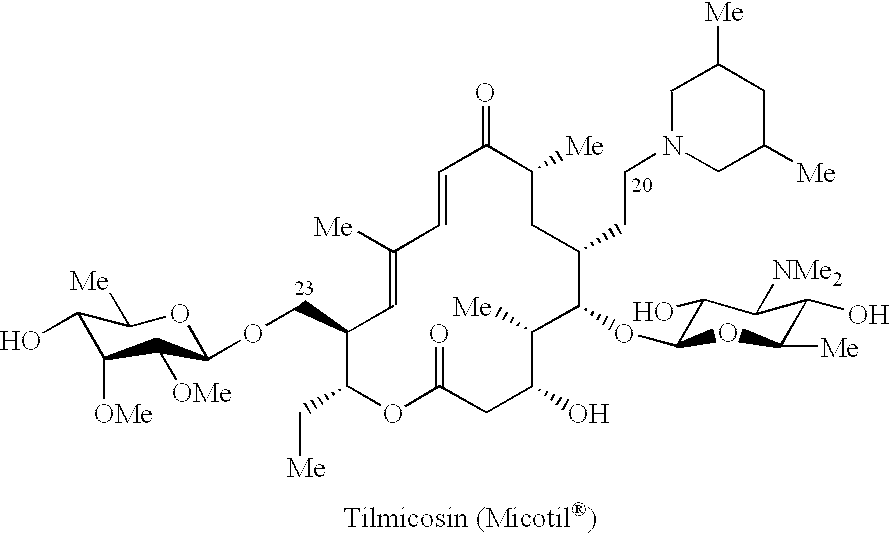
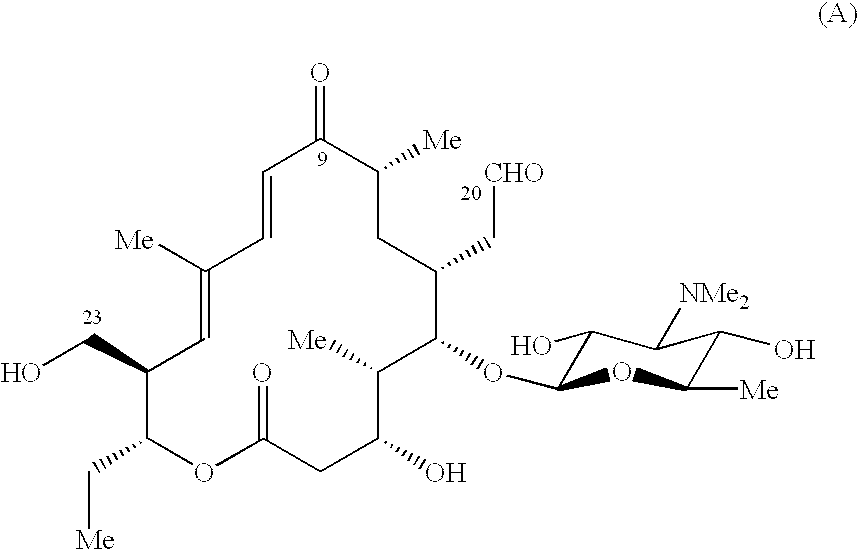
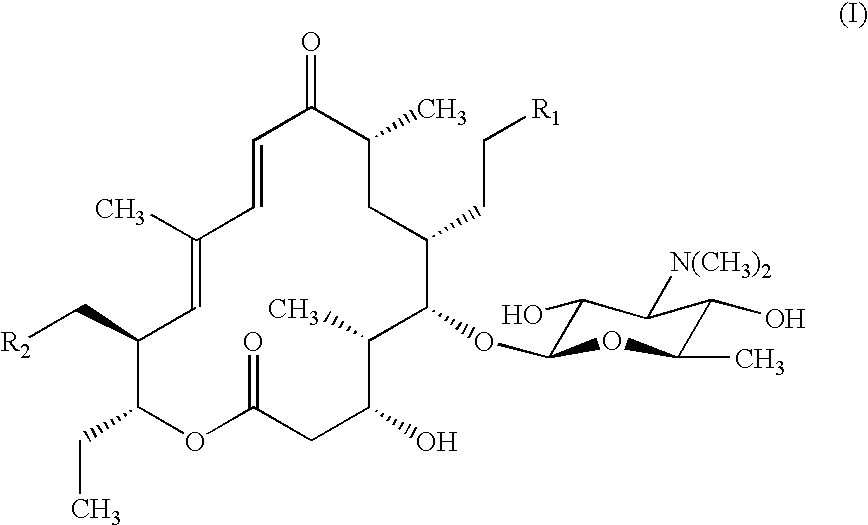
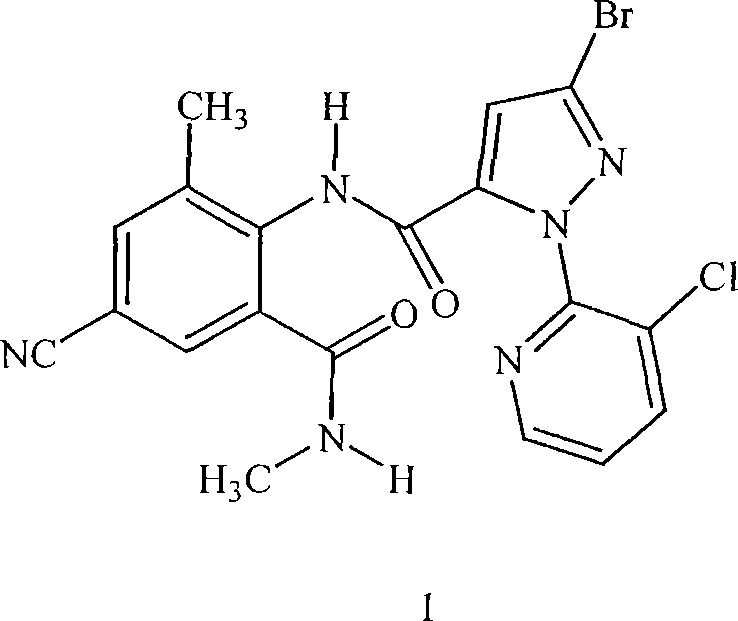
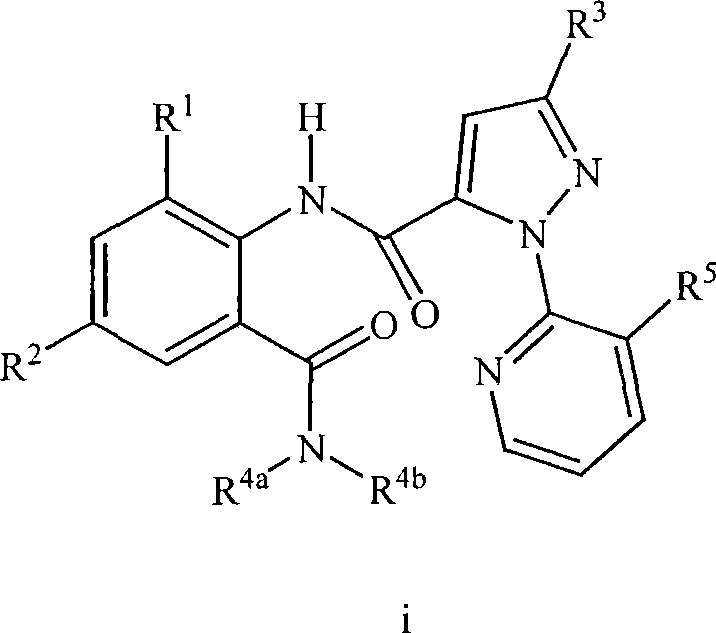
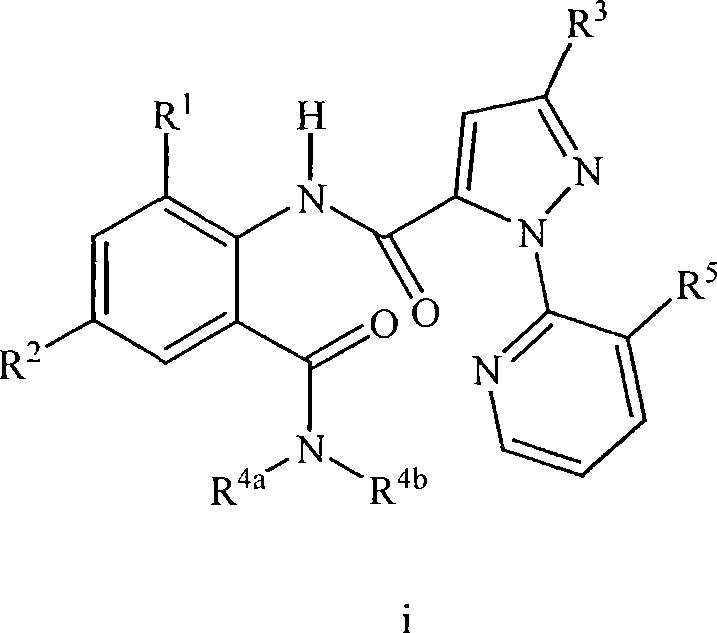
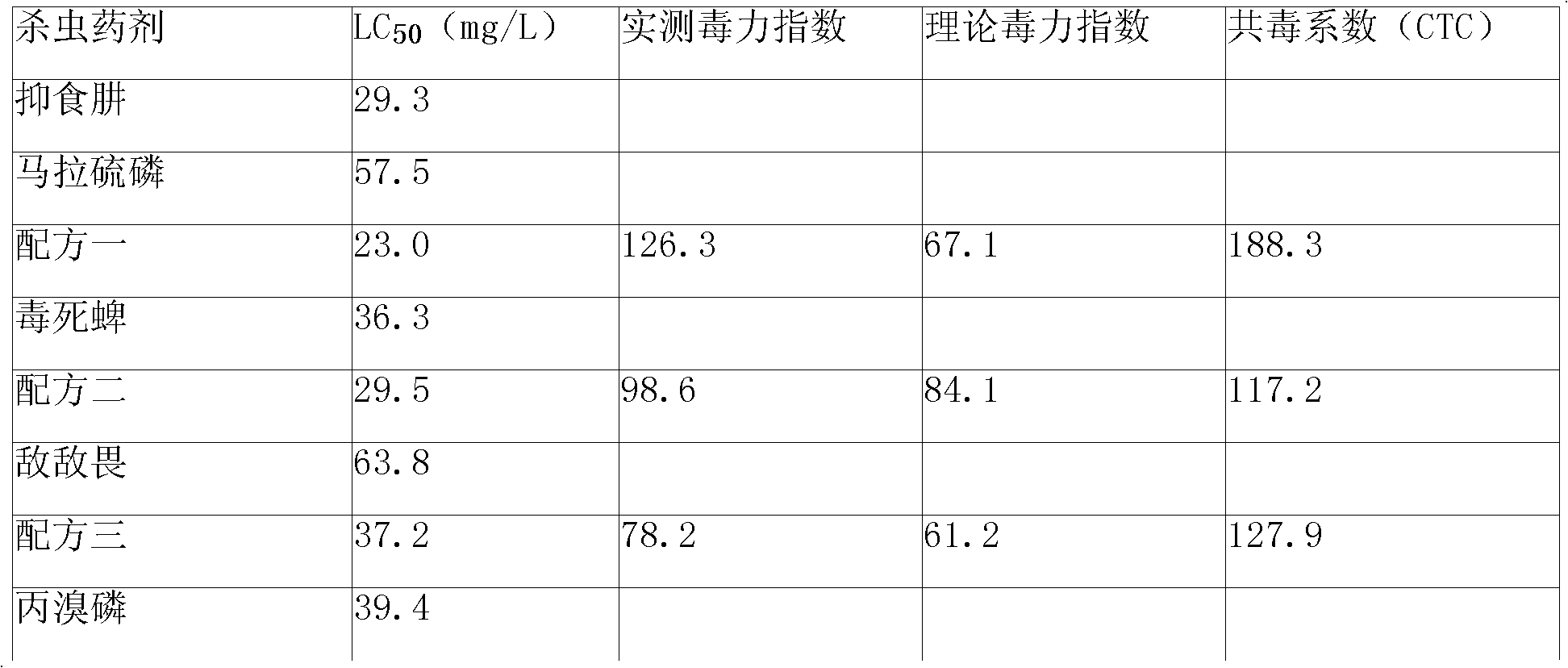
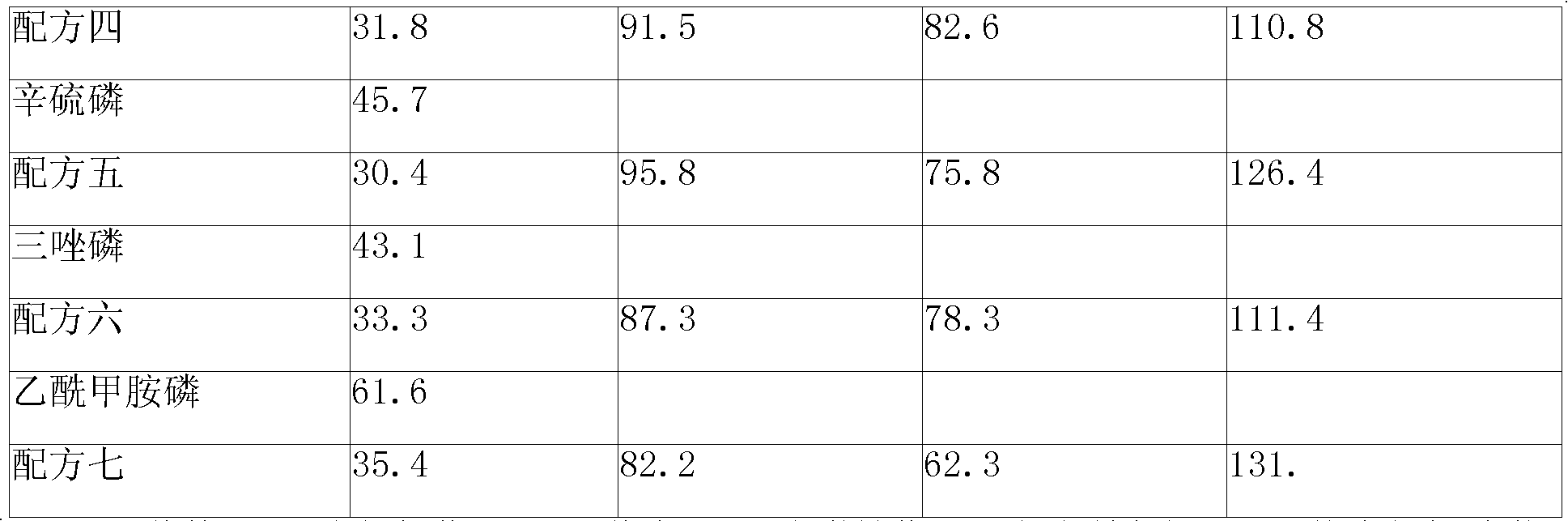
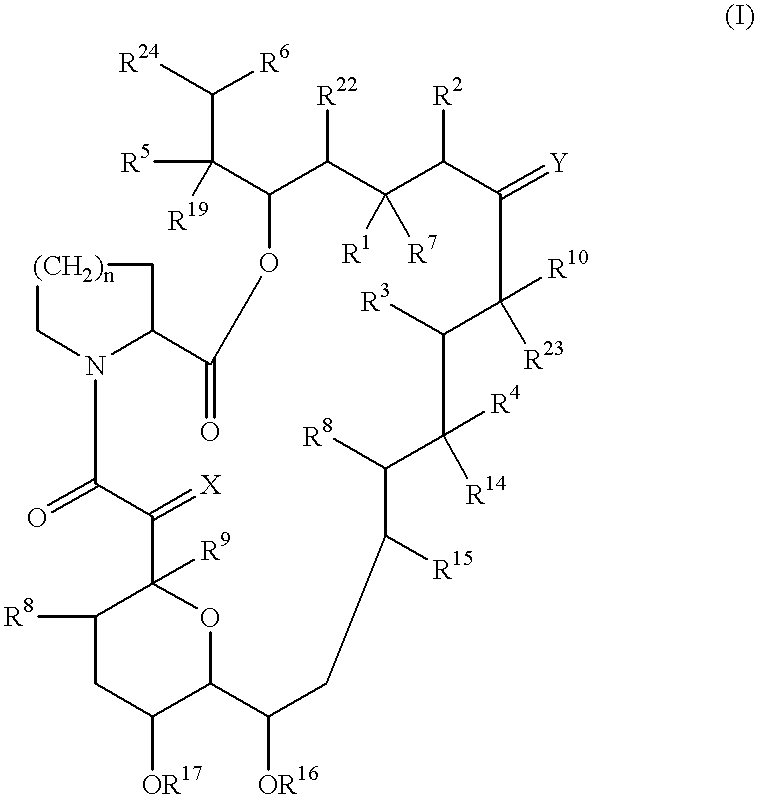
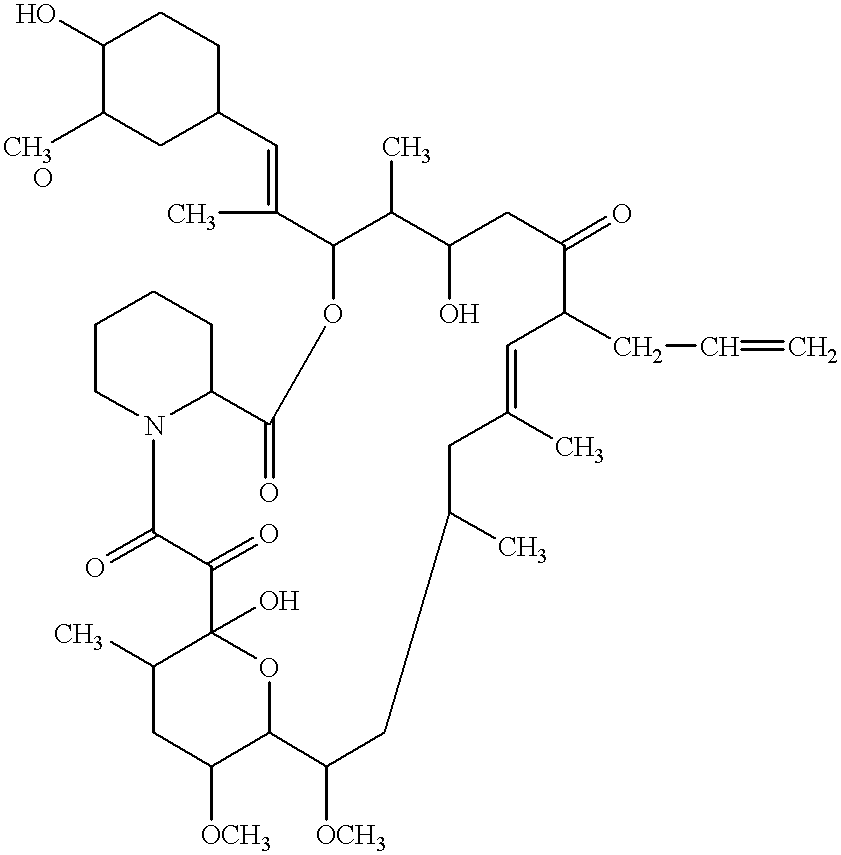
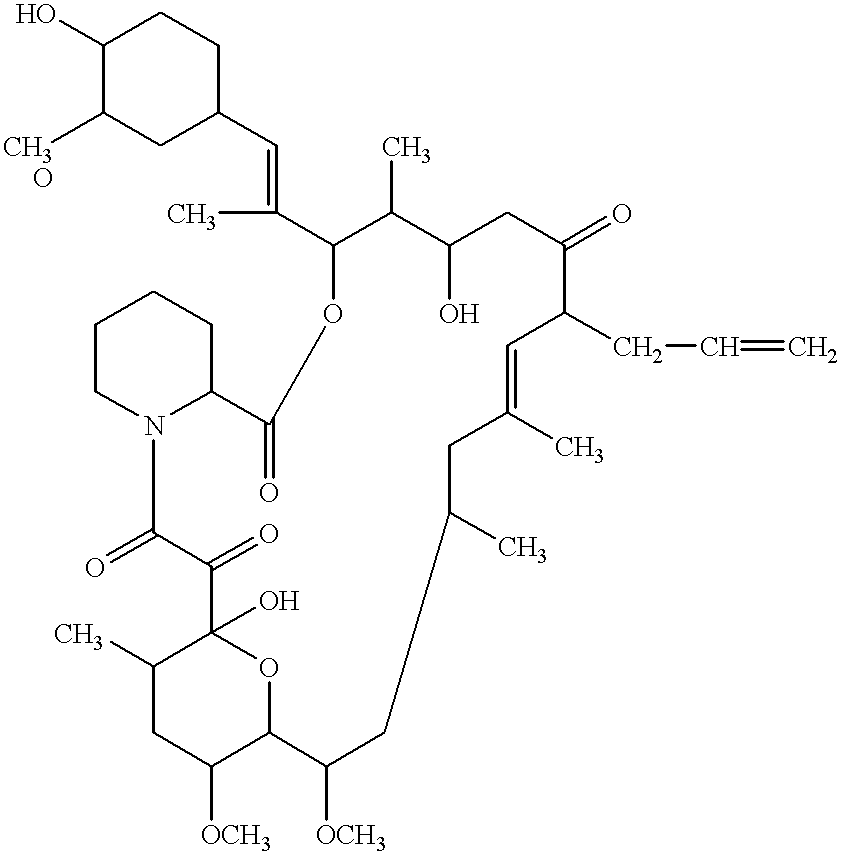
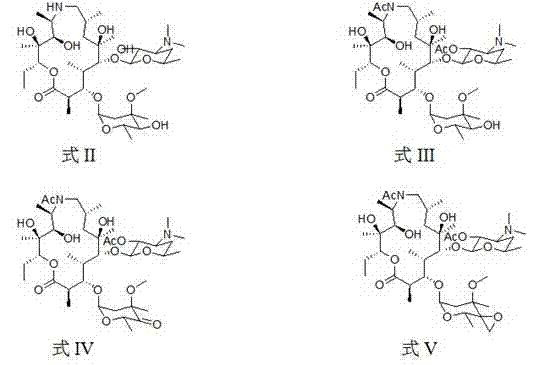
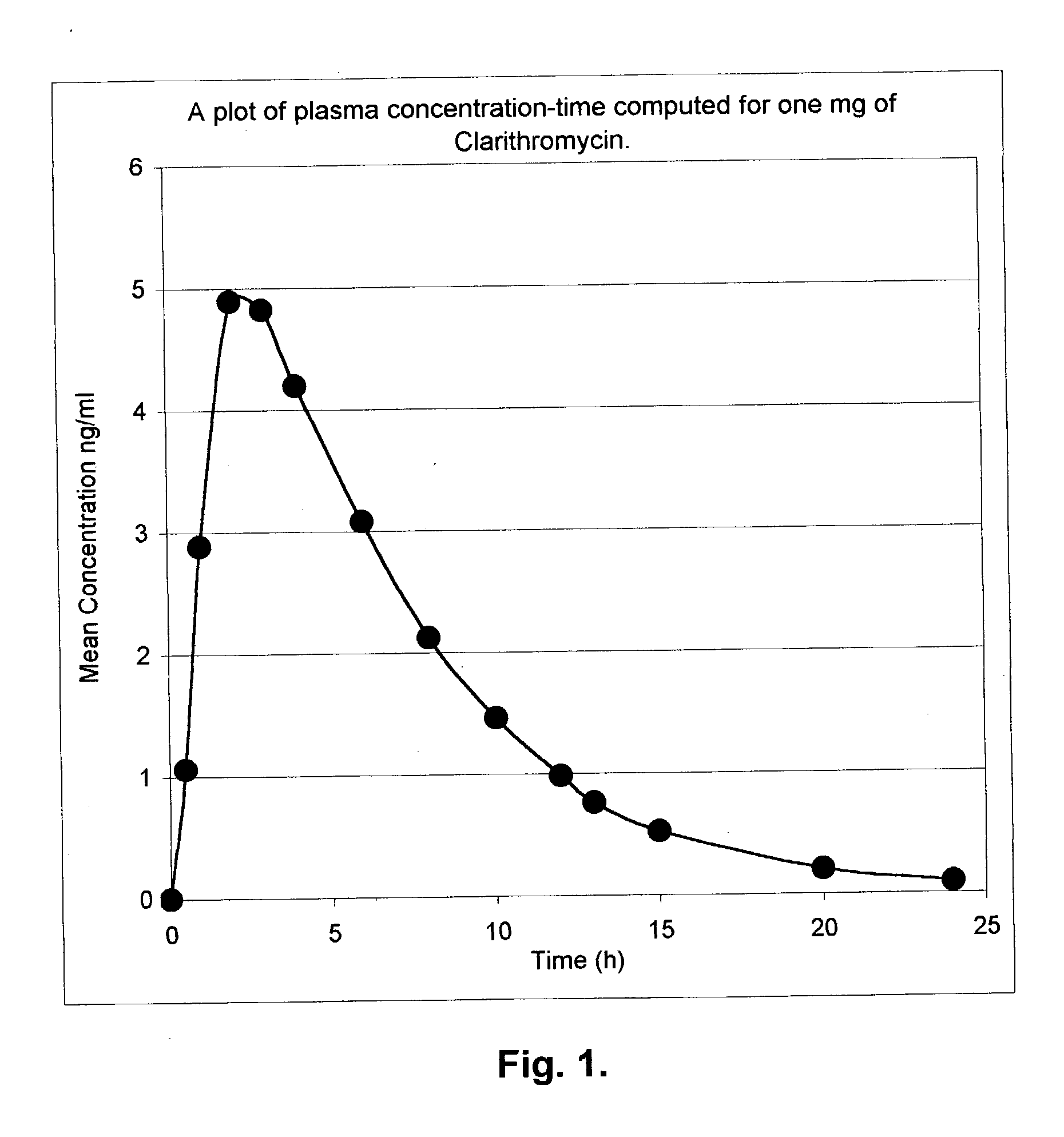
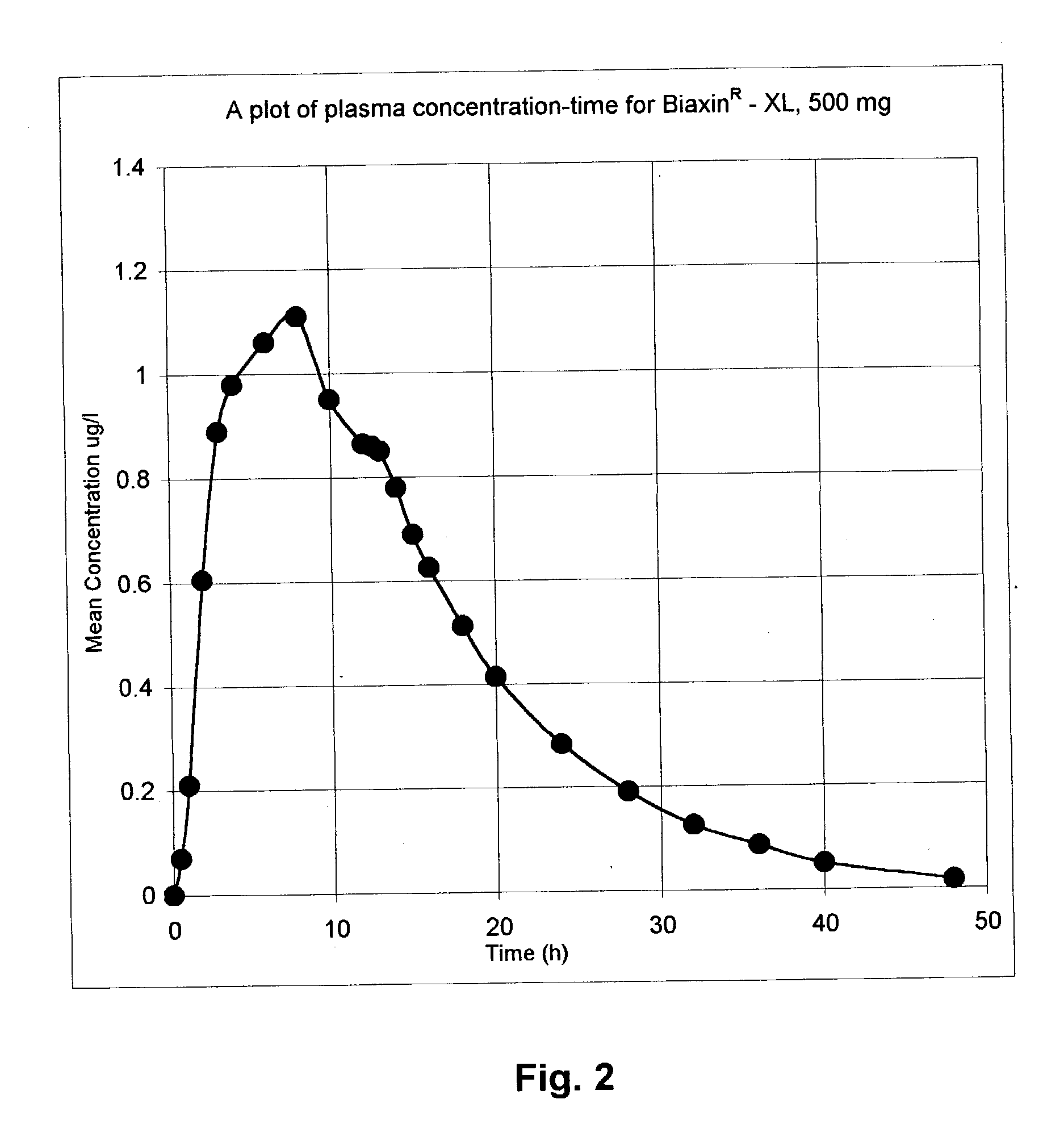
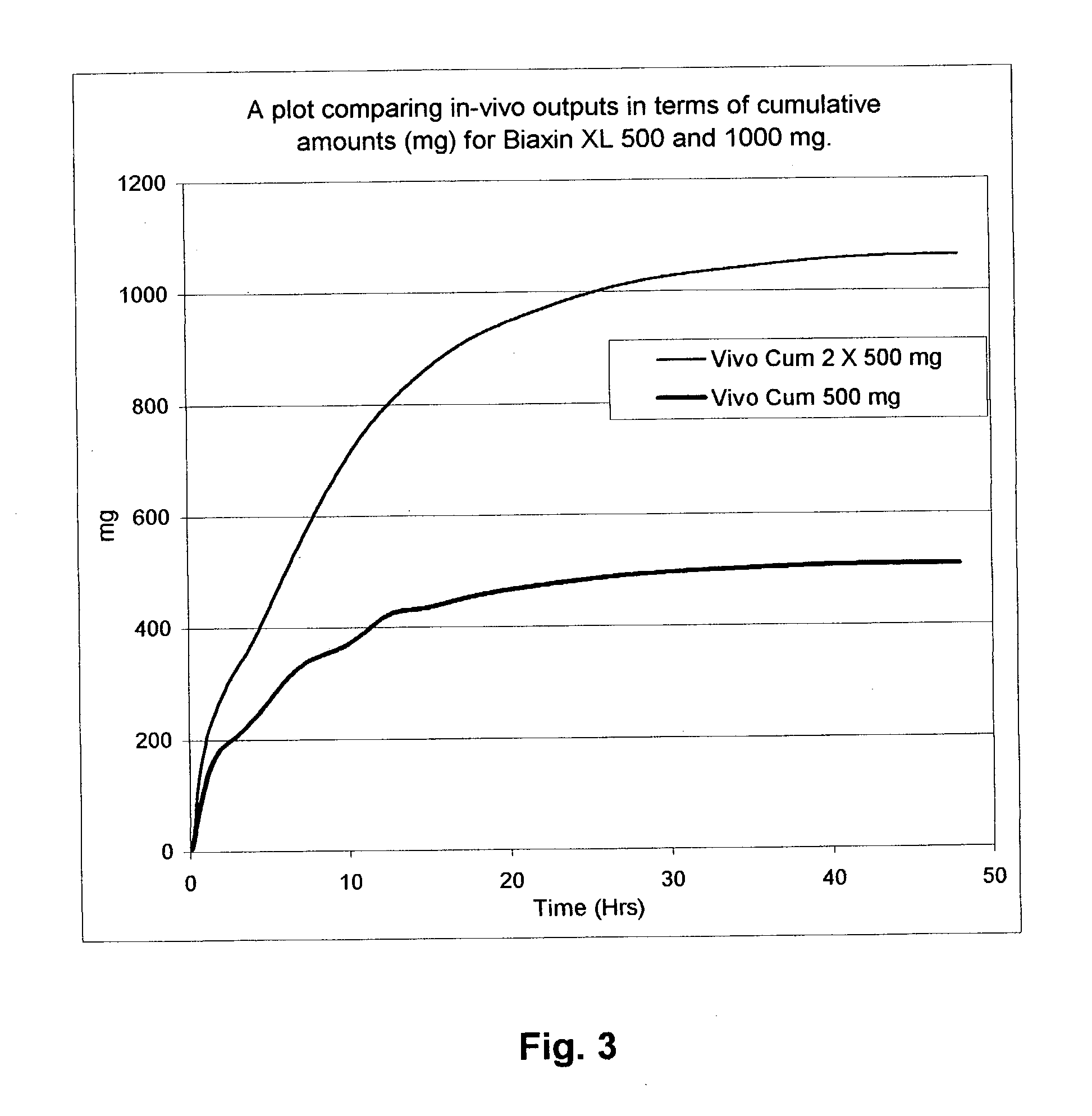
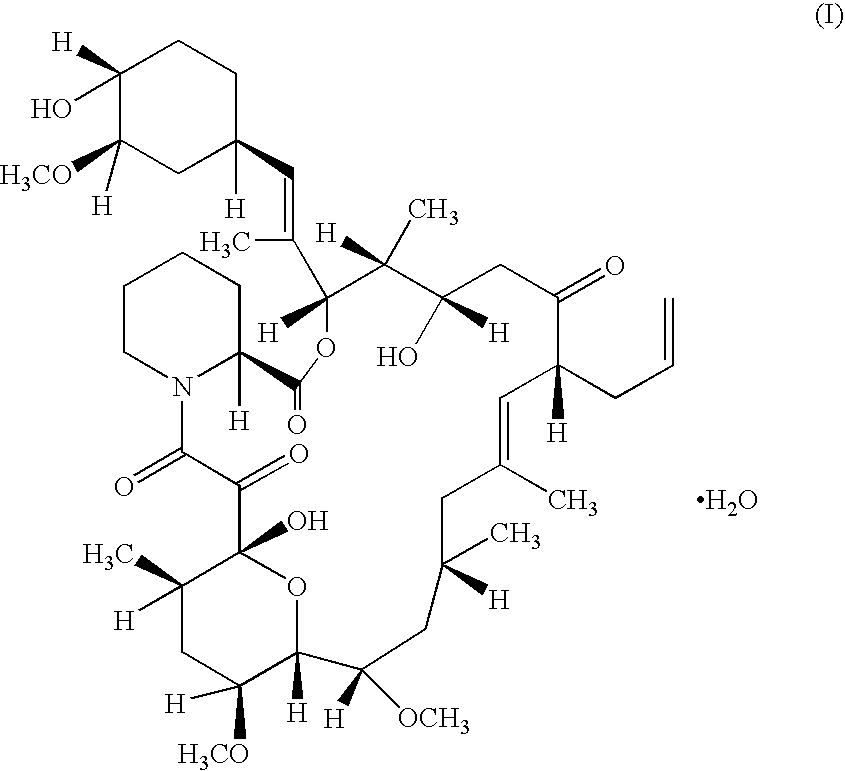
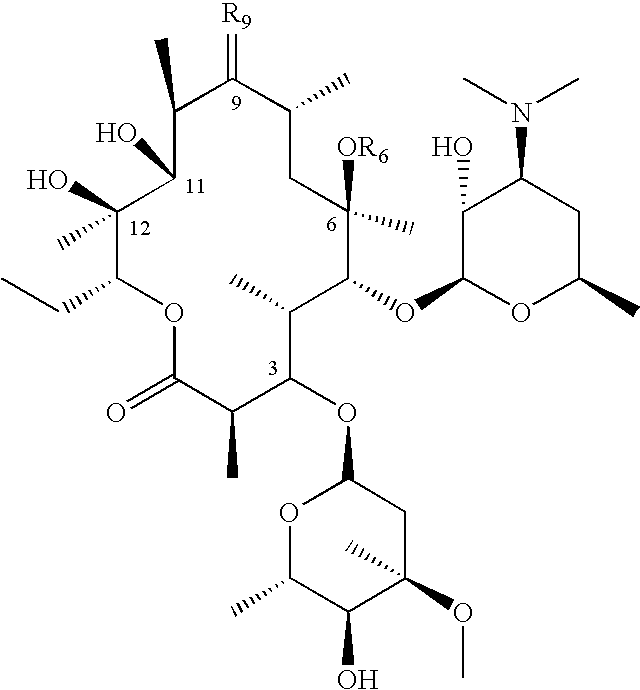
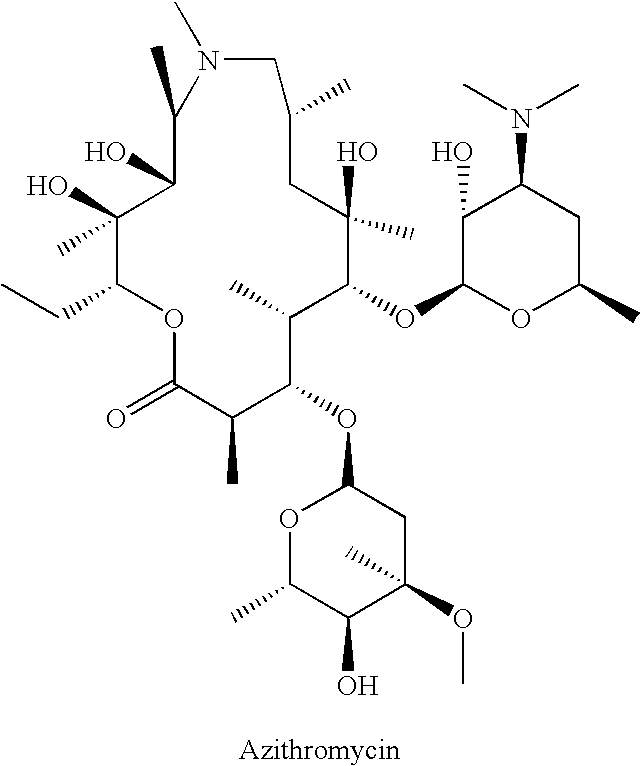
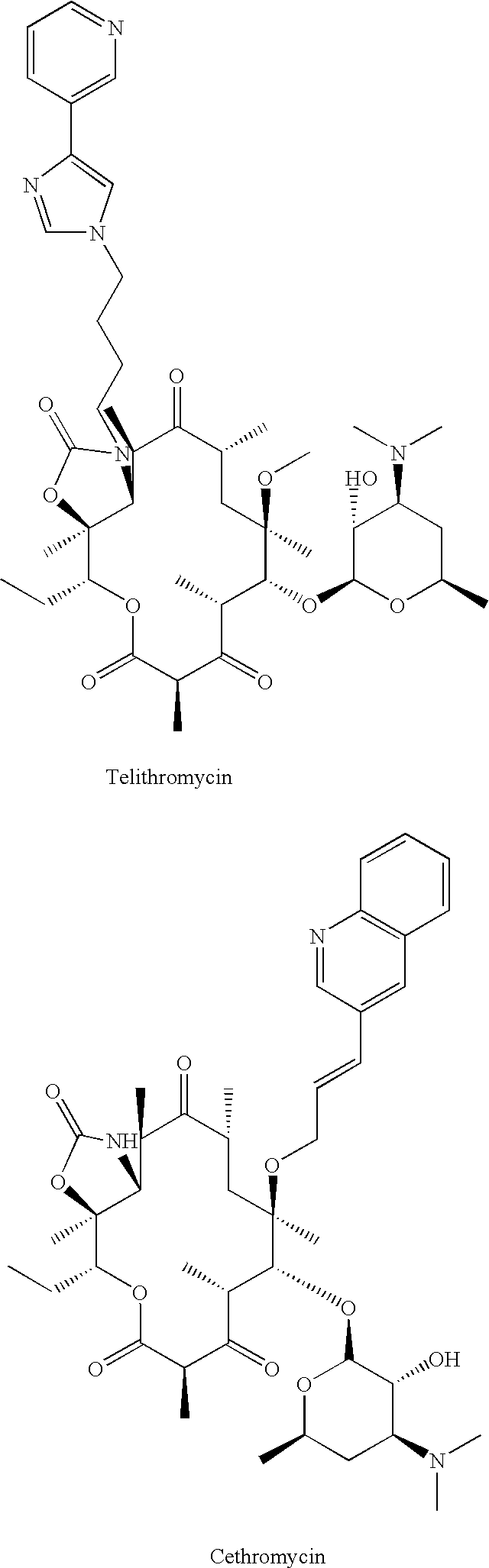
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
521 results about "Macrolide resistance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
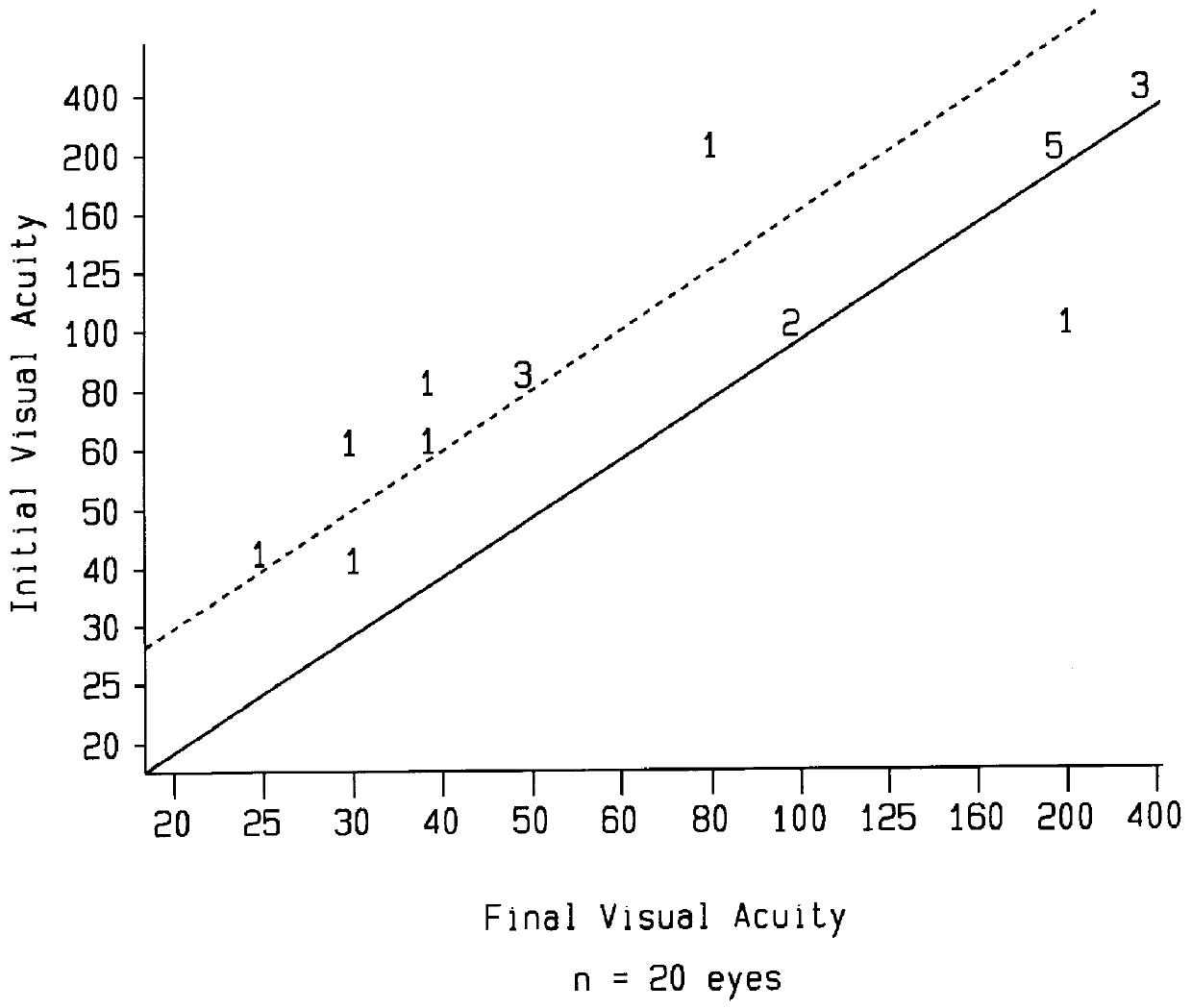
