalyst of 1,5-cyclooctadiene iridium chloride dipolymer ([Ir(COD)Cl]2). The preparation method of a 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole product is mild in reaction conditions, and the yield of the product is not lower than 70 percent; the preparation method is mild in reaction conditions, is green and is high in reaction efficiency and is more suitable for large-scale production requirements, and a prepared novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
Carboxylic acid derivatization beta-carboline and preparation method thereof
InactiveCN108191863AStrong toleranceMild reaction conditionsOrganic chemistryOrganic solventCarboxylic acid
The invention relates to carboxylic acid derivatization beta-carboline and a preparation method of the carboxylic acid derivatization beta-carboline. The method comprises the steps that organic solvent is added into the mixture of 6-methyl-beta carbazoline, metal-salt catalyst and initiator, oxygen is introduced, and after stirring reaction is conducted, the reaction is terminated; filtering is conducted, and solid crude products are collected; further purification of the crude products is conducted through recrystallization and silica gel column chromatography to obtain products. According tothe method, reaction conditions are mild, the application range of substrates is wide, and a large number of beta-carboline derivatives with diversified structures can be obtained.
Owner:NINGBO UNIV
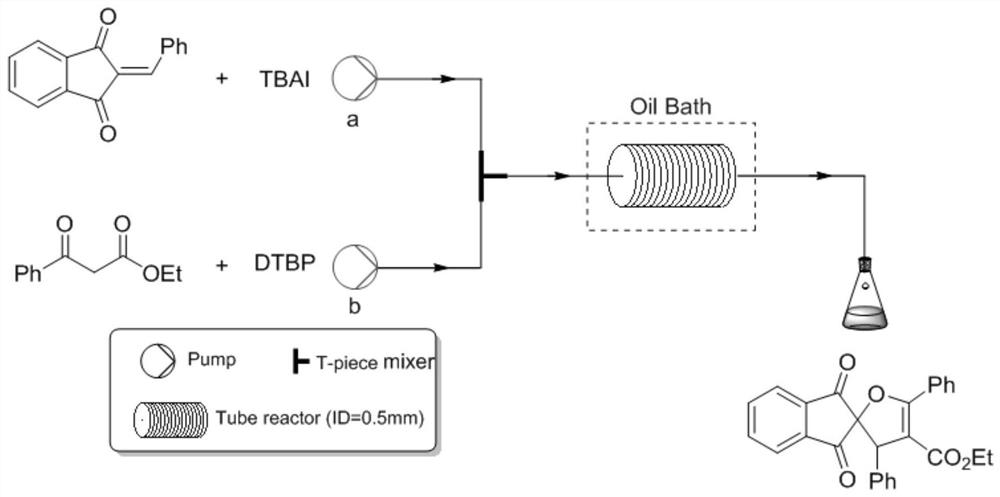
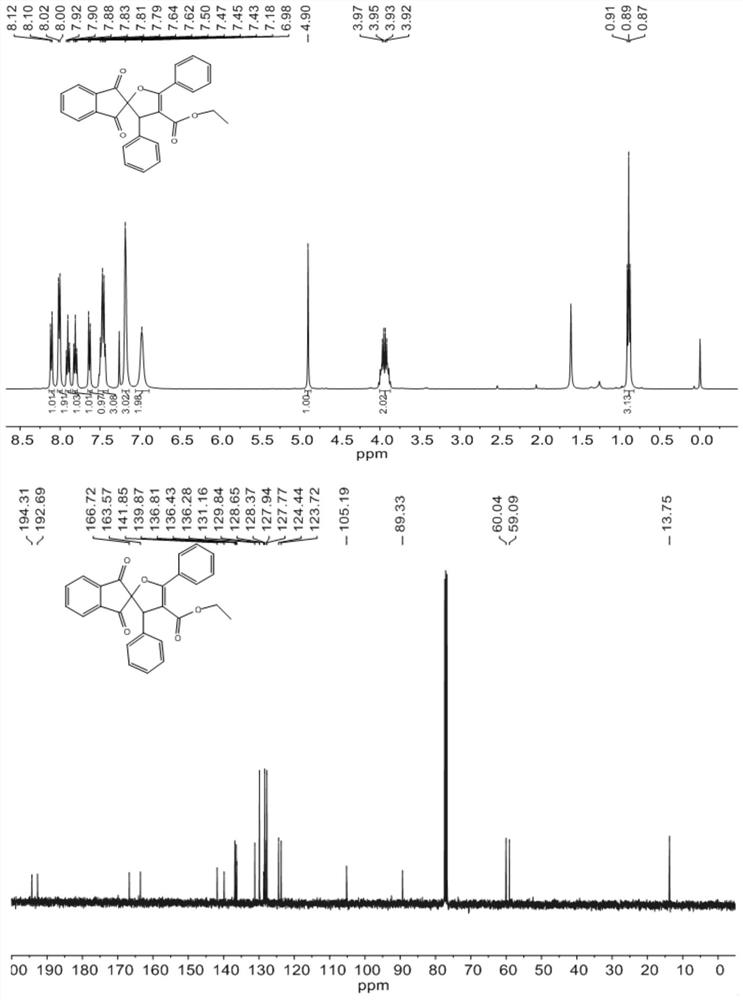
Method for synthesizing dihydrofuran containing 1, 3-indandione spiro skeleton by using micro-channel reaction device
PendingCN112209907AAvoid multi-step reactionsAtom utilization is highOrganic chemistryChemical/physical/physico-chemical microreactorsFuranPtru catalyst
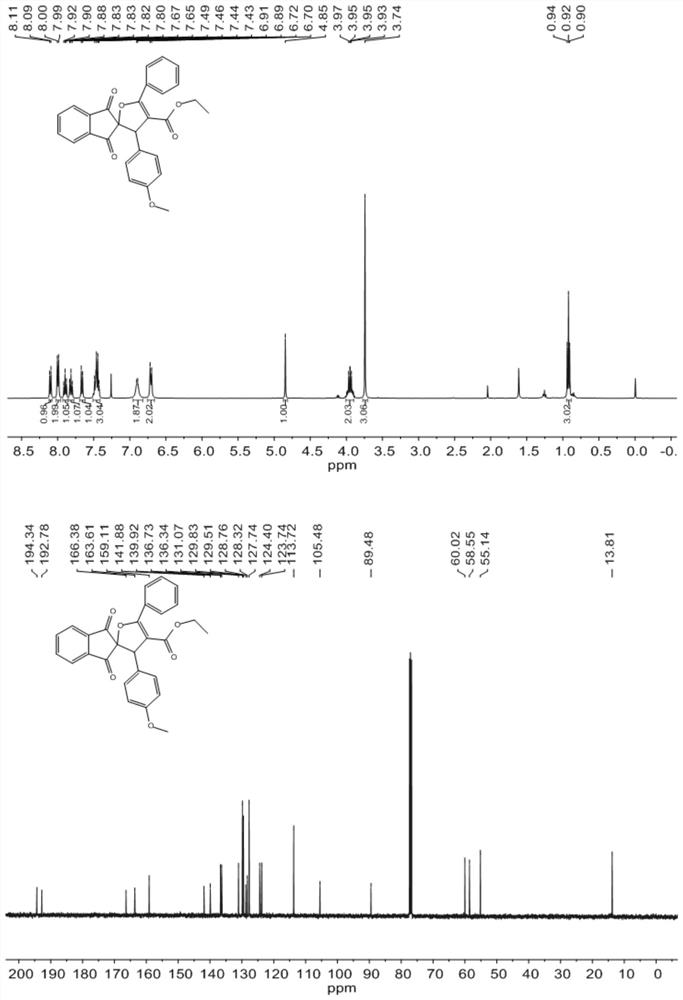
The invention discloses a method for synthesizing a dihydrofuran compound containing a 1, 3-indandione spiro skeleton as shown in a formula III by using a micro-channel reaction device, which comprises the following steps: by using a 2-benzylidene-1, 3-indandione compound I and a benzoyl ethyl acetate compound II as reaction raw materials, carrying out continuous reaction by using the micro-channel reaction device to prepare the dihydrofuran compound containing a 1, 3-indandione spiro skeleton. The micro-channel reaction device comprises a feeding pump, a micro-mixer and a micro-reactor whichare sequentially connected through a pipeline. Compared with the prior art, the new dihydrofuran containing the 1, 3-indandione spiro skeleton is prepared by taking the 2benzylidene 1, 3-indandione compound as the substrate for the first time, and the method avoids multi-component reaction, uses a non-metal catalyst and a low-toxicity solvent, and is a quick, efficient, green and environment-friendly synthetic product, wherein R1 is selected from halogenated benzene, C1-C4 alkyl benzene, C1-C4 alkoxy benzene, nitrobenzene, furan or naphthalene, and R2 is selected from halogenated benzene, C1-C4 alkyl benzene, C1-C4 alkoxy benzene, nitrobenzene, furan, thiophene, pyridyl or naphthalene.
