Preparation method of ezetimibe and its intermediate
A technology for ezetimibe and intermediates, which is applied in the field of preparation of ezetimibe and its intermediates, can solve the problems of unsuitability for industrial production, harsh reaction conditions, low optical purity, etc., and achieve d.e. value and yield High efficiency, mild reaction conditions and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
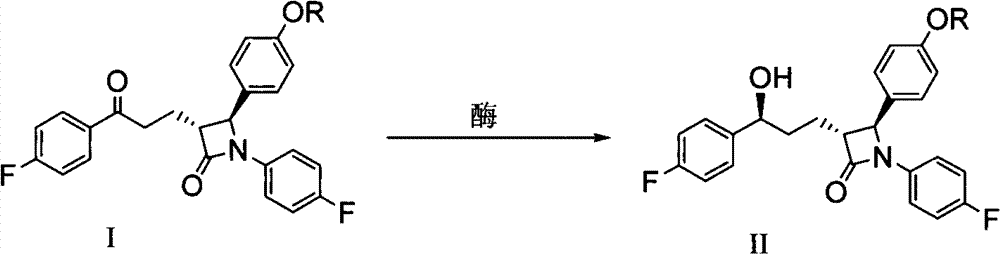
Image
Examples
Embodiment 17
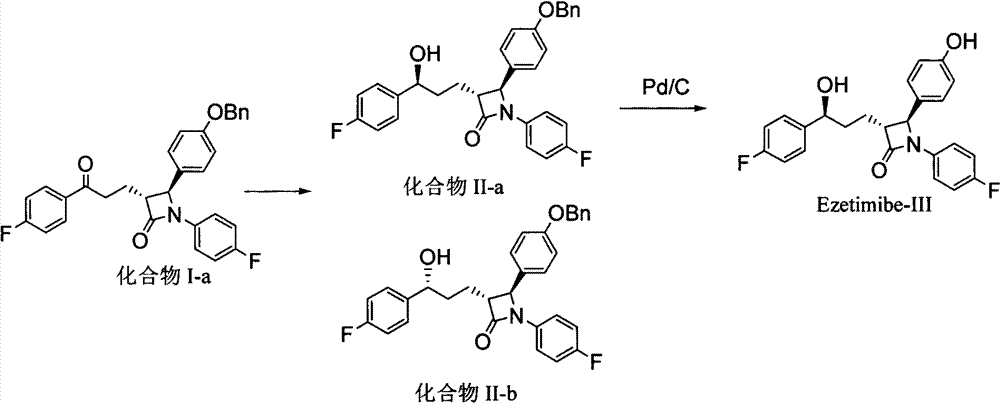
[0062] Example 17 Synthesis of Ezetimibe
[0063] Add 3 g of palladium carbon (10% Pd / C) to the reaction solution obtained in Example 8, add 5 psi of hydrogen at room temperature, stir for 5 to 8 hours, then filter and concentrate to obtain a crude solid, which is then recrystallized with tert-butanol and water , 3.8 g of pure ezetimibe can be obtained with a purity of 99% and a two-step yield of 92%.
[0064] 1 H NMR (400MHz, DMSO-d6): δ9.82(s, 1H), 9.16(s, 1H), 7.57-7.55(m, 2H), 7.31-7.29(m, 2H), 7.14-7.10(m, 4H), 7.0-6.95(m, 2H), 6.66-6.63(m, 2H), 5.22(d, J=4.4, 1H), 4.50-4.49(m, 1H), 2.80-2.75(m, 1H), 1.64-1.52 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com