Antibiotic treatment of age-related macular degeneration
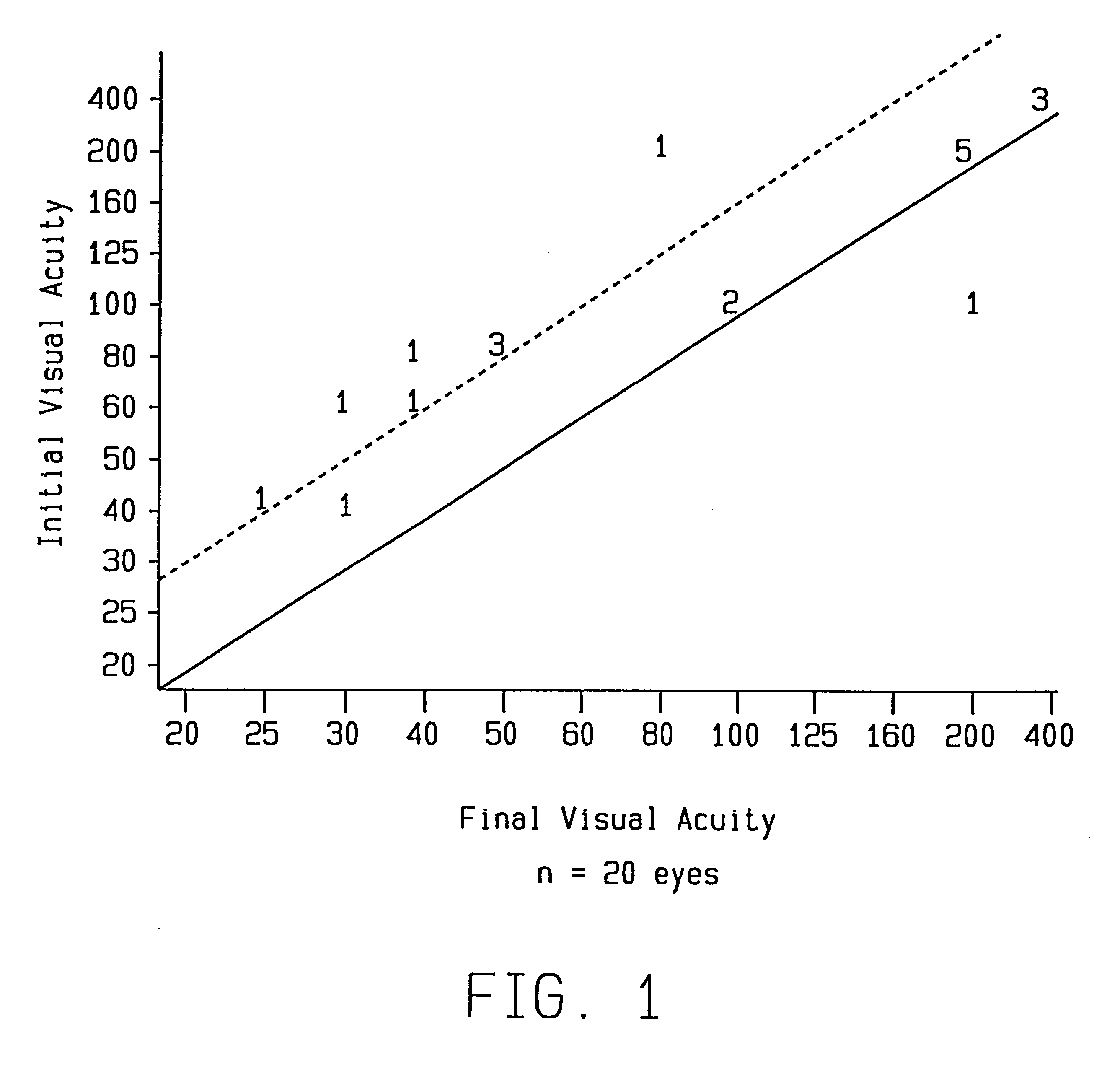
InactiveUS6015803AImprove visual acuityBiocideTetracycline active ingredientsAntibiotic YMacrolide resistance
A method is provided for the treatment of age-related macular degeneration by administering various antibiotics, such as tetracycline and its derivatives, rifamycin and its derivatives, macrolides, and metronidazole, to a patient in a therapeutically effective amount.
Owner:WIROSTKO BARBARA
Novel antibacterial agents
Owner:MERCK SHARP & DOHME CORP +1
Macrolide antibiotics and treatment and prophylaxis of pasteurellosis using the same
ActiveUS6514946B1High antibacterial activityReduced activityAntibacterial agentsBiocideTreatment fieldAntibacterial activity
20,23-disubstituted mycaminosyltylonolide derivatives and use of the same in the field of the prophylaxis and treatment of pasteurellosis are disclosed. The di-substituents are peperidino optionally substituted with one or two methyl groups. The derivatives have selective antibacterial activity against Pasteurella.