Owner:NANJING ADVANCED BIOLOGICAL MATERIALS & PROCESS EQUIP INST CO LTD
Method for synthesizing pyridine compound by using microchannel reaction device
ActiveCN113307766AAvoid multi-step reactionsAvoid reactionOrganic chemistryChemical/physical/physico-chemical microreactorsFuranPtru catalyst
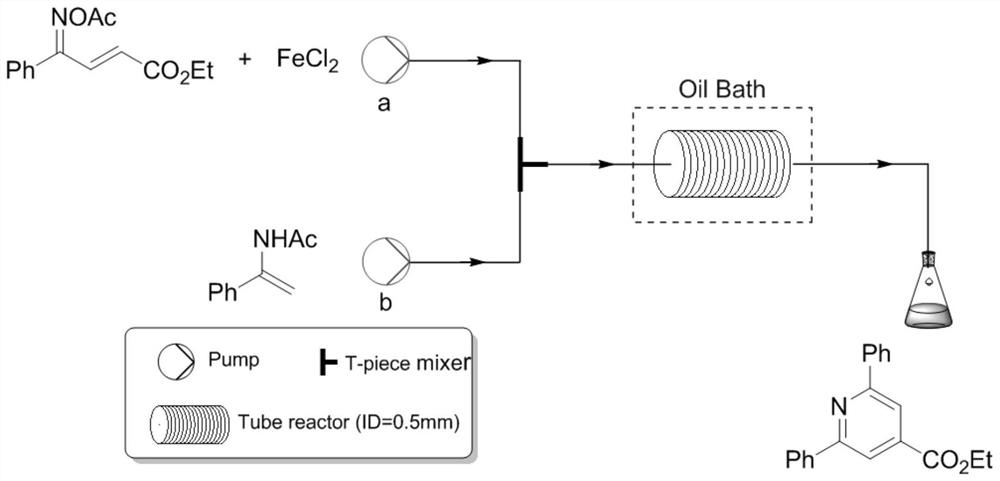
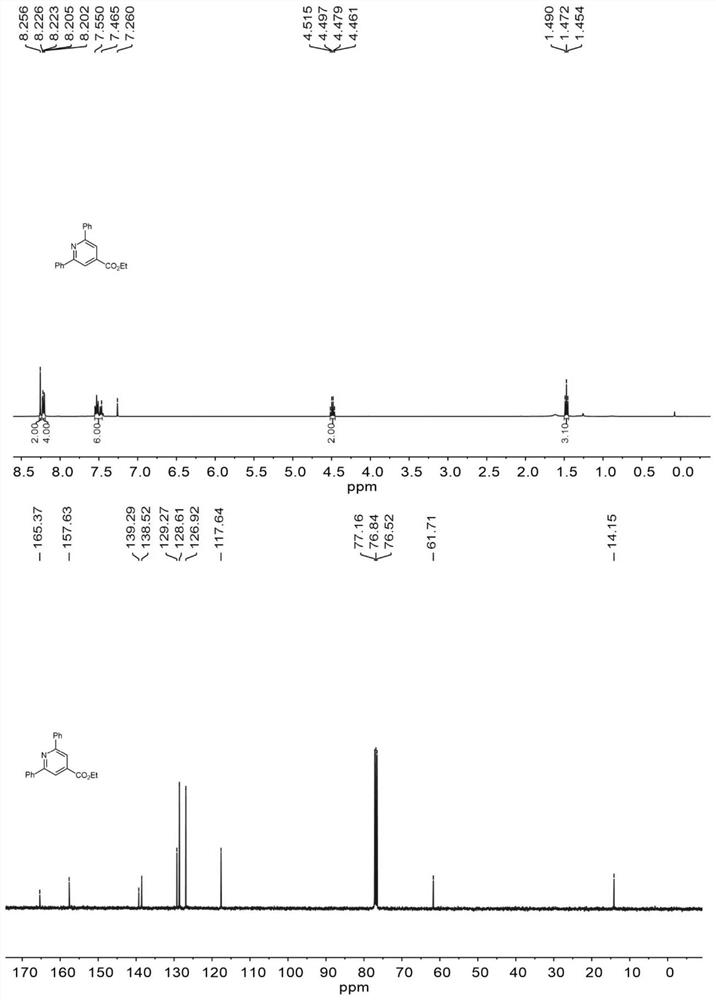
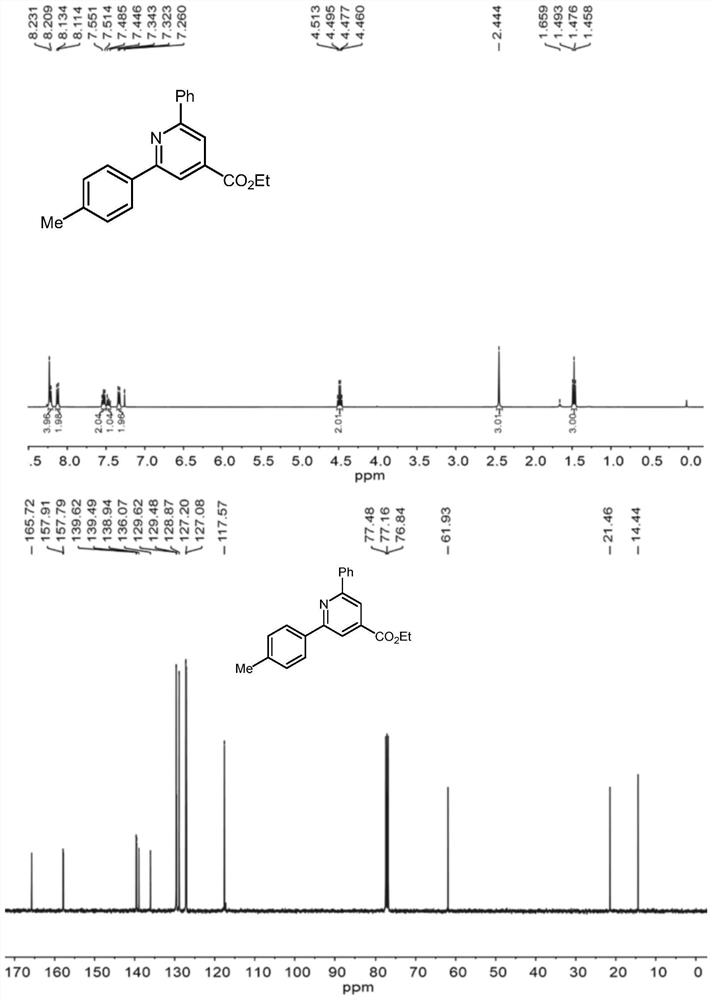
The invention discloses a method for synthesizing a pyridine compound shown as III by using a micro-channel reaction device, which comprises the following steps: taking an alpha, beta-unsaturated ketoxime ester compound I and an N-acetyl amide compound II as reaction raw materials, adding a catalyst, and continuously reacting by using the micro-channel reaction device to prepare the pyridine compound. Compared with the prior art, the novel pyridine compound is prepared by taking the alpha, beta-unsaturated ketoxime ester compound and the N-acetyl amide compound as substrates, multi-component reaction is avoided, and the product is rapidly and efficiently synthesized by using the metal catalyst. Wherein R1 and R2 are independently selected from non-substituted or substituted phenyl, furyl, naphthyl or C1-C5 alkyl; and the substituted phenyl group is selected from phenyl groups substituted by halogen, C1-C5 alkyl groups or C1-C5 alkoxy groups.
Owner:NANJING ADVANCED BIOLOGICAL MATERIALS & PROCESS EQUIP INST CO LTD
Method for preparing p-phenylenediamine
InactiveCN105418438ASimple processHigh yieldOrganic compound preparationAmino compound preparationReaction temperatureHigh pressure
The invention belongs to the technical field of fine chemical engineering, and mainly relates to a preparation method for p-phenylenediamine. The preparation method for the high-purity p-phenylenediamine comprises the steps that a nitro selection reduction reaction is performed in organic solvent under high pressure by taking p-dinitrobenzene as a raw material, taking selenium as a catalyst and taking alkali as a catalyst promoter, wherein the reaction temperature ranges from 80 DEG C to 150 DEG C, the reaction pressure ranges from 1 MPa to 10 MPa, and the reaction time ranges from 2 hours to 10 hours; cooling is performed until the room temperature is achieved, after degassing is performed, oxygen or air is introduced, stirring is performed for 1-2 hours, filtering is performed, cooling crystallization is performed on filtrate, suction filtration is performed, and after vacuum drying is performed on filter cakes, the finished p-phenylenediamine is obtained.
