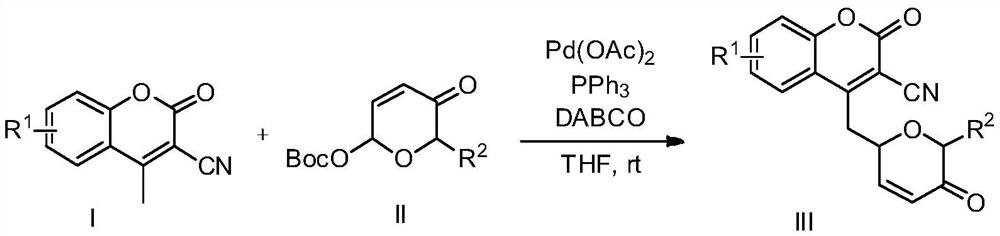
Preparation method of novel dihydropyrone coumarin compound
A dihydropyrone-based, dihydropyrone-based technology, applied in the field of preparation of new dihydropyrone-based coumarin compounds, to achieve high stereoselectivity, mild reaction conditions, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
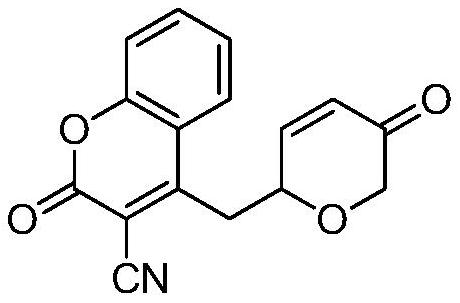
[0016] Example 1: Preparation of 4-(2-(5-oxo-5,6-dihydro-2H-pyranyl)methyl)-3-cyanocoumarin
[0017] In air, the Pd(OAc) 2 (0.01mmol), PPh 3 (0.015mmol), DABCO (0.3mmol) were dissolved in THF (4mL), added 2-(5-oxo-5,6-dihydro-2H-pyranyl) tert-butyl carbonate (0.2mmol) and 3- Cyano-4-methylcoumarin in the mixture. The mixture was stirred at room temperature for 12 h, then filtered, spin-dried and separated by column chromatography to obtain 35 mg of white solid product, yield 63%
[0018]
[0019] Mp = 174-176°C. 1 H NMR (400MHz, CDCl 3 )δ=7.85(dd, J=8.3,1.4Hz,1H),7.73(ddd,J=8.7,7.4,1.5Hz,1H),7.43(ddd,J=7.1,3.6,1.1Hz,2H),7.13 (dd, J=10.5,1.7Hz,1H),6.28(dd,J=10.5,2.3Hz,1H),4.82(ddd,J=8.7,4.3,2.2Hz,1H),4.24(d,J=16.4 Hz, 1H), 4.05(dd, J=16.4, 1.8Hz, 1H), 3.60–3.38(m, 2H). 13 C NMR (126MHz, CDCl 3 )δ=195.9, 161.9, 156.5, 153.7, 147.6, 135.4, 128.1, 127.4, 125.1, 118.2, 117.7, 113.6, 103.5, 73.1, 36.5, 15.1. HRMS (ESI) m / z calcd for C 16 h 11 NO 4 (M+Na) + 304.0586,fo...
Embodiment 2
[0020] Example 2: Preparation of 6-methoxy-4-(2-(5-oxo-5,6-dihydro-2H-pyranyl)methyl)-3-cyanocoumarin
[0021] In air, the Pd(OAc) 2 (0.01mmol), PPh 3 (0.015mmol), DABCO (0.3mmol) was dissolved in THF (4mL), added tert-butyl 2-(5-oxo-5,6-dihydro-2H-pyranyl)carbonate (0.2mmol) and 3 - Cyano-4-methyl-6-methoxycoumarin (0.3 mmol) in the mixture. The mixture was stirred at room temperature for 12 h, then filtered, spin-dried and separated by column chromatography to obtain 37 mg of a white solid product with a yield of 60%.
[0022]
[0023] Mp=178-180°C. 1 H NMR (400MHz, CDCl 3 )δ=7.36(d,J=9.1Hz,1H),7.30(dd,J=9.1,2.8Hz,1H),7.23(d,J=2.8Hz,1H),7.13(dd,J=10.5,1.7 Hz,1H),6.27(dd,J=10.5,2.3Hz,1H),4.82(ddd,J=8.4,4.5,2.1Hz,1H),4.25(d,J=16.4Hz,1H),4.06(dd ,J=16.4,1.8Hz,1H),3.88(s,3H),3.52–3.36(m,2H). 13 C NMR (126MHz, CDCl 3 )δ=193.2, 161.3, 156.6, 156.6, 148.1, 148.0, 128.3, 122.6, 118.8, 118.5, 113.7, 109.6, 103.9, 72.9, 71.4, 56.1, 36.2. HRMS (ESI) m / z calcd for C 17 h 13...
Embodiment 3
[0024] Example 3: Preparation of 3-cyano-4-(2-(5-oxo-5,6-dihydro-2H-pyranyl)methyl)6-methylcoumarin
[0025] In air, the Pd(OAc) 2 (0.01mmol), PPh 3 (0.015mmol), DABCO (0.3mmol) was dissolved in THF (4mL), added tert-butyl 2-(5-oxo-5,6-dihydro-2H-pyranyl)carbonate (0.2mmol) and 3 - Cyano-4,6-dimethylcoumarin (0.3 mmol) in the mixture. The mixture was stirred at room temperature for 12 h, then filtered, spin-dried and separated by column chromatography to obtain 38 mg of a white solid product with a yield of 65%.
[0026]
[0027] Mp=198-200℃. 1 H NMR (400MHz, CDCl 3)δ=7.54(dd, J=14.0,5.6Hz,2H),7.32(d,J=8.4Hz,1H),7.11(dd,J=10.5,1.7Hz,1H),6.29(d,J=2.3 Hz, 1H), 4.81(ddd, J=8.8, 4.2, 2.1Hz, 1H), 4.25(d, J=16.4Hz, 1H), 4.06(dd, J=16.4, 1.8Hz, 1H), 3.46(dd ,J=16.2,6.8Hz,2H),2.47(s,3H). 13 C NMR (126MHz, CDCl 3 )δ=193.2, 161.4, 156.6, 152.0, 148.0, 136.6, 135.3, 128.3, 126.3, 117.7, 117.6, 113.7, 103.5, 72.9, 71.4, 35.9, 21.1. HRMS (ESI) m / z calcd for C 17 h 13 NO 4 (M+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com