Owner:ZH BISEIBUTSU KAGAKU KENYKU KAI
Mixtures of anthranilamide invertebrate pest control agents
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-cyano-2-methyl-6[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, an N-oxide, or a salt thereof, Formula (1) and (b) at least one invertebrate pest control agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC AGRO SINGAPORE PTE LTD +1
Plant insecticidal protective agent and application thereof
ActiveCN101796949AImprove insecticidal effectHigh speedBiocideAnimal repellantsMacrolide resistanceToxicology
The invention belongs to the technical field of the farm chemical, and in particular relates to a plant insecticidal protective agent and an application thereof. The invention provides the plant insecticidal protective agent which is recomposed by food suppressing hydrazine and organic phosphorus (I), pyrethroids insecticides (ii), insect growth regulation agent (III), Macrolides (IV), heterocyclic insecticidal component (V), carbamates (VI) or Nereistoxin pesticide and the application thereof. The recomposed insecticidal composite remarkably improves the insecticidal effect, at least has four acting ways at the same time such as touch out, stomach toxicity, internal absorption and antifeedant so as to adequately protect the plant, favors the delay of the production of the drug resistanceof the insects through different acting ways, and enlarges the pest control spectrum. The composite can be widely applicable to the control of different chewing mouthparts, piercing-sucking mouthparts, lapping mouthparts and rasping mouthparts pests.
Owner:QINGDAO STAR CROPSCI
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Macrolide dosage forms