Owner:王晓伟
Preparation and catalytic application of donor-acceptor type ionic porous polymer
ActiveCN111978516AImprove catalytic performanceMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPerylene derivativesFine chemical
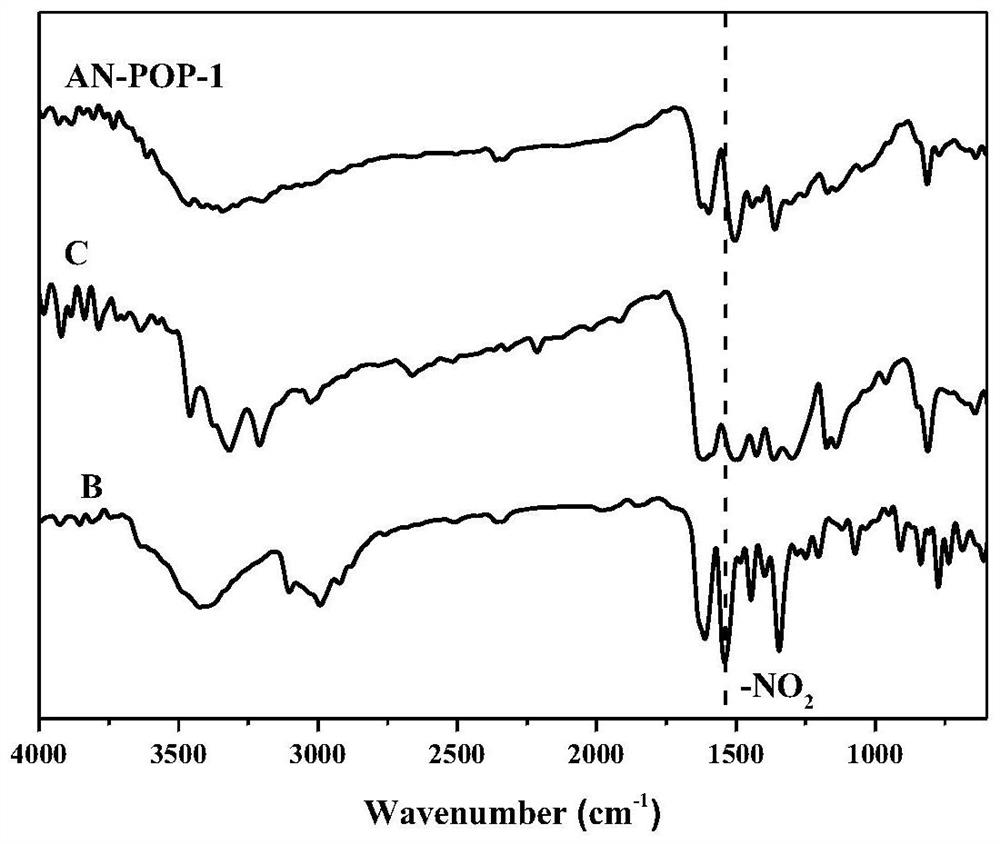
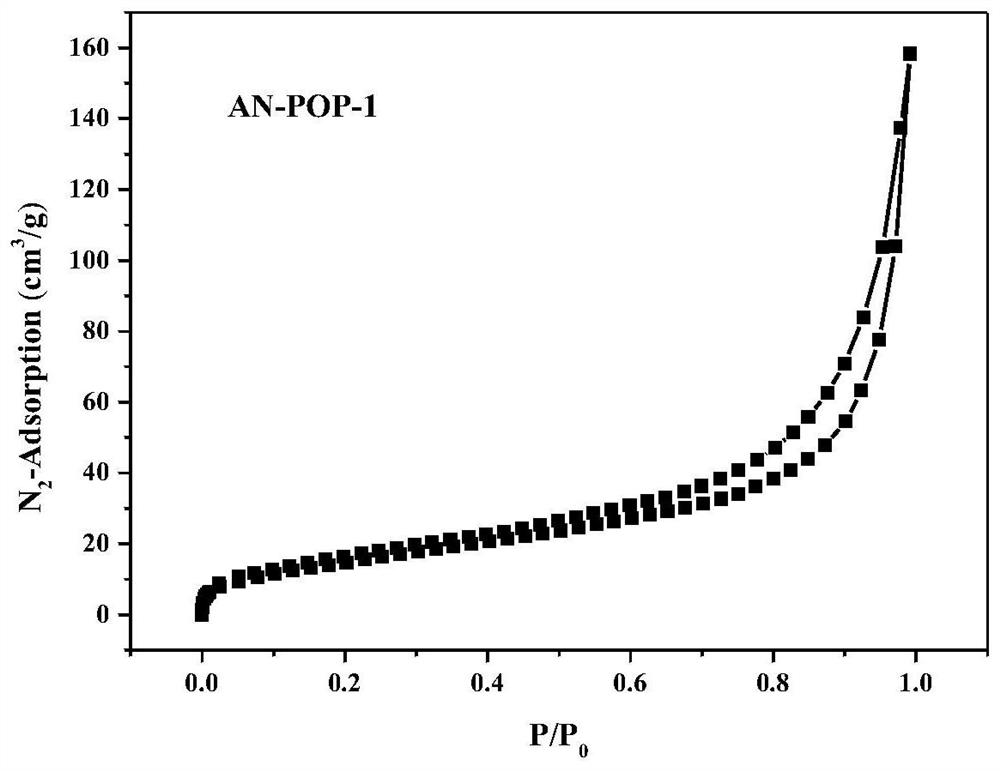
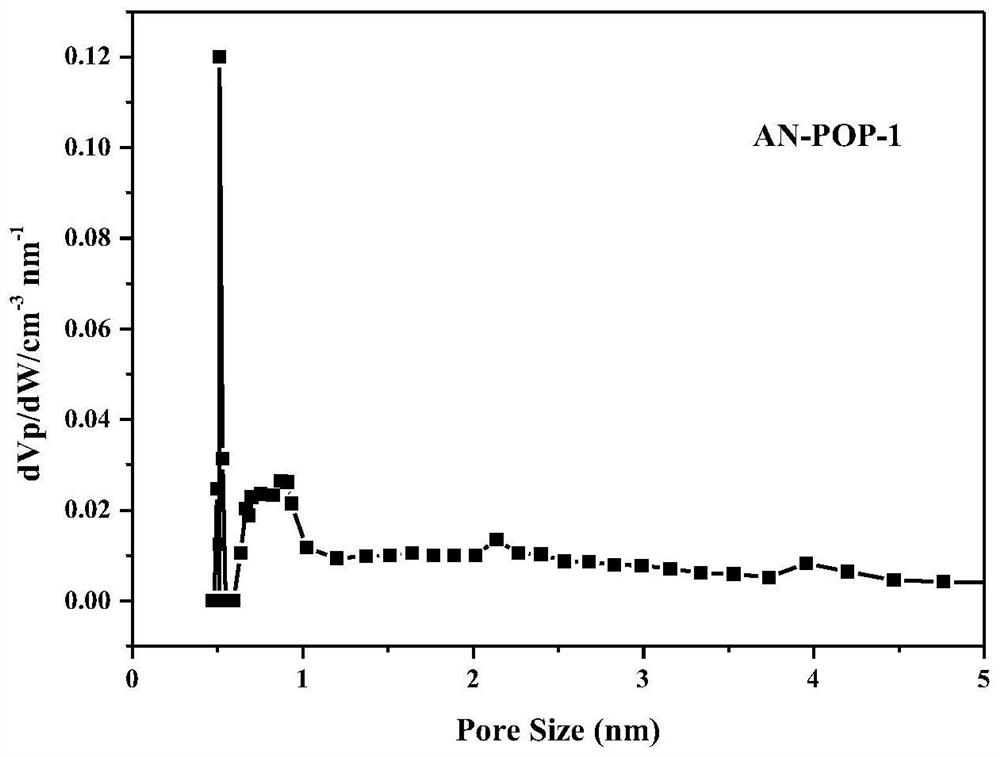
The invention belongs to the technical field of fine chemical engineering, and discloses preparation and catalytic application of a donor-acceptor type ionic porous polymer. The polymer AN-POP-1 is prepared through a Zincke reaction. An anthracene ring structure is introduced into 1,1'-bis(2,4-dinitrophenyl)-[9,10-bis(3-pyridyl)anthracene]-1,1'-diammonium dichloride, so that the photocatalytic performance is improved; the preparation method comprises the following steps: adding 1,1'-bis(2,4-dinitrophenyl)-[9,10-bis(3-pyridyl)anthracene]-1,1'-diammonium dichloride and 1,3,5-tri(4-aminophenyl)triazine C into a schlenk tube, and reacting for 48-72 hours under the protection of nitrogen at 100-150 DEG C to obtain the polymer AN-POP-1 catalyst. The AN-POP-1 polymer prepared by the invention hasgood chemical and thermal stability and relatively high catalytic performance, and the catalytic yield of a benzylamine derivative can reach 90% or above.
Owner:DALIAN UNIV OF TECH
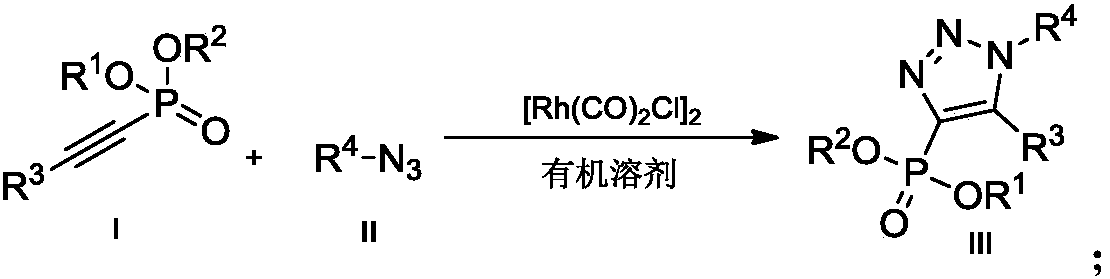
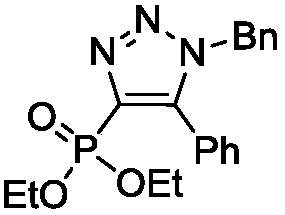
Preparation method of novel 4-phosphoryl-1, 4, 5-trisubstituted 1, 2, 3-triazole
ActiveCN108558944AMild reaction conditionsReaction conditions greenGroup 5/15 element organic compoundsAzideOrganic synthesis
The invention belongs to the technical field of organic synthesis, and provides a preparation method of novel 4-phosphoryl-1, 4, 5-trisubstituted 1, 2, 3-triazole. According to the preparation method,in an organic solvent, under the action of a tetracarbonyl rhodium dichloride dimer [Rh(CO)2Cl]2 catalyst, an alkynyl phosphate compound and azide are catalyzed to prepare the 4-phosphoryl-1, 4, 5-trisubstituted 1, 2, 3-triazole. By the preparation method of the 4-phosphoryl-1, 4, 5-trisubstituted 1, 2, 3-triazole product, provided by the invention, a reaction condition is mild and the product yield is not lower than 67%. With the preparation method, the reaction condition is mild and environmentally friendly, the reaction efficiency is high, a large-scale production requirement is met better, and the prepared 4-phosphoryl-1, 4, 5-trisubstituted 1, 2, 3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
Preparation method of sulfophenyl pyrazolone and intermediate thereof
ActiveCN105646356AMild reaction conditionsSafe reaction conditionsSulfonic acid esters preparationEsterification reactionHydrolysis
The invention discloses a preparation method of sulfophenyl pyrazolone. The method consists of: taking a compound (I) as the raw material, in the presence of alkali, subjecting the compound (I) and a compound (II) to substitution reaction, then conducting oxidation by an oxidizing agent to obtain a compound (III), esterfying the compound (III) to obtain a compound (IV), subjecting the compound (IV) to thionation to obtain a compound (VI), conducting oxidation and hydrolysis (or hydrolysis, oxidation) on the compound (VI) to obtain a compound (VII), subjecting the compound (VII) to acyl chlorination, subjecting the obtained acyl chloride (VIII) and 1, 3-dimethyl-5-hydroxypyrazole to esterification reaction so as to obtain a compound (IX), and finally carrying out rearrangement on the compound (IX) to obtain a compound (X). The method provided by the invention has the advantages of cheap and easily available raw materials, high reaction conversion rate, few three wastes, and is beneficial to industrial production.
