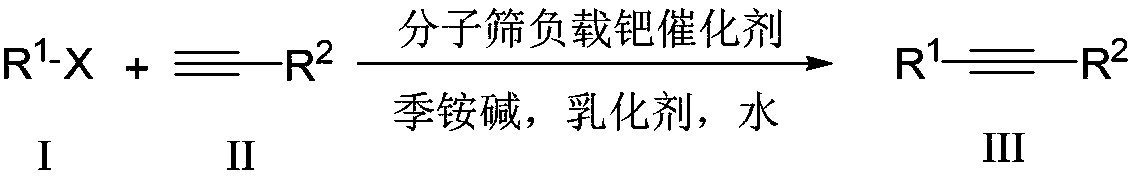
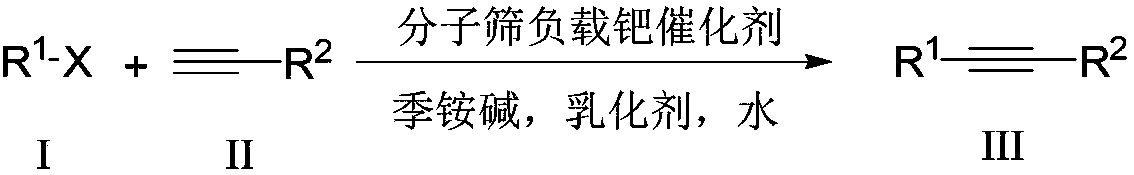
Aqueous phase catalyzed Sonogashira cross-coupling reaction and separation and purification method
A technology of cross-coupling reaction and purification method, which is applied in the field of Sonogashira cross-coupling reaction catalyzed by trace amounts of palladium loaded on molecular sieves. Good recyclability, good recyclability, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 0.121g (0.5mmol) of 4-methoxyiodobenzene, 0.072mL (0.65mmol) of phenylacetylene, 0.01g of Pd(Ⅱ)@Y (where Pd is 0.16mmol), 0.185mL of 25% tetraethyl Aqueous ammonium hydroxide solution (tetraethylammonium hydroxide is 1 mmol), 0.5 mL Triton X-100, and 3 mL water were added to the reaction flask, reacted at 100 ° C for 4 minutes, stopped the reaction, naturally cooled to room temperature, and filtered , the recovered filter cake can be directly used as a catalyst for further catalysis. The filtrate from which Pd(II)@Y was removed was left standing at room temperature for 1 hour, and a pale yellow solid precipitated out, which was then fully crystallized at -2°C for 2 hours, filtered and washed with water to obtain pure 1-methoxy-4- (Phenylethynyl)benzene, the yield was 97.0%. To the filtrate from which 1-methoxy-4-(phenylethynyl)benzene was removed, 4-methoxyiodobenzene, phenylacetylene and recovered Pd(II)@Y were added directly, and the reaction was repeated as describe...
Embodiment 2
[0019] In this example, replace Pd(II)@Y in Example 1 with 0.005g Pd(0)@Y (where Pd is 0.000016mmol), and react at 100°C for 4 hours, other steps are the same as in Example 1 , and pure 1-methoxy-4-(phenylethynyl)benzene was obtained in a yield of 97.0%.
Embodiment 3
[0021] In this example, the 4-methoxy iodobenzene in Example 1 was replaced with an equimolar iodobenzene, and reacted at 100°C for 3 minutes, and the other steps were the same as in Example 1 to obtain pure 1,2-diphenyl Acetylene, its yield was 98.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com