ActiveUS20120064124A1Preventing the inflammatory phase of healingReduces and eliminates adverse toxic effectOrganic active ingredientsSenses disorderDrug deliveryMacrolide resistance
Provided is a drug delivery composition comprising at least one polymer and at least one active agent; wherein the active agent is present in crystalline form on at least one region of an outer surface of the composition and wherein active agent surface content is adjusted to provide a selected active agent release profile.
Owner:MT ACQUISITION HLDG LLC
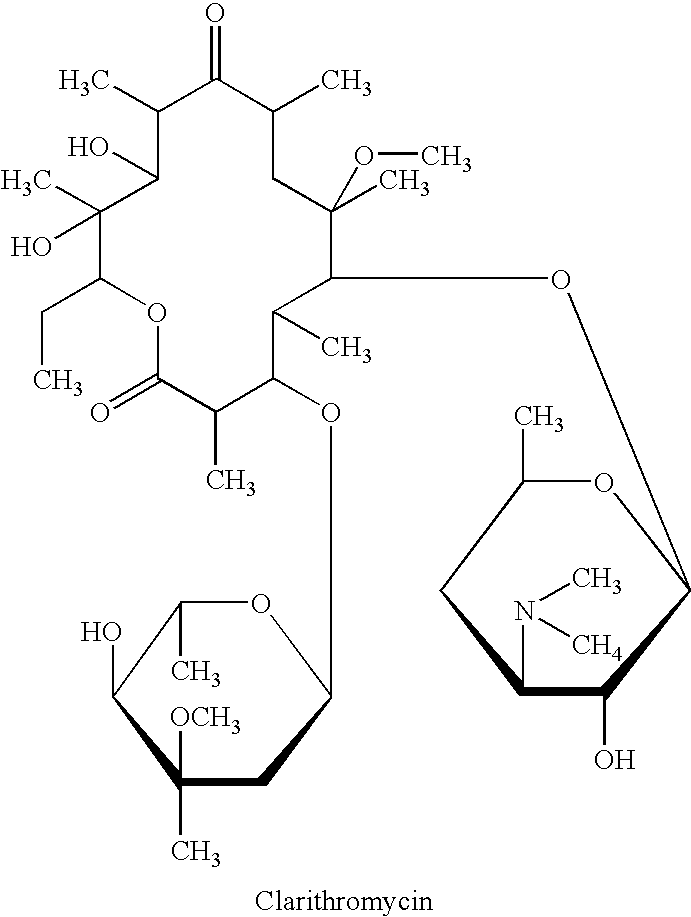
Nanoparticulate clarithromycin formulations
InactiveUS20070015719A1Improve bioavailabilityAntibacterial agentsPowder deliveryNanoparticleClarithromycin
The present invention is directed to compositions comprising nanoparticulate macrolides such as clarithromycin, or a salt or derivative thereof, having improved bioavailability. The nanoparticulate macrolide particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of infection and related diseases.
Owner:ELAN PHRMA INT LTD
Use of macrolide compounds for the treatment of dry eye
InactiveUS6872383B2Good treatment effectSuperior improving effect on dry eye symptomsBiocideSynthetic polymeric active ingredientsMacrolide resistancePharmacology
Owner:SUCAMPO
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Antibiotic treatment of age-related macular degeneration
A method is provided for the treatment of age-related macular degeneration by administering various antibiotics, such as tetracycline and its derivatives, rifamycin and its derivatives, macrolides, and metronidazole, to a patient in a therapeutically effective amount.
Owner:WIROSTKO BARBARA
Method for simultaneously detecting multi-kind pesticide residues in bee products
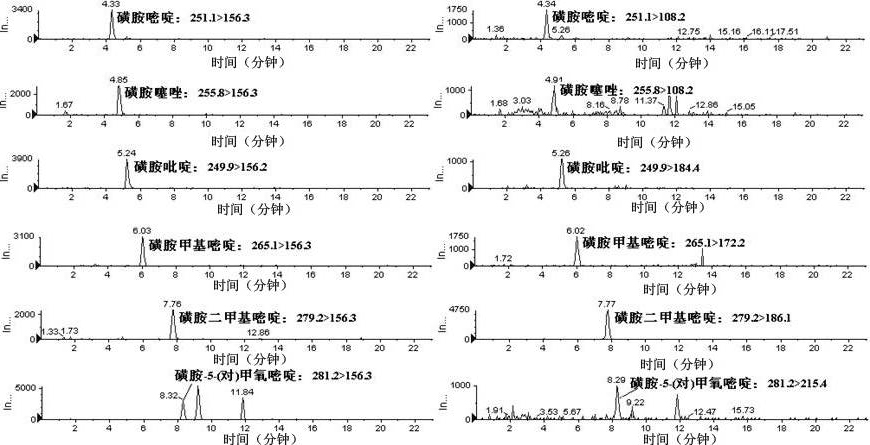
InactiveCN101358953ASolve the problem of matrix effectFast wayComponent separationRetention timePhosphate
The present invention relates to a method of simultaneously detecting a plurality of agro-veterinary drug residues in bee products. The extracted liquid trichloroacetic acid or perchloric acid and the extracted liquid acetate, phosphate or borate solution are added into a sample; the pH value is controlled between 4.5 and 9.0; the mixed solution is centrifuged, the filtrate is added into a solid phase extraction column to be extracted, the extraction column is eluted and dried, the column is washed by oxalic acid-methanol solution, the volume of the eluent is defined by the aqueous solution of methanol, the eluent is added into liquid chromatography-tandem mass spectrometry to be analyzed and tested, the acquired chromatographic peak is contrasted with the known standard chromatographic peak of the drug, and according to the retention time and the abundance of the mass spectrum ions, the specific name of the detected drug is determined. The method only requires one pre-treatment of the sample, and thus can simultaneously extract 11 classes and more than 60 kinds of veterinary drug residues, such as sulfonamides, quinolones, macrolides, lincomycins, nitroimidazoles, beta-lactams, tetracyclines, chloromycetins, trinethoprims, chlordimeform, triadimenol and the like, the efficiency of analysis is high, and the detection cost is greatly reduced.
Owner:中华人民共和国江苏出入境检验检疫局
Use of macrolide compounds for treating glaucoma
Owner:ASTELLAS PHARMA INC
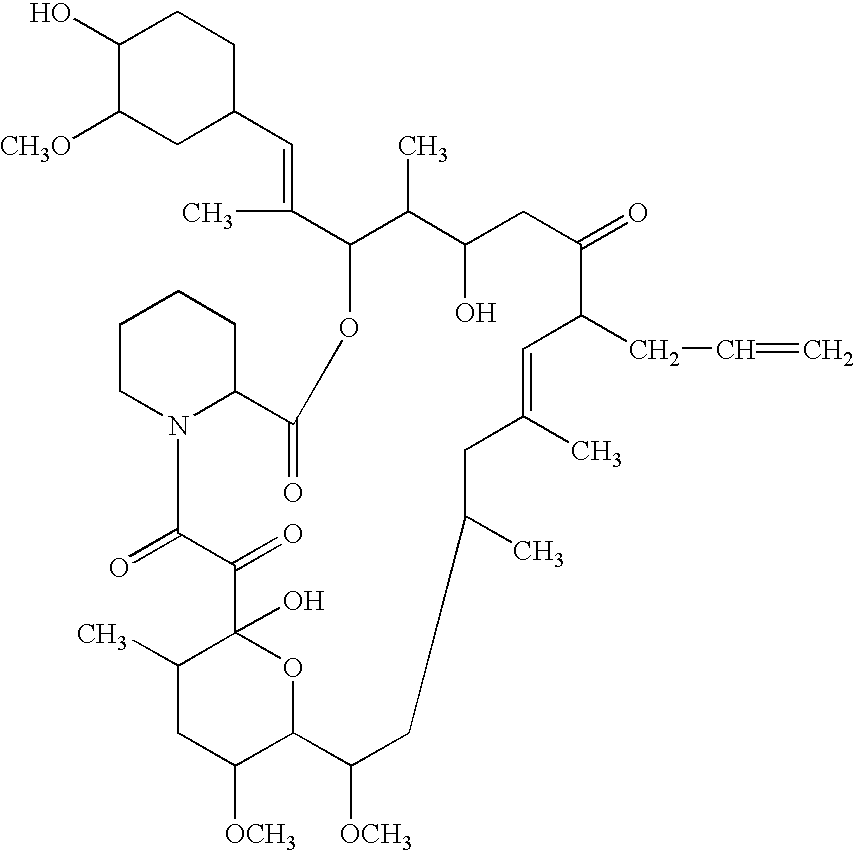
A new method for preparing telamycin
ActiveCN102260306AAvoid catalytic hydrogenation methodsReduce manufacturing costSugar derivativesSugar derivatives preparationEpoxyAzithromycin
The invention discloses a novel method for preparing tulathromycin and relates to a semi-synthetic macrolide antibiotic. The method comprises the following steps: simultaneously protecting 2'-hydroxyl and 6a-amino of desmethyl azithromycin with acetyl, then carrying out oxidation and epoxidation on 4''-hydroxy, then removing the protecting groups under alkaline alcohol solution conditions, and carrying out nucleophilic addition on 4''-epoxy group with n-propylamine to obtain the target compound tulathromycin. Compared with the prior art, the method for preparing tulathromycin has the advantages of simple process, mild conditions, high yield and the like, and is beneficial to industrial production.
Owner:SHANDONG LUKANG SHELILE PHARMA
Anthelmintic macrocyclic lactone compositions
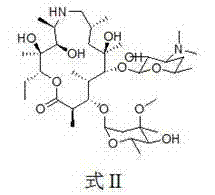
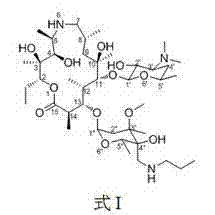
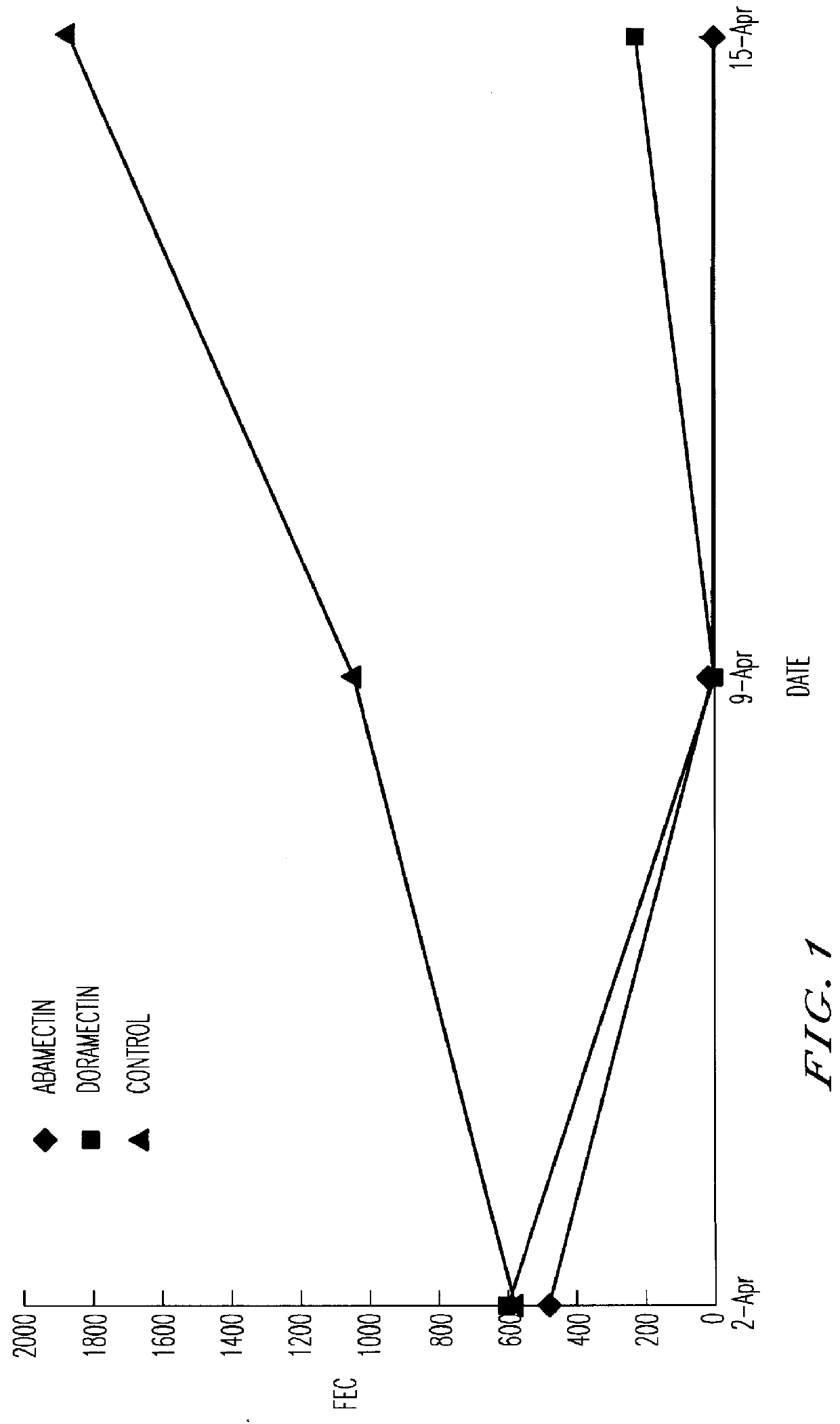
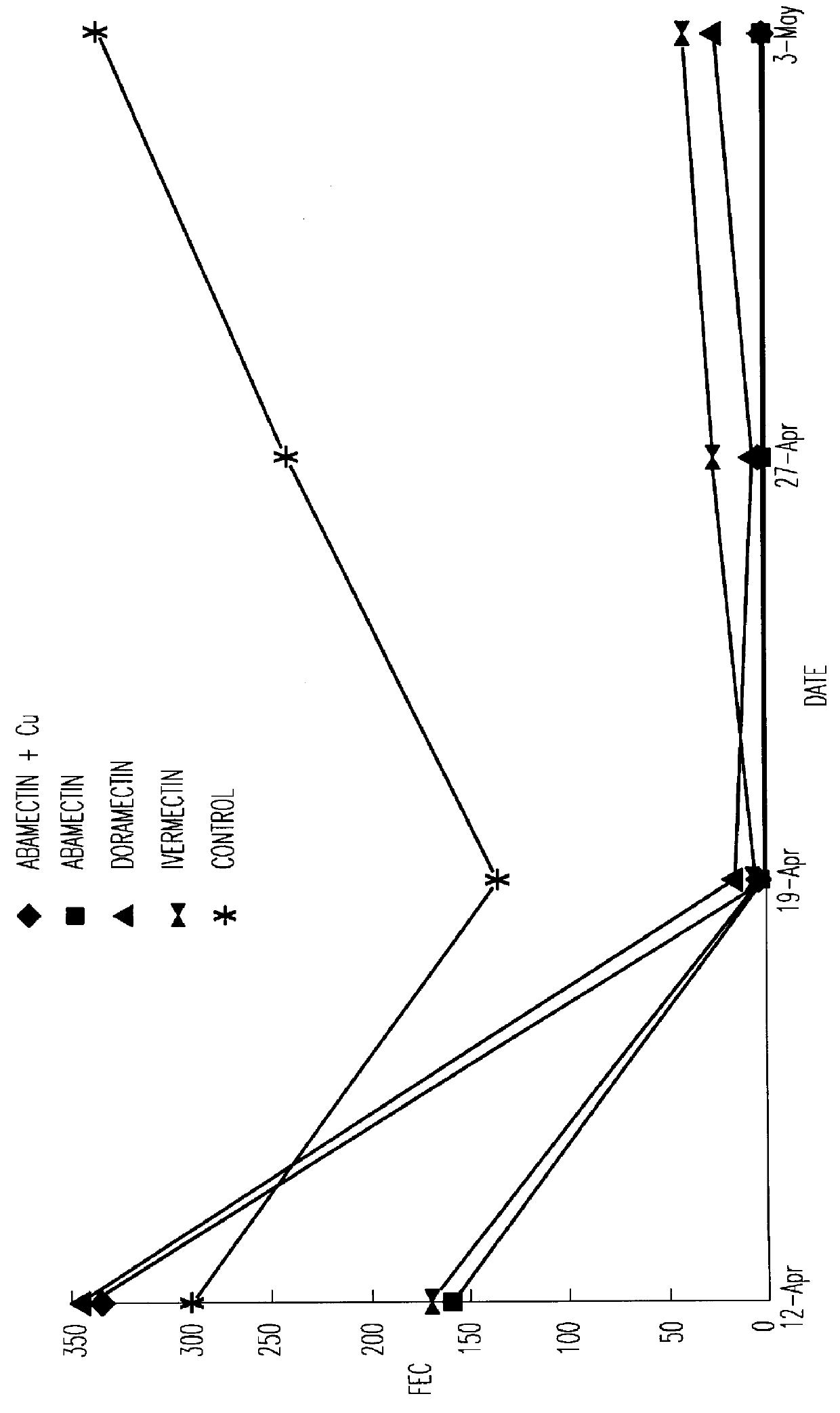
PCT No. PCT / NZ96 / 00099 Sec. 371 Date Mar. 25, 1998 Sec. 102(e) Date Mar. 25, 1998 PCT Filed Sep. 19, 1996 PCT Pub. No. WO97 / 11709 PCT Pub. Date Apr. 3, 1997A composition comprising an anthelmintic chosen from the class of macrocyclic lactones including but not limited to the avermectins, ivermectin, doramectin, abamectin, milbemycin and moxidectin, together with a vegetable oil and a co-solvent chosen from the group comprising alcohols having four or more carbons atoms. The compositions of the inventions may be formulated as injections, drenches or for topical administration and are suitable for treating helminthiasis in animals.
Owner:MERIAL INC
Rate-controlled delivery of macrolides
InactiveUS20030091627A1EffectiveEliminate the problemBiocideCarbohydrate active ingredientsCyclodextrinClarithromycin
There is disclosed the formulation of a poorly soluble macrolide antibiotic, such as clarithromycin together with beta-cyclodextrin, and optionally a dicarboxylic acid wherein the particles of the formulation are prepared using microfluidization techniques in a particle size in the range of from 5 to 15 microns.
Owner:SHARMA VINAY
Pharmaceutical gel formulations
A pharmaceutical gel composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) one or more gel forming agents; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Compounds, compositions as carriers for steroid/nonsteroid anti-inflammatory; antienoplastic and antiviral active molecules