Owner:ZHEJIANG ZHUJI UNITED CHEM
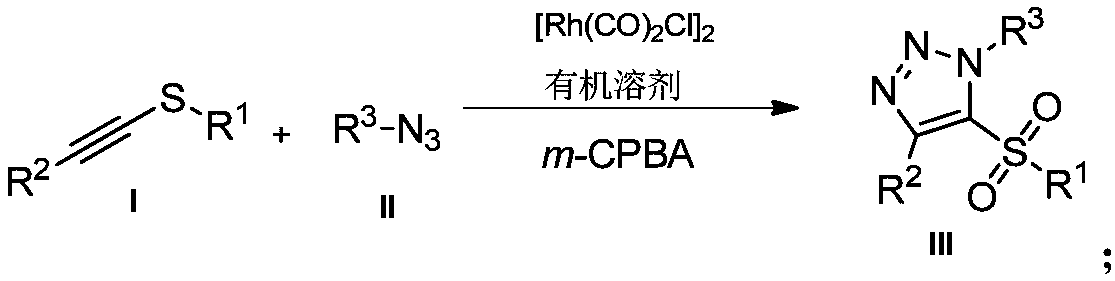
Novel preparation method of 5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole
InactiveCN109232449AMild reaction conditionsReaction conditions greenOrganic chemistryOrganic solvent3-chloroperoxybenzoic acid
The invention belongs to the technical field of organic synthesis and provides a novel preparation method of 5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole. The novel preparation method comprises thesteps: catalyzing a thioethyne compound to react with azide in an organic solvent under the action of a rhodium carbonyl chloride dimer catalyst; then, adding 3-chloroperoxybenzoic acid; and preparing5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole by using a 'one-pot method'. The preparation method of 5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole is mild in reaction condition and the yield of notlower than 55%. The preparation method is mild in reaction condition, green, high in reaction efficiency and more meets the requirement for large-scale production, and the prepared 5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
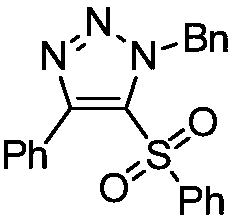
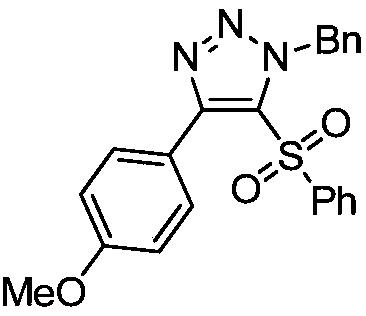
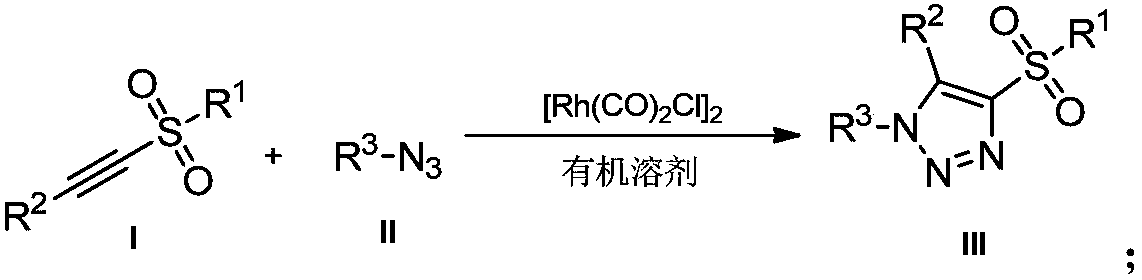
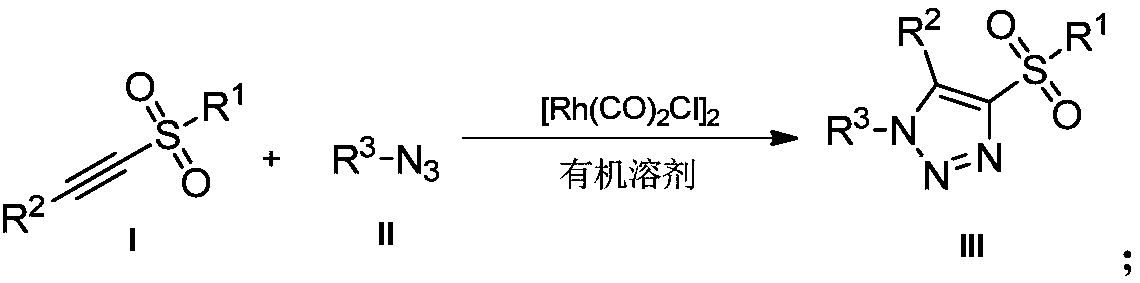
Novel method for preparing 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole
InactiveCN109096214AMild reaction conditionsReaction conditions greenOrganic chemistryOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis and provides a novel method for preparing 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole. The novel method comprises the following step: in an organic solvent, under the action of a tetracarbonyl rhodium chloride dimer catalyst, catalyzing a hydrocarbon compound in sulfonyl and nitrine, thereby obtaining the 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole. The method for preparing the 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole, which is provided by the invention, is gentle in reaction condition and has a product yield which is not less than 70%. The method is gentle and green in reaction condition, high in reaction efficiency and applicable to on-scale production requirements, and the prepared 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
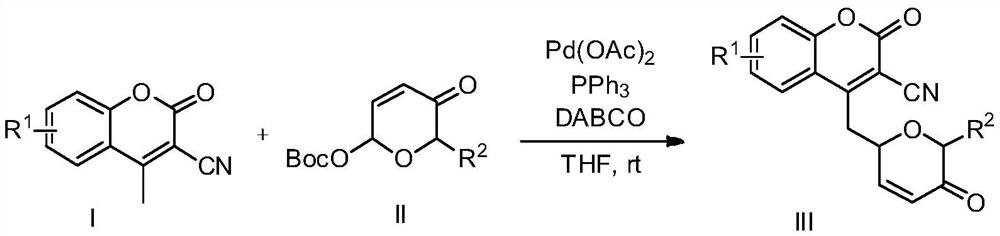
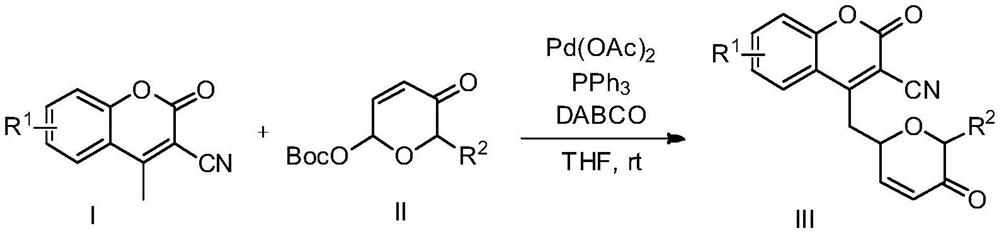
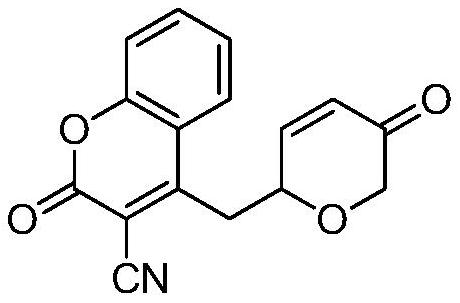
Preparation method of novel dihydropyrone coumarin compound
InactiveCN112409345AMild reaction conditionsHigh regional selectivityOrganic chemistryOrganic synthesisCatalytic effect
The invention belongs to the technical field of organic synthesis, and relates to a preparation method of a novel dihydropyrone coumarin compound. The preparation method comprises the following steps:in tetrahydrofuran, takingtriphenyl phosphine as a ligand and1,4-diazabicyclo[2.2.2] octane as an alkali, and under the catalytic action of Pd(OAc)2, reacting a dihydropyrone compound with a 3-cyano-4-methyl coumarin compound to prepare the 3-cyano-4-(6-dihydropyrone) methyl coumarin. The preparation method is mild in reaction condition, and the product yield is not lower than 52%. The inventionprovides a preparation method for obtaining dihydropyrone coumarin with excellent regioselectivity and high stereoselectivity by a simple and effective method. The preparation method is mild in reaction condition, green, high in reaction efficiency and more suitable for large-scale production requirements, and the prepared dihydropyrone coumarin compound is a compound with potential physiologicalactivity.
Owner:DALIAN UNIV OF TECH
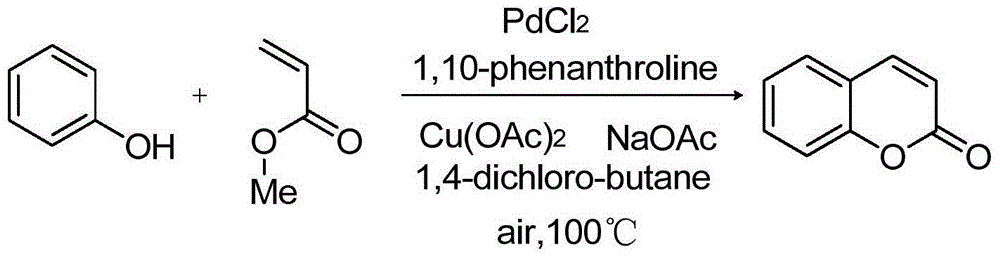
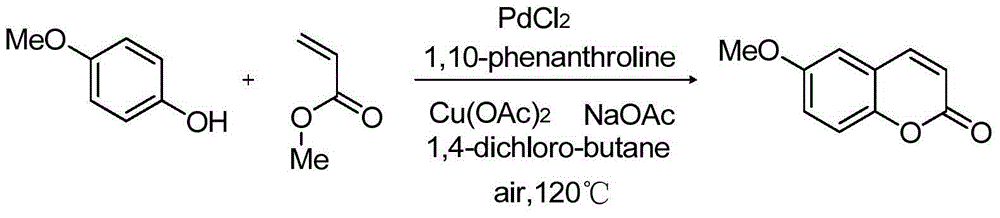
Synthetizing method of coumarin compound
InactiveCN104447654AReaction conditions greenImprove conversion rateOrganic chemistryPhenol CompoundC c coupling
The invention provides a synthetizing method of a coumarin compound. The coumarin compound is mildly and efficiently prepared by using a phenols compound with a substituent group and methyl acrylate as raw materials, performing a reaction in 1,4-dichlorobutane under the action of a catalyst of Pa(II)-Cu(II), a ligand and weak alkali, and performing a C-C coupling reaction. The synthetizing method disclosed by the invention is simple and convenient, is easy to operate, is high in conversion rate, and is higher in yield; fewer by-products are generated in a synthetizing process, the synthetizing method conforms to the requirements of green chemistry, and the synthetizing method is mild and efficient, so that the synthetizing method is suitable for industrial production.
