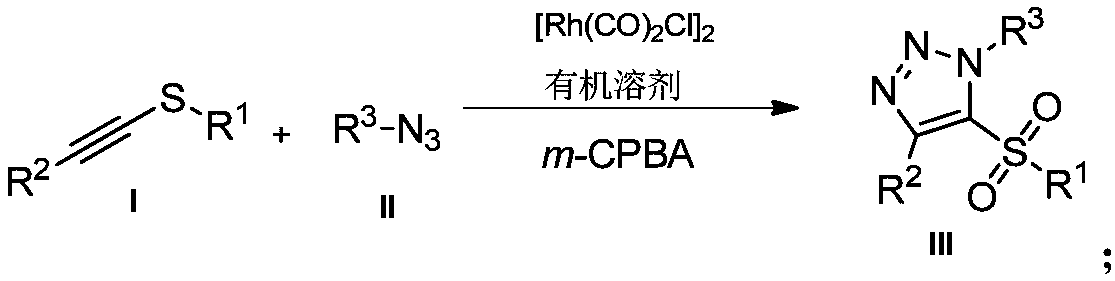
Novel preparation method of 5-sulfonyl-1,4,5-trisubstituted 1,2,3-triazole
A sulfonyl and tri-substituted technology, applied in the field of organic synthesis, can solve the problems such as no public report on the preparation technology, and achieve the effects of high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
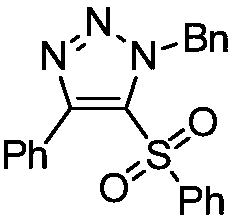
[0021] Example 1: Preparation of 5-benzenesulfonyl-(1-benzyl)-4-phenyl-1H-1,2,3-triazole
[0022] Under air, 1-phenylethynylphenylsulfide (0.2mmol, 42.0mg) was dissolved in chloroform (2mL), and benzyl azide (0.3mmol, 40.2mg) and [Rh(CO) 2 Cl] 2 (0.005mmol, 1.9mg), stirred the reaction mixture at 40°C, reacted for 12h, then added m-chloroperoxybenzoic acid (0.6mmol, 103.5mg), reacted at room temperature for 3h, and separated by column chromatography after the reaction to obtain 62mg of a yellow solid product , yield 80%.
[0023]
[0024] Mp=139-141°C. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.78(d, J=8.0Hz, 2H), 7.58(t, J=8.0Hz, 1H), 7.52(t, J=8.0Hz, 1H), 7.46-7.38(m, 4H) ,7.21(t,J=8.0Hz,1H),7.19-7.13(m,2H),6.84-6.79(m,4H),4.34(t,J=8.0Hz,2H),3.12(t,J=8.0 Hz,2H). 13 C NMR (100MHz, CDCl 3 ): δ145.2, 140.9, 139.6, 136.4, 133.5, 130.5, 129.8, 129.0, 128.8, 128.6, 128.6, 127.8, 127.1, 124.1, 49.9, 36.3. HRMS (ESI-TOF) m / z calcd for C 22 h 19 N 3 o 2 S(M+Na) + 412.1090,foun...
Embodiment 2
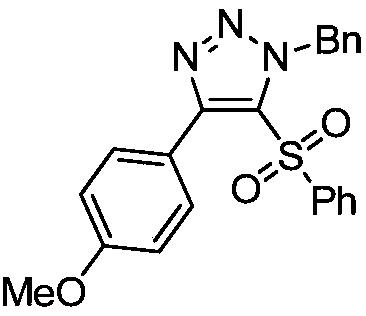
[0025] Example 2: Preparation of 5-benzenesulfonyl-(1-benzyl)-4-p-methoxyphenyl-1H-1,2,3-triazole
[0026] Under air, 1-p-methoxyphenylethynylphenylsulfide (0.2mmol, 48.0mg) was dissolved in chloroform (2mL), and then benzyl azide (0.3mmol, 40.2mg) and [Rh(CO ) 2 Cl] 2 (0.005mmol, 1.9mg), stirred the reaction mixture at 40°C, reacted for 12h, then added m-chloroperoxybenzoic acid (0.6mmol, 103.5mg), reacted at room temperature for 3h, and separated by column chromatography after the reaction to obtain a colorless liquid product 61 mg, yield 75%.
[0027]
[0028] 1 HNMR (400MHz, CDCl 3 , TMS): δ7.57(d, J=8.0Hz, 2H), 7.42(t, J=8.0Hz, 1H), 7.35-7.33(m, 5H), 7.18-7.12(m, 4H), 6.96( d, J=12.0Hz, 2H), 6.06(s, 2H), 3.88(s, 3H). 13 C NMR (100MHz, CDCl 3 ): δ160.7, 150.1, 140.0, 134.7, 133.9, 131.8, 131.5, 128.9, 128.8, 128.5, 128.1, 127.0, 121.1, 113.6, 55.3, 54.5. HRMS (ESI-TOF) m / z calcd for C 22 h 19 N 3 o 3 S(M+Na) + 428.1039, found 428.1043.
Embodiment 3
[0029] Example 3: Preparation of 5-benzenesulfonyl-(1-benzyl)-4-p-bromophenyl-1H-1,2,3-triazole
[0030] Under air, 1-p-bromophenylethynylphenylsulfide (0.2mmol, 57.6mg) was dissolved in chloroform (2mL), and benzyl azide (0.3mmol, 40.2mg) and [Rh(CO) 2 Cl] 2 (0.005mmol, 1.9mg), stirred the reaction mixture at 40°C, reacted for 12h, then added m-chloroperoxybenzoic acid (0.6mmol, 103.5mg), reacted at room temperature for 3h, and separated by column chromatography after the reaction to obtain 64mg of white solid product , yield 71%.
[0031]
[0032] Mp=141-143°C. 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.57(dd,J=12.0,8.0Hz,4H),7.44(t,J=8.0Hz,1H),7.36-7.34(m,5H),7.20-7.13(m,4H), 6.06(s,2H). 13 C NMR (100MHz, CDCl 3 ): δ149.0, 139.7, 134.5, 134.2, 132.5, 131.7, 131.4, 129.0, 128.9, 128.6, 128.1, 127.8, 127.0, 124.3, 54.5. HRMS (ESI-TOF) m / z calcd for C 21 h 16 BrN 3 o 2 S(M+Na) +476.0039,found 476.0042.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com