InactiveUS7157433B2Elevate tissue concentrationReduced effectBiocideSenses disorderViral diseaseCompound (substance)
The present invention relates (a) to new compounds represented by Formula I:wherein M represents a macrolide subunit (macrolide moiety) derived from macrolide possessing the property of accumulation in inflammatory cells, V represents an anti-inflammatory steroid or nonsteroid subunit, or an antineoplastic or antiviral subunit and L represents a linking group covalently linking M and V; (b) to their pharmacologically acceptable salts, prodrugs and solvates, (c) to processes and intermediates for their preparation, and (d) to their use in the treatment of inflammatory / neoplastic / viral diseases and conditions in humans and animals.
Owner:GLAXOSMITHKLINE ISTRAZIVACKI CENTAR ZAGREB D O O
Pharmaceutical ointment formulations
A pharmaceutical ointment composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) an ointment base; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant or a pharmaceutically acceptable salt or ester thereof through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Method of treating tuberculosis
Macrolide and ketolides, and compositions containing the same, useful in the treatment of tuberculosis are disclosed. Methods of treating tuberculosis using the macrolides and ketolides, and compositions containing the same, also are disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Microcapsules containing macrolide lactones abamectin, milbemectin, avermectins, milbemycins, emamectins, ivermectins and mectins in general
Microencapsulated formulations of macrolide lactones (abamectin, milbemectin, milbemycins emamectin, avermectins, ivermectins) wherein the active ingredient is protected from UV-degradation, with exceptional release characteristics resembling those of an emulsion concentrate or, if desired, of long-lasting effect; further with appropriate rheological properties, and with reduced toxicity. The invention provides a unique microencapsulation process for the chemical stability and biological activity of mectins, e.g. abamectin, and provides microcapsules of mectins to be used in formulations CS, WG / CS, ZC, EC / CS and any formulation type containing microcapsules and combination with other biologically active ingredients.
Owner:GAT MICROENCAPSULATION AG
Method for simultaneously measuring various drug residues in honey by utilizing liquid chromatogram tandem mass spectrum isotope dilution method
The invention relates to a method for detecting various drug residues in honey, in particular relating to a method for simultaneously measuring various drug residues in honey by utilizing a liquid chromatogram tandem mass spectrum isotope dilution method. The method provided by the invention comprises the following steps: directly diluting by virtue of a phosphate buffer solution the pH value of which is equal to 8; carrying out HLB (Hydrophile Lipophile Balance) extraction and purification; carrying out measurement by utilizing the liquid chromatogram tandem mass spectrum isotope dilution method (LC-MS / MS); and quantifying by utilizing the internal standard method and external standard method of isotope internal standard dilution; and measuring low-limit sulfanilamide drugs to be 1.0 mu g / kg, nitromidazoles drugs to be 1.0 mu g / kg, carbostyril drugs to be 2.0 mu g / kg, macrolide drugs to be 3.0 mu g / kg, lincosamides drugs to be 2.0 mu g / kg and praziquantel to be 0.3 mu g / kg. The method provided by the invention is simple, convenient and rapid and has the advantages of small resource consumption and low detection cost; the front processing procedure, drug variety and instruments for measurement can be better complementary with the existing method; and the method provided by the invention is suitable for the simultaneous measurement requirements of various drugs in the honey and can provide a powerful technical guarantee for maintaining the food safety and guaranteeing that Chinese honey is successfully exported.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
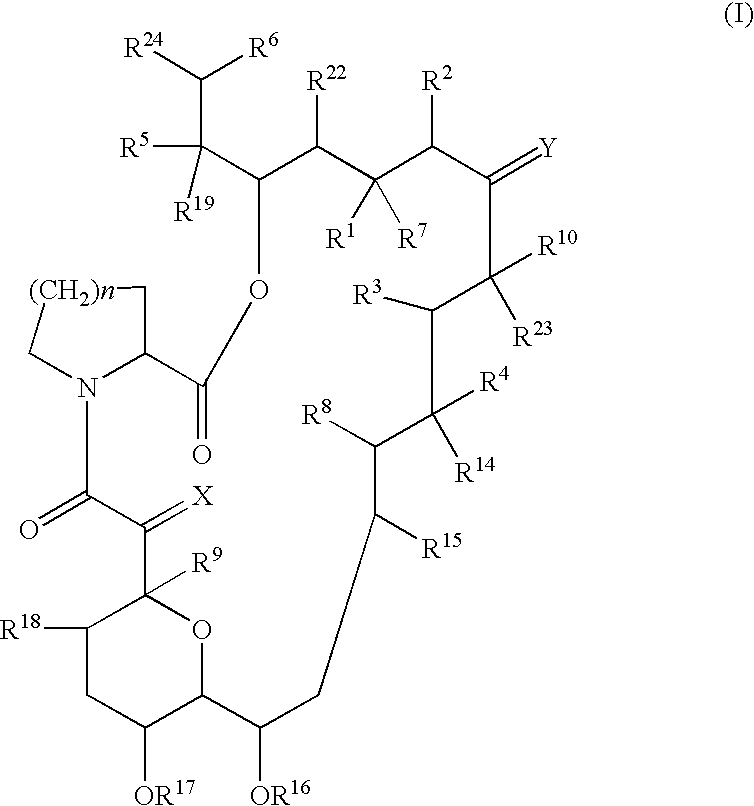
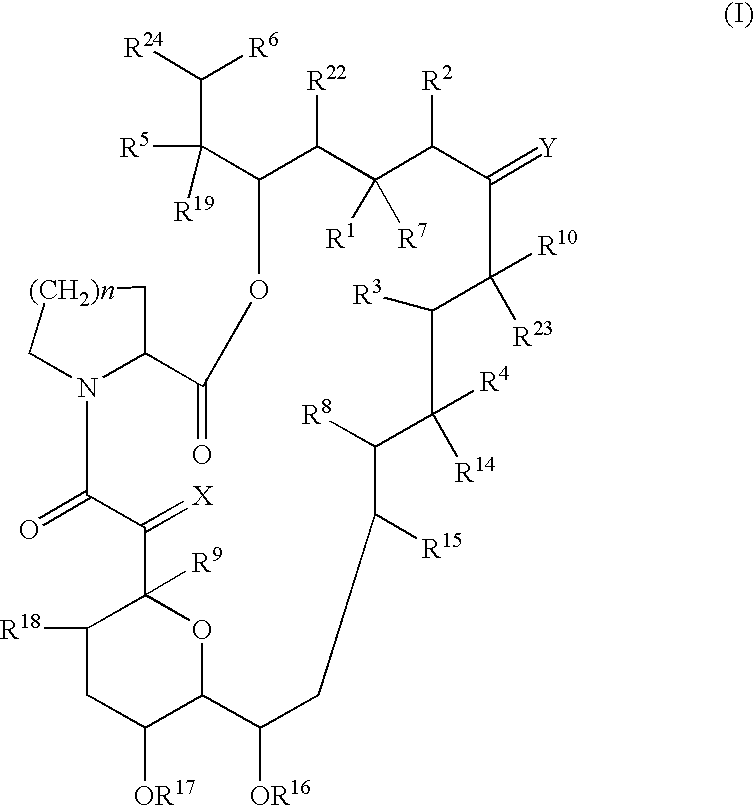
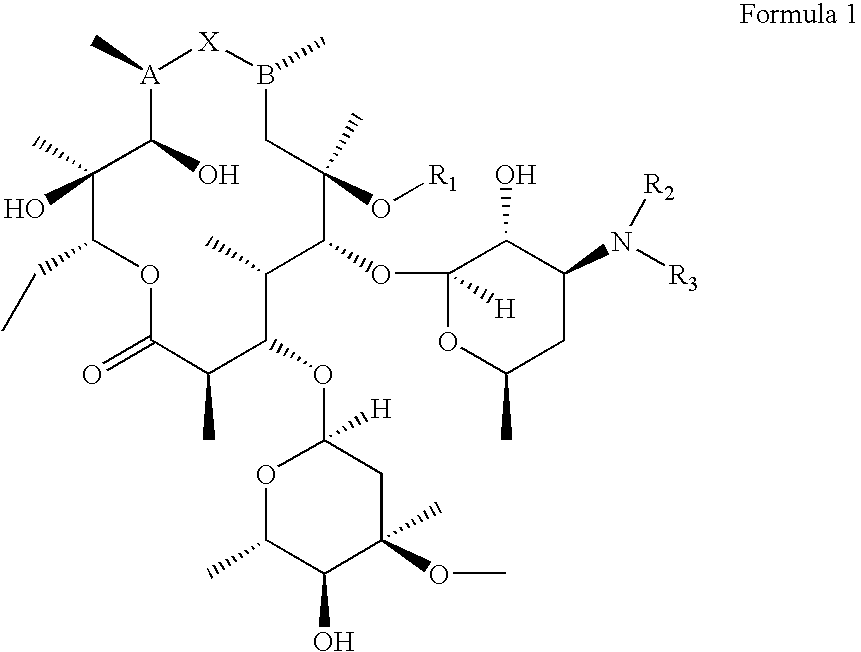
Preparation and utility of substituted erythromycin analogs
InactiveUS20070281894A1Decreased inter-individual variation in plasma levelsSignificant clinical effectAntibacterial agentsBiocideChemical synthesisMicroorganism
The present disclosure is directed to novel macrolide antibiotics of Formula 1 and pharmaceutically acceptable salts and prodrugs thereof; and the chemical syntheses and medical uses of these novel macrolide antibiotics for the treatment and / or management of infections caused by various aerobic and anaerobic gram-positive and gram-negative microorganisms as well as various mycobacteria.