Owner:ANHUI HYEA AROMAS
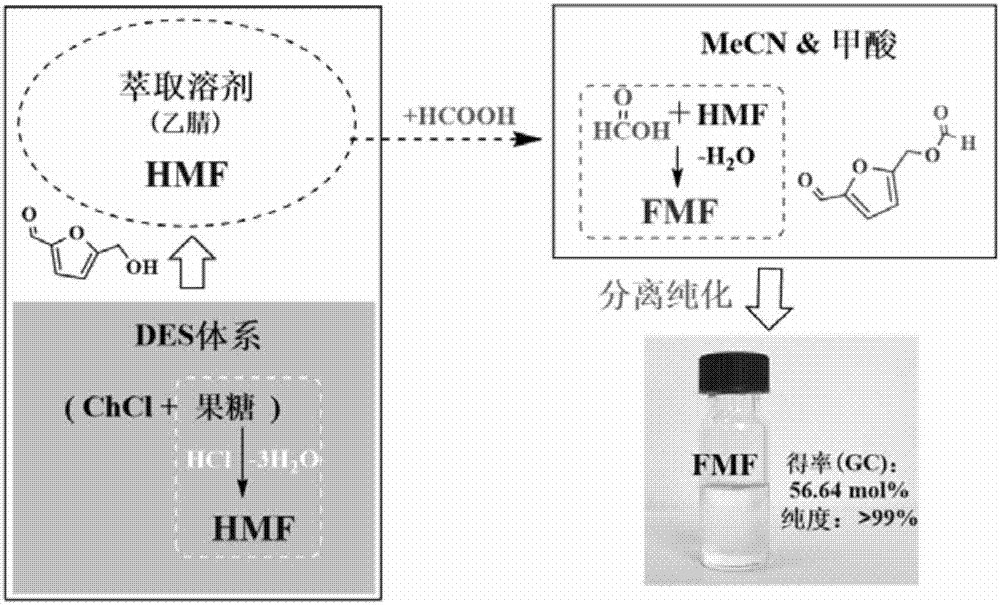
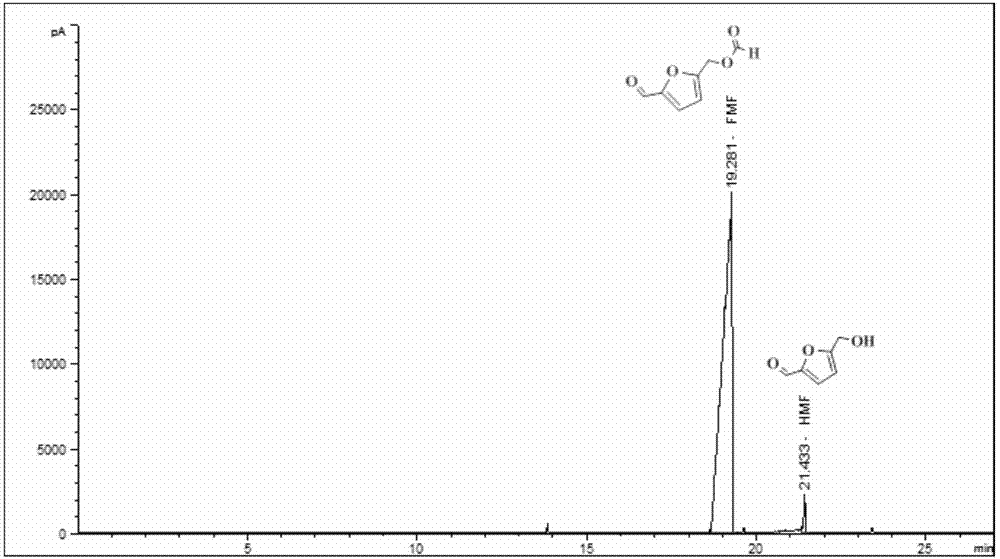
Method for preparing 5-formyloxy methylfurfural from fructose
InactiveCN106995423AWide variety of sourcesIncrease the amount of feedOrganic chemistrySodium bicarbonateSolvent
The invention discloses a method for preparing 5-formyloxy methylfurfural from fructose, and relates to 5-formyloxy methylfurfural. The method specifically comprises the following steps of mixing the fructose and choline chloride into a reaction container, using hydrochloric acid as a catalyst, using a polarity organic solvent as a reaction extracting agent, and heating to react; after reaction is finished, naturally cooling, fetching out reaction liquid, adding a certain amount of anhydrous formic acid into the reaction liquid, and continuing to heat to react; after reaction is finished, relieving pressure and distilling to remove the solvent, using the extracting agent to continuously extract the residues, combining the extracting agent, and relieving pressure and distilling to recycle the extracting agent; under the certain condition, distilling the residual liquid, using a saturated sodium bicarbonate water solution to wash distilling matter, and drying anhydrous magnesium sulfate, so as to obtain the 5-formyloxy methylfurfural with purity more than 99%. The method has the advantages that the reaction condition is mild, the reaction system is green and recyclable, the feeding amount is high, the yield rate is high, a new path is provided for preparation of the 5-formyloxy methylfurfural, and the added value of biomass raw materials is further improved.
Owner:XIAMEN UNIV
Photochemical catalytic synthesis method of aryl olefin compounds
ActiveCN111423299AHigh GC yieldAvoid non-selective degradation reactionsOrganic compound preparationOrganic chemistry methodsOrganic synthesisAlkene
The invention belongs to the field of photochemical organic synthesis, and particularly relates to a photochemical catalytic synthesis method of an aromatic olefin compound, which comprises the following steps: in the presence of light, a photocatalyst, a cocatalyst, a ligand, alkali and a hydrogen donor, carrying out C = C reduction reaction on an aromatic alkyne compound to obtain the aromatic olefin compound. The highest yield of the reaction system product can reach 83%.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
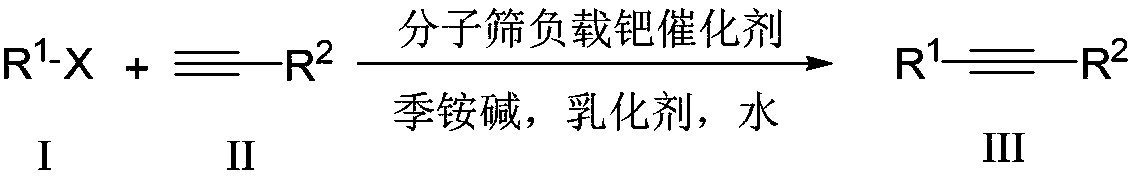
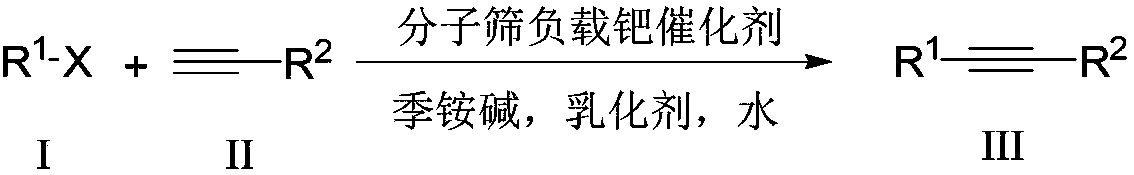
Aqueous phase catalyzed Sonogashira cross-coupling reaction and separation and purification method
ActiveCN109265324AFast processAvoid volatile lossMolecular sieve catalystOrganic compound preparationPurification methodsFiltration
The invention discloses an aqueous phase catalyzed Sonogashira cross-coupling reaction and separation and purification method. The method includes adopting molecular sieve-supported palladium as a catalyst, water as a solvent and quaternary ammonium base as a base to conduct high-efficiency palladium catalysis carbon-carbon bond cross coupling reaction to synthesize an alkyne compound. At higher temperature, catalysis can be completed in 3 minutes, so that a catalyst can conduct catalysis on a fluid bed. The catalyst can be directly reused after being separated in the recycling process, and can be reused for more than 10 times without reducing the activity. An emulsifier and the quaternary ammonium base can also be recycled. A catalytic product can be directly crystallized and precipitatedin the system, and a pure product is obtained by a filtration washing method. The step of column chromatography is omitted, and the experimental operation is simple and safe. In addition, the reaction system has good universal applicability to a reaction substrate and has great practical application value.
Owner:SHAANXI NORMAL UNIV
Diaryl acetate compound and preparation method thereof
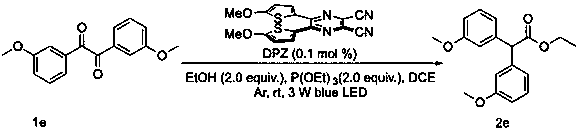
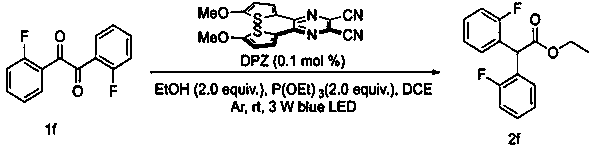
ActiveCN110981720AMild reaction conditionsImprove practicalityOrganic compound preparationCarboxylic acid esters preparationColumn chromatographyPhoto catalysis
Owner:HENAN UNIVERSITY
Dynamic and static combined stirring system and process for preparing chromium salt by using chromite liquid phase oxidation method
ActiveCN110947319ASimple structureReasonable designAluminium silicatesTransportation and packagingChromic saltFluid phase
The invention discloses a dynamic and static combined stirring system. The system comprises a stirrer and further comprises a plurality of static stirring paddles parallel to a stirring shaft of the stirrer, the static stirring paddles are arranged around the stirring shaft, and stirring blades are installed at the bottom of the stirring shaft. In addition, the invention further discloses a process for preparing a chromium salt through chromite liquid phase oxidation by adopting the dynamic and static combined stirring system. The invention provides a novel process for preparing a chromium salt by using the chromite liquid phase oxidation method, the key process problems of solid-liquid separation in the chromite leaching process, separation of the chromium salt in a high-alkali medium andconversion of an intermediate product into a series of chromium salts are innovatively solved, and the method has a great industrial application prospect.
