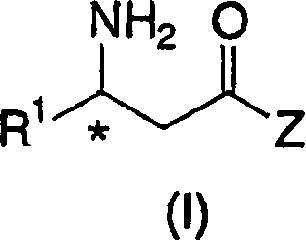
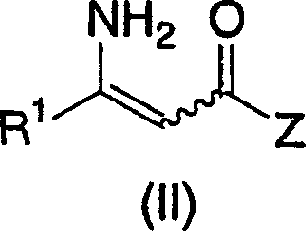
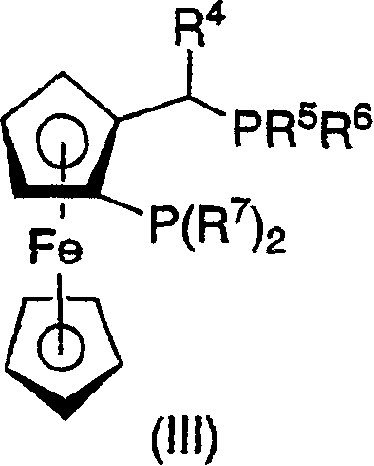
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
A compound and alkyl technology, applied in the field of preparation of chiral β-amino acid derivatives by asymmetric hydrogenation, can solve problems such as difficult synthesis of reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098]
[0099] (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine- 7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine (2-5)
[0100] Preparation of 3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazine, hydrochloride (1-4)
[0101] Reaction scheme 1
[0102]
[0103]
[0104] Step A : Preparation of bishydrazide (1-1)
[0105] Hydrazine (20.1 g, 35 wt % solution in water, 0.22 mol) was fainted with 310 mL of acetonitrile. 31.5 g of ethyl trifluoroacetate (0.22 mol) were added over 60 minutes. Increase the internal temperature from 14°C to 25°C. The resulting solution was aged at 22-25°C for 60 minutes. The solution was cooled to 7°C. 17.9 g of 50 wt% aqueous NaOH (0.22 mol) and 25.3 g of chloroacetyl chloride (0.22 mol) were added simultaneously at a temperature below 16°C over 130 minutes. When the reaction was complete, the mixture was vacuum distilled at 26-27 Hg and 27-30° C. to remove water and ethanol. D...
Embodiment 2
[0159]
[0160] (3S)-3-Amino-3-(6-methylhydropyridin-3-yl)propionic acid methyl ester (3-2)
[0161] Add (1,5-cyclooctadiene)rhodium(I) chloride dimer {[Rh(cod)Cl] to a 7 mL bottle under nitrogen atmosphere 2} (14.2 mg, 0.029 mmol) and (R,S)-t-Bu Josiphos (31.3 mg, 0.058 mmol). Degassed methanol (1 mL) was then added and the catalytic complex was stirred at room temperature for 45 minutes. In a separate 2-mL bottle, the enaminoester 3-1 (0.1 g, 0.5 mmol) was dissolved in 0.9 mL of distilled 2,2,2-trifluoroethanol. Add 0.1 mL of the prepared catalyst solution to the same bottle to obtain a 1 mol% catalyst loading and a 90 / 10 mixture of 2,2,2-trifluoroethanol / methanol. The hydrogenation bottle was then sealed and transferred to a hydrogenation vessel under nitrogen. After degassing with hydrogen 3 times, the enaminoester was hydrogenated under 90-psig hydrogen at 50°C for 13.5 hours. The yield by HPLC was 88%, and the optical purity was 89% ee.
[0162] 1 H-NMR (400M...
Embodiment 3-6
[0164]
[0165] Ex.
[0166] A: Reaction condition: 0.15mol% [Rh(cod)Cl] 2 ; 0.33mol% (R, S)-t-BuJosiphos, 50°C, 100psig H 2 . B Determined yield; c Determined by chiral HPLC using AS-RH or AD-RH chiral columns, eluting with 25-40% acetonitrile / water as mobile phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com