Owner:AUSPEX PHARMA INC
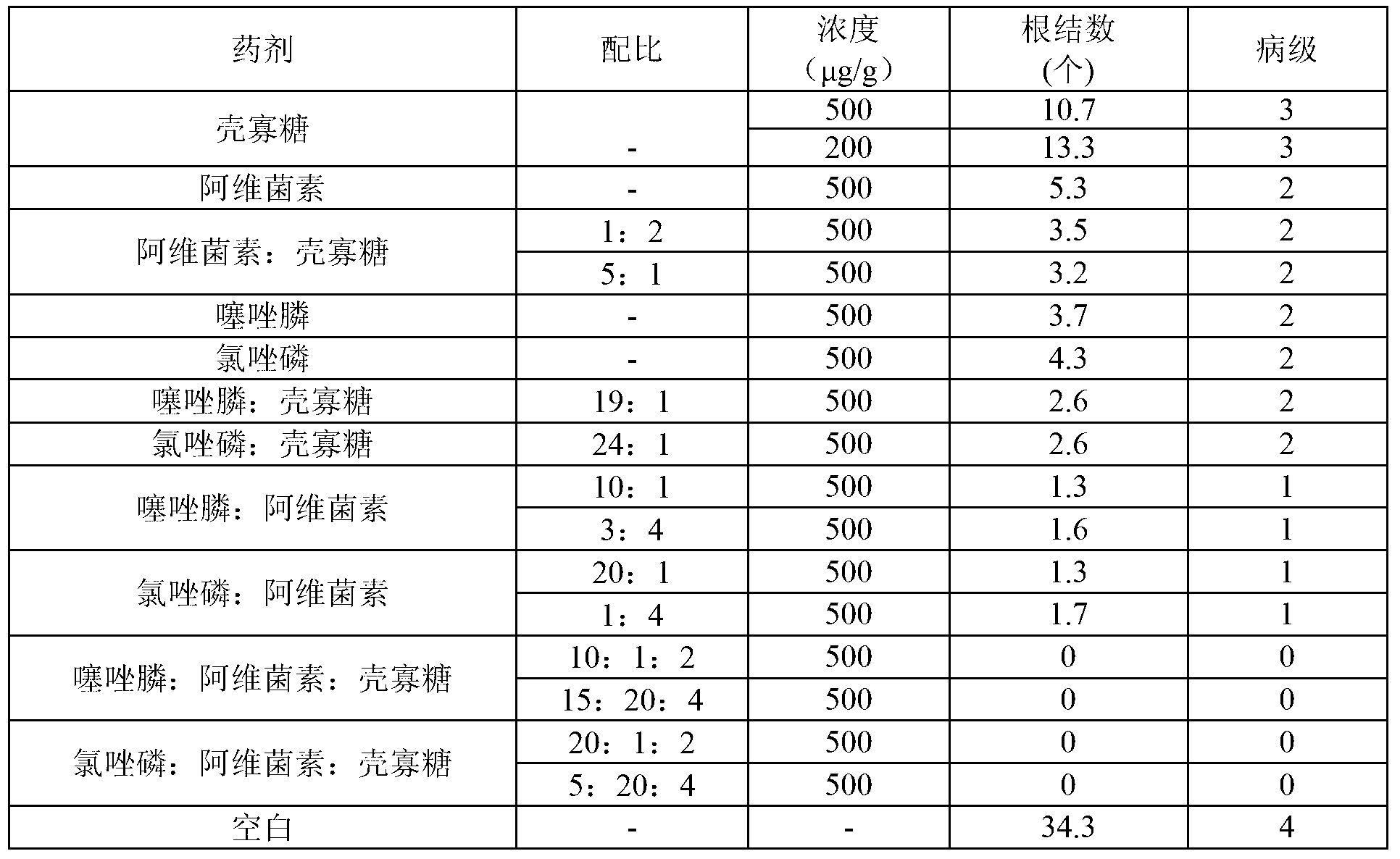
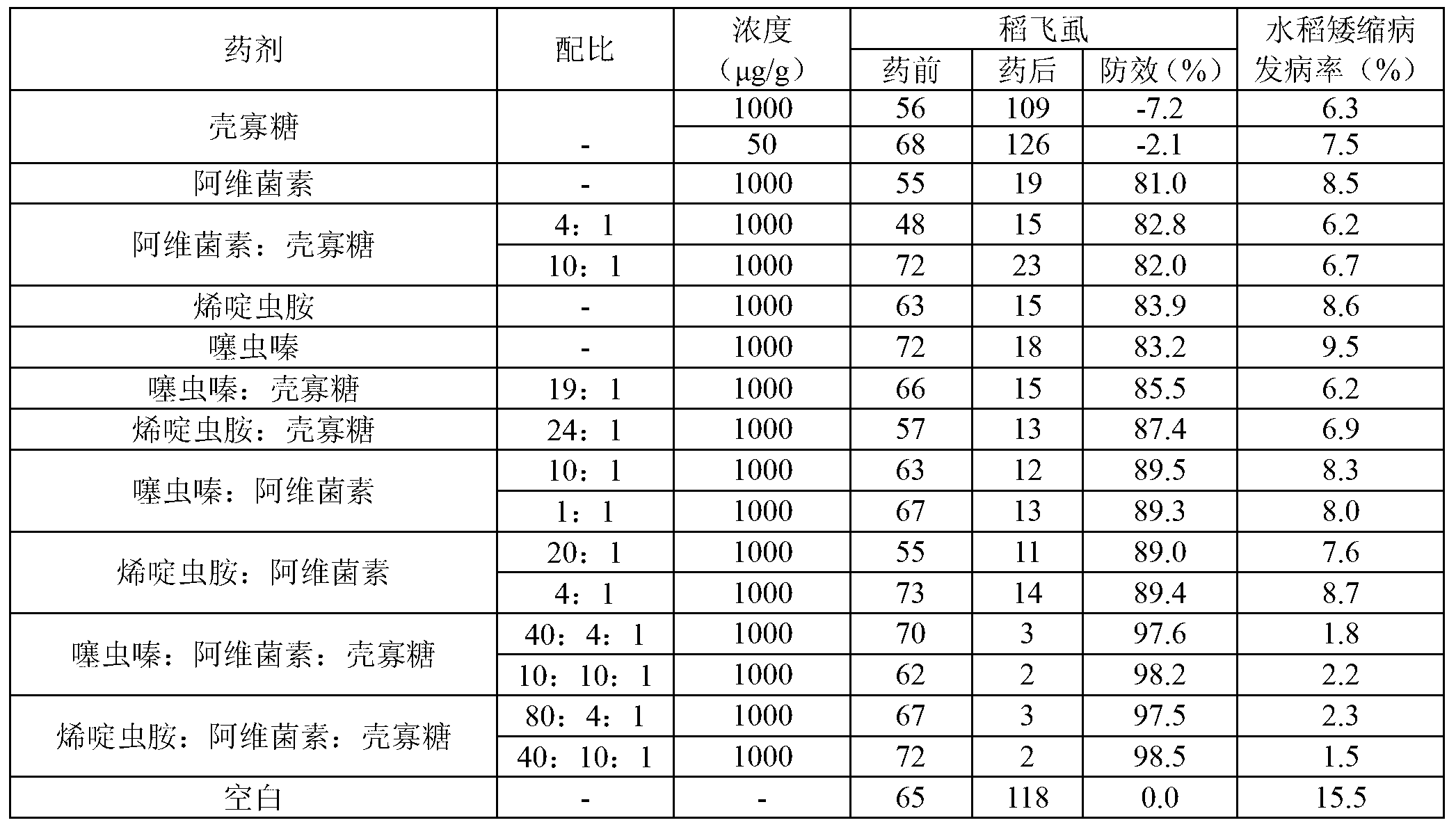