Owner:CHONGQING UNIV OF TECH
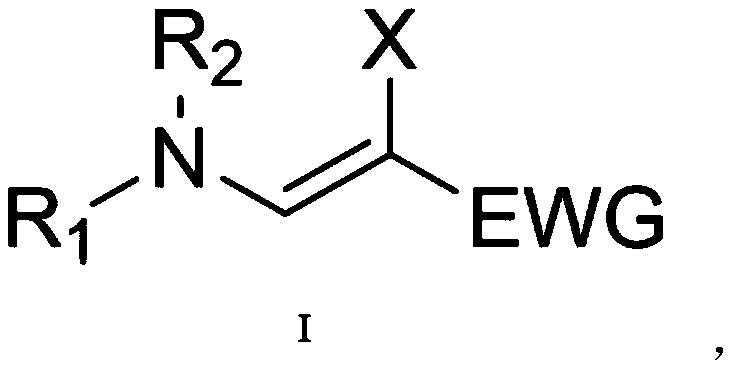
Beta-halogenated enamine acid ester compound and preparation method thereof
ActiveCN111423296AHigh reactivityHigh Synthetic AvailabilityOrganic compound preparationOrganic halogenationMorpholineEnamine
The invention discloses a beta-halogenated enamine acid ester compound and a preparation method thereof. The structure of the beta-halogenated enamine acid ester compound is shown as a formula I whichis described in the specification. In the formula I, R1 and R2 are selected from alkyl groups, morpholine, pyrrole, benzyl groups and hydrogen; X is Br or Cl; and EWG is an electron withdrawing group. The preparation method comprises the following steps: sequentially mixing dimethylformamide, an alkyne-terminated compound and secondary amine or derivatives thereof, carrying out stirring, mixing and sufficient reacting, adding triethylene diamine and N-halogenated imide, carrying out stirring and sufficient reacting at 0-50 DEG C, performing quenching with saturated edible salt water, and allowing a quenched product to pass through a column for purification so as to obtain the beta-halogenated enamine acid ester compound. According to the method, the enamine acid ester compound with high activity is prepared by utilizing metal catalysis-free multi-component reactions, operation is simple and convenient, assembly efficiency is high, automation can be easily realized, and the used raw materials are cheap and easy to obtain; and the method has the advantages of simple, mild and green reaction conditions and good substrate applicability, and can realize high yield in virtue of most amino compounds (especially the secondary amine).
Owner:JIANGSU UNIV OF SCI & TECH
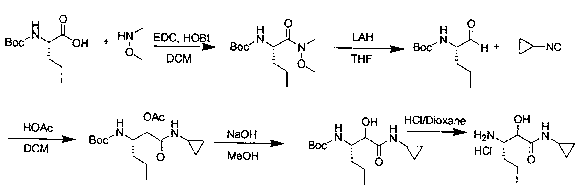
Preparation method of (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride
InactiveCN102702015AEasy to operateMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationHexacosenoic acidPharmaceutical Substances
The invention discloses a preparation method of (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride, and belongs to the technical field of preparation of a medical intermediate. The chiral product of the (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride is obtained by performing the five reaction steps, including epoxidation, resolution, ring opening, esterification and amination, on trans-2-hexenoic acid serving as an initial raw material, wherein water is used as a reaction solvent in the first step of epoxidation. By adoption of the technology, the preparation method has the advantages of convenience in operation, mild reaction conditions, cheap and readily available used raw materials, low toxicity, high product purity reaching over 99.4 percent, high e.e. (Enantionmeric Excess) value reaching over 99.5 percent, low equipment requirement and suitability for large-scale industrial production.
Owner:ZHEJIANG LANGHUA PHARMA
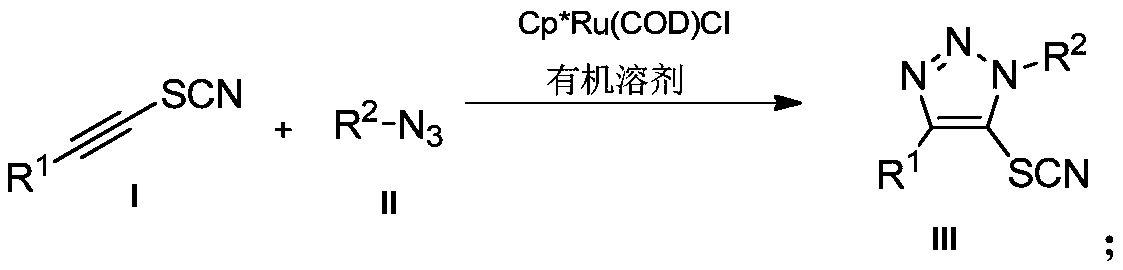
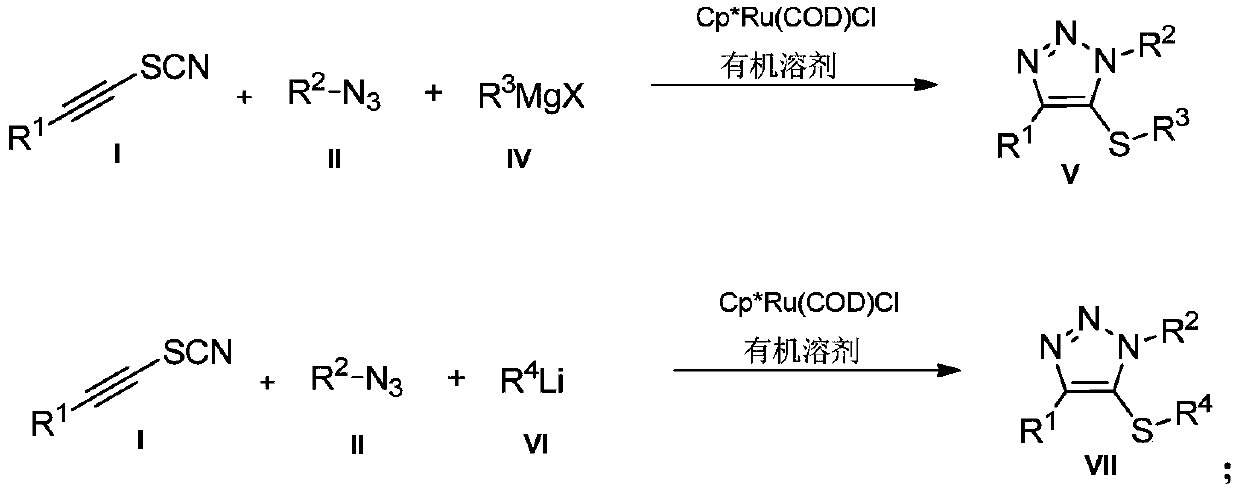
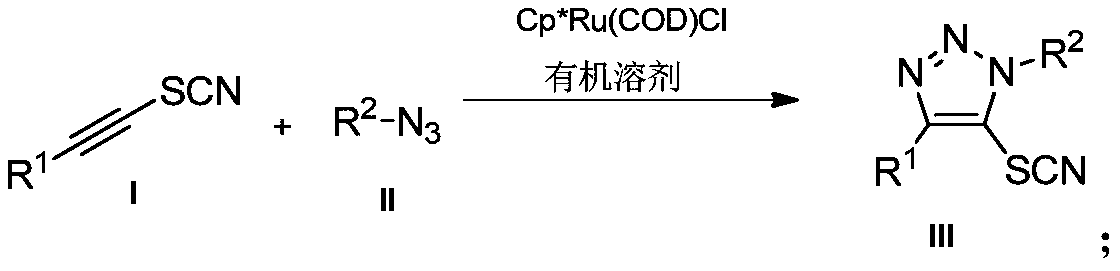
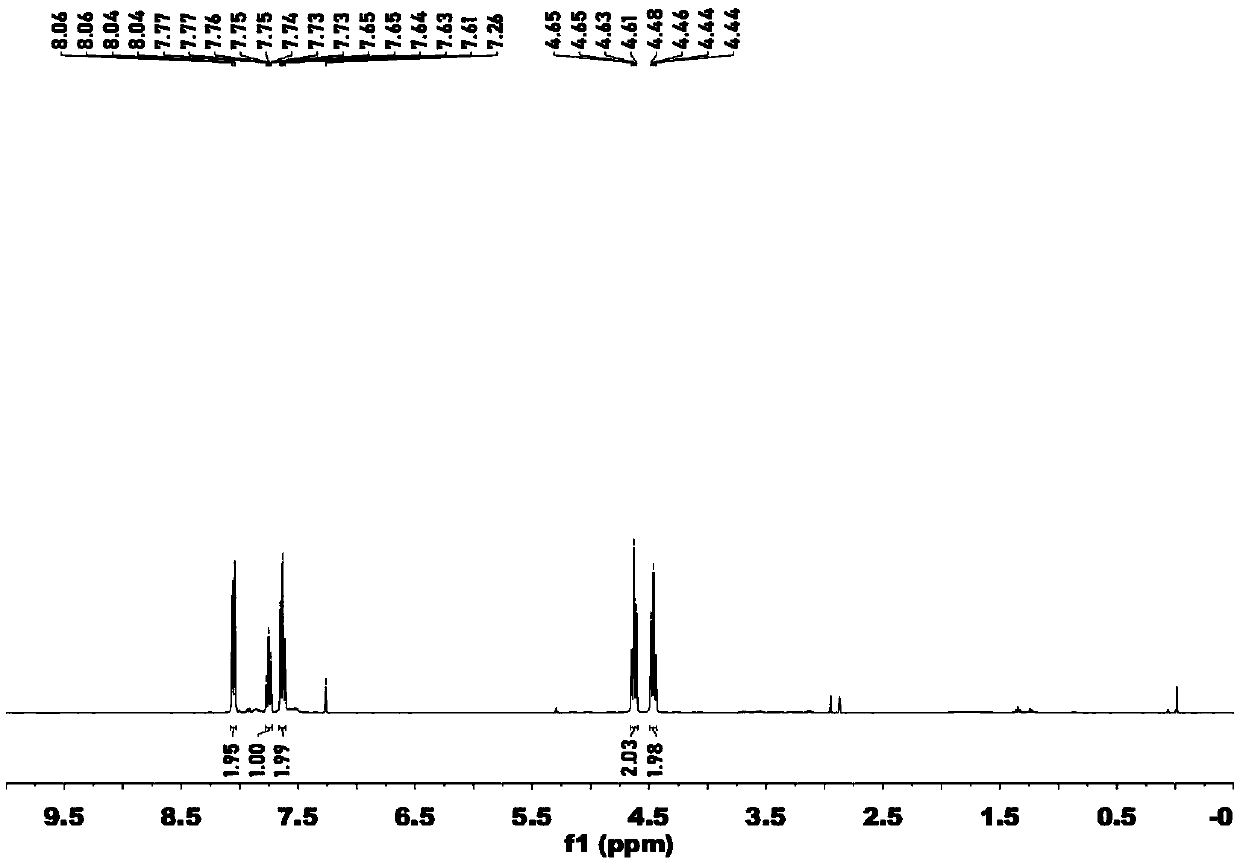
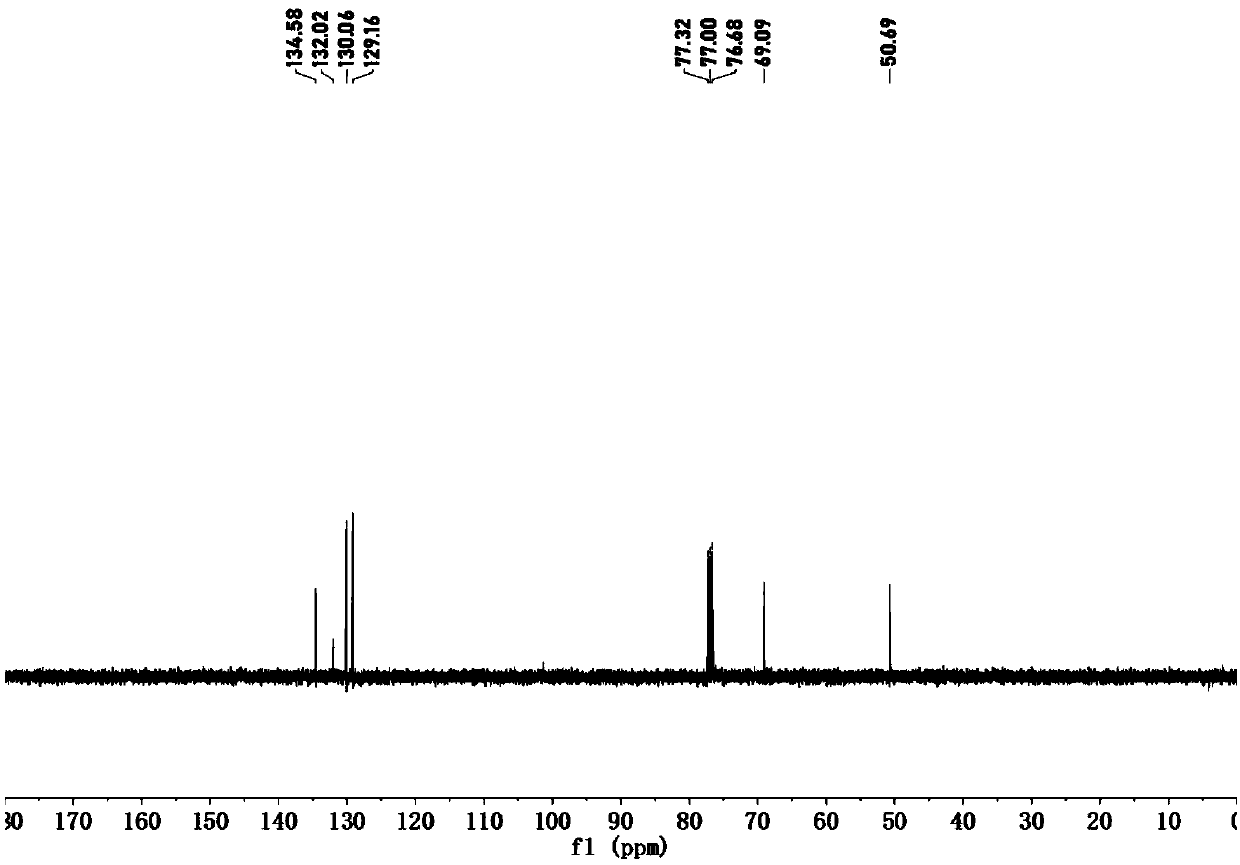
Preparation method and application of novel 5-thiocyanate substituted 1,4,5-trisubstituted 1,2,3-triazole
InactiveCN110590685AMild reaction conditionsReaction conditions greenOrganic chemistryOrganic synthesisThio-
The invention belongs to the technical field of organic synthesis, and provides a preparation method and application of a novel 5-thiocyanate substituted 1,4,5-trisubstituted 1,2,3-triazole. The preparation method of the 5-thiocyanate substituted 1,4,5-trisubstituted 1,2,3-triazole and a preparation method of 5-thio-1,4,5-trisubstituted 1,2,3-triazole have mild reaction conditions, and the productyields are not less than 62%. The preparation methods have the mild reaction conditions, are environmentally friendly and have high reaction efficiency, and the methods are more suitable for large-scale production requirements; and the prepared 5-thio-1,4,5-trisubstituted 1,2,3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
1,2-oxygen azetidines compound and preparation method and application thereof
The invention discloses a 1,2-oxygen azetidines compound and preparation method and application thereof. The N-hydroxyphthalimide is reacted with 1,2-dibromoethane and triethylamine to obtain compoundIII; the compound III is reacted with hydrobromic acid to obtain yellow compound IV; the compound IV, the pyridine and halide R1X are reacted to obtain the compound V; the compound V is reacted withsodium hydride to obtain 1,2-oxygen azetidines compound. The 1,2-oxygen azetidines compound can be used for aminomethylation or hydroxymethylation of the 1-pyrimidyl indole compound; and the reactionprocess has the advantages of being good in region selectivity, high in yield, simple in process condition, mild in reaction condition, green, high in atom economy, and wide in substrate applicable range.
Owner:NANJING UNIV OF TECH
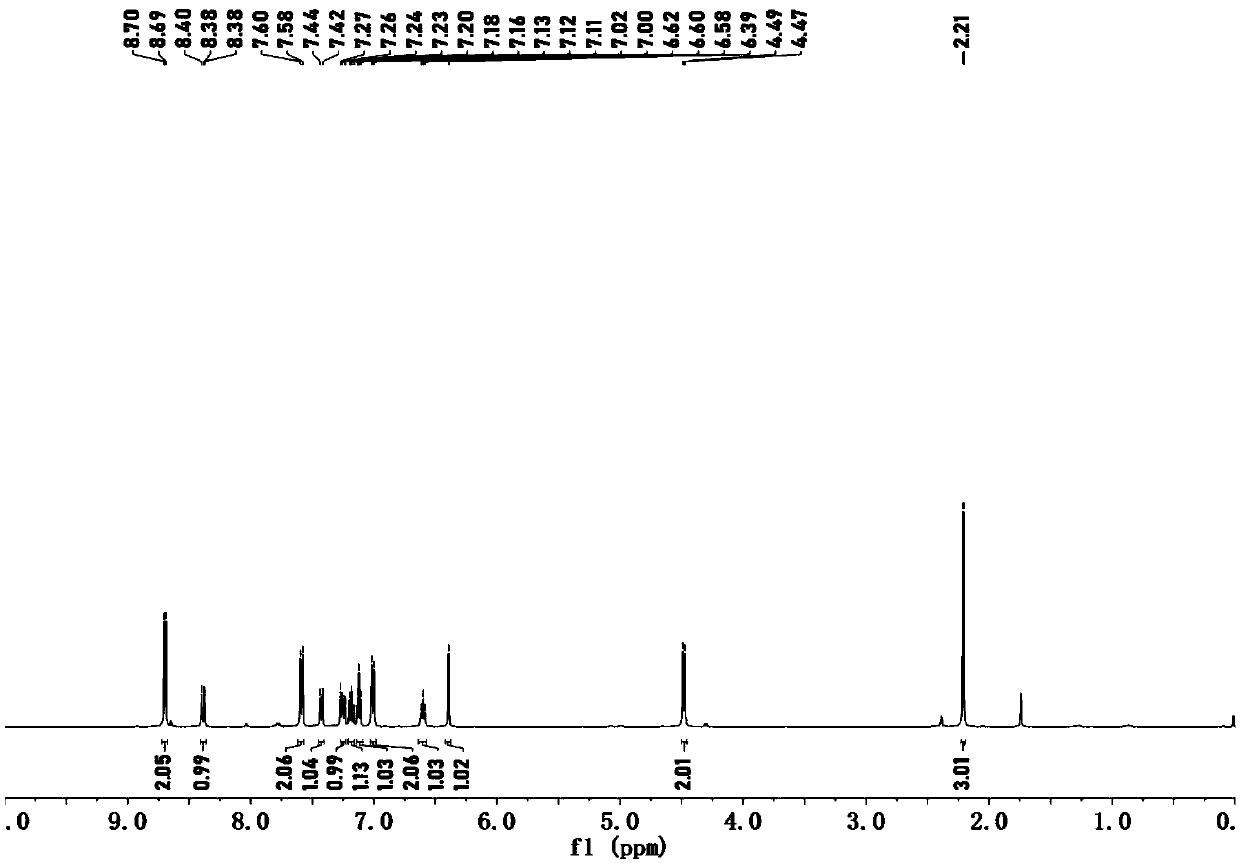
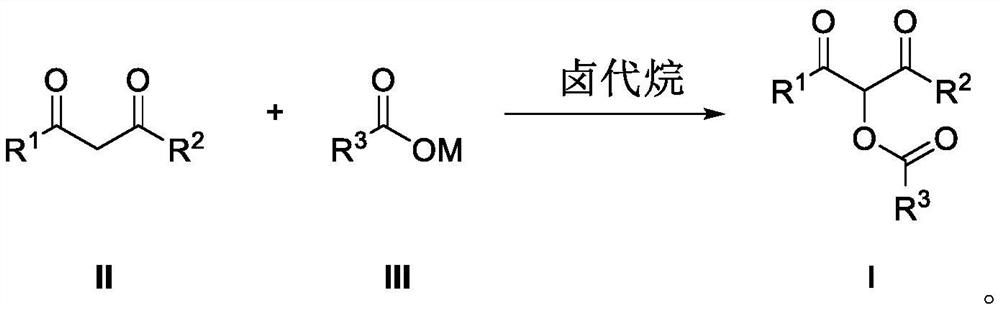
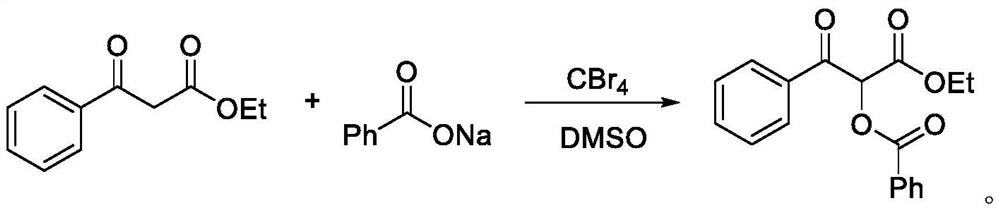
Preparation method of alpha-acyloxy ketone compound
PendingCN113200850AMild reaction conditionsEasy to buildPreparation by caroxylic acid/anhydride-hydrocarbon reactionCarboxylic acid ester formation/introductionCarboxylateKetone
The invention discloses a preparation method of an alpha-acyloxy ketone compound, which comprises the following steps of adding a 1, 3-dicarbonyl compound, carboxylate and a catalyst alkyl halide into an organic solvent, and stirring and reacting for 0.5-1 hour at the temperature of 20-30 DEG C to obtain a reaction product, namely a mixture, performing purification treatment on the mixture to obtain alpha-acyloxy ketone, wherein the molar ratio of the 1, 3-dicarbonyl compound to the carboxylate to the alkyl halide is 1: 1: 1. According to the method, carboxylate with low cost is selected as an acyloxylation reagent, reaction conditions are mild and green, a 1, 3-dicarbonyl compound is taken as a raw material, and a novel method for efficiently, simply and conveniently constructing a C-O bond is successfully realized through activation of a carbonyl alpha-position C-H bond and subsequent cascade reaction, so that a series of alpha-acyloxyketone compounds are obtained.
Owner:WEINAN NORMAL UNIV
Nickel/copper catalyst and preparation method thereof, and method for directly preparing 1,2-hexanediol from cellulosan by using nickel/copper catalyst
InactiveCN103055870BHigh catalytic activityLarge specific surface areaOrganic compound preparationHydroxy compound preparationFiberCarrying capacity
The invention relates to a supported nickel / copper catalyst and a preparation method thereof, and a method for preparing 1,2-hexanediol from cellulosan by using the supported nickel / copper catalyst. The weight ratio of nickel to copper in the catalyst is between 1:10 and 10:1. Based on the total weight of the catalyst, the carrying capacity of the nickel is 0.05 to 40 percent, and the carrying capacity of the copper is 0.05 to 50 percent; meanwhile, the preparation process for the catalyst is simple; and the method for preparing 1,2-hexanediol from cellulosan by using the catalyst can be used for highly selectively preparing 1,2-hexanediol, and compared with conventional hexylene routes, the method has significant advantages of reproducible raw materials, environment-friendly reaction process and the like.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI +1
A kind of preparation method of sulfophenylpyrazolone and its intermediate
ActiveCN105646356BMild reaction conditionsSafe reaction conditionsSulfonic acid esters preparationThio-Chloride
The invention discloses a preparation method of sulfophenylpyrazolone. The method uses compound (I) as a raw material, carries out a substitution reaction with compound (II) in the presence of a base, and then undergoes oxidation with an oxidant to prepare compound (III), compound (III) is esterified to obtain compound (IV), compound ( IV) Compound (VI) is obtained by thioreaction, compound (VI) is then oxidized, hydrolyzed (or hydrolyzed, oxidized) to obtain compound (VII), and compound (VII) is subjected to acid chloride to obtain acid chloride (VIII) and 1 , 3-dimethyl-5-hydroxypyrazole undergoes an esterification reaction to obtain compound (IX), and finally compound (IX) is rearranged to obtain compound (X). The beneficial effects of the invention are mainly reflected in the following: the raw materials are cheap and easy to obtain, the reaction conversion rate is high, the three wastes are few, and the industrial production is favorable.
Owner:ZHEJIANG ZHUJI UNITED CHEM
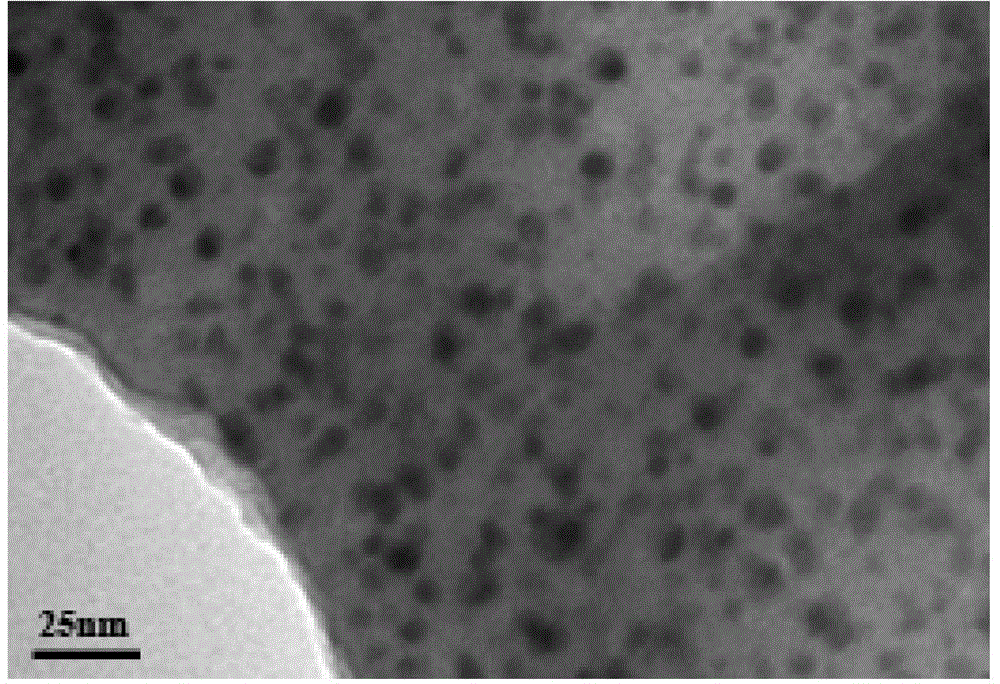
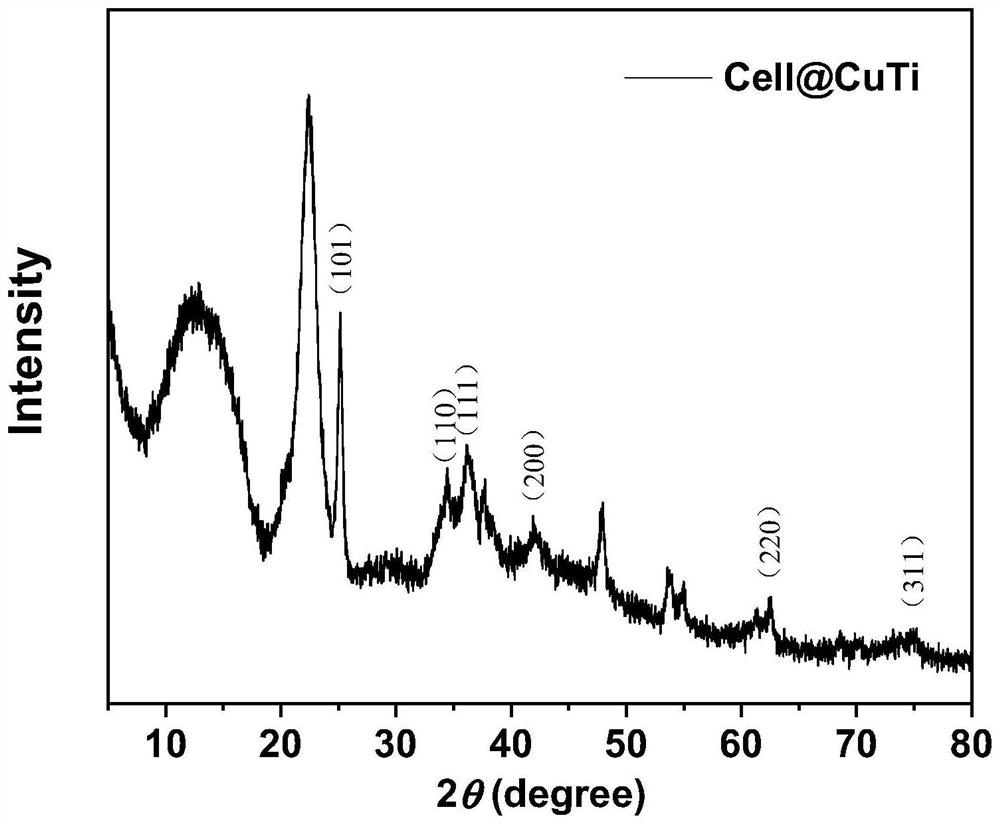
Cellulose-loaded heterojunction catalytic material and method for preparing chiral boride from cellulose-loaded heterojunction catalytic material
ActiveCN114570428AGood biocompatibilityEasy to separateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystOrganosolv
The invention discloses a cellulose supported heterojunction catalytic material and a method for preparing chiral boride, and the method for preparing the chiral boride comprises the following steps: mixing alpha, beta-unsaturated ester I, bis (pinacolato) diboron, a catalytic material Cell (at) CuTi and a ligand, adding toluene and water, and carrying out asymmetric boronation reaction of the alpha, beta-unsaturated ester. The catalytic material Cell (at) CuTi has high catalytic activity and stable properties, can be applied to catalysis of asymmetric boron addition reactions of different types of alpha, beta-unsaturated esters, and has the advantages of small catalyst dosage, mild reaction conditions, no need of using a large amount of organic solvents, high product yield and high enantioselectivity; the reaction is carried out at room temperature in an air environment, anhydrous and anaerobic operation is not needed, and the method is simple, convenient and wide in application and has the advantage of a one-pot method; and the catalytic material can be repeatedly used and has potential industrial application value.
Owner:HUBEI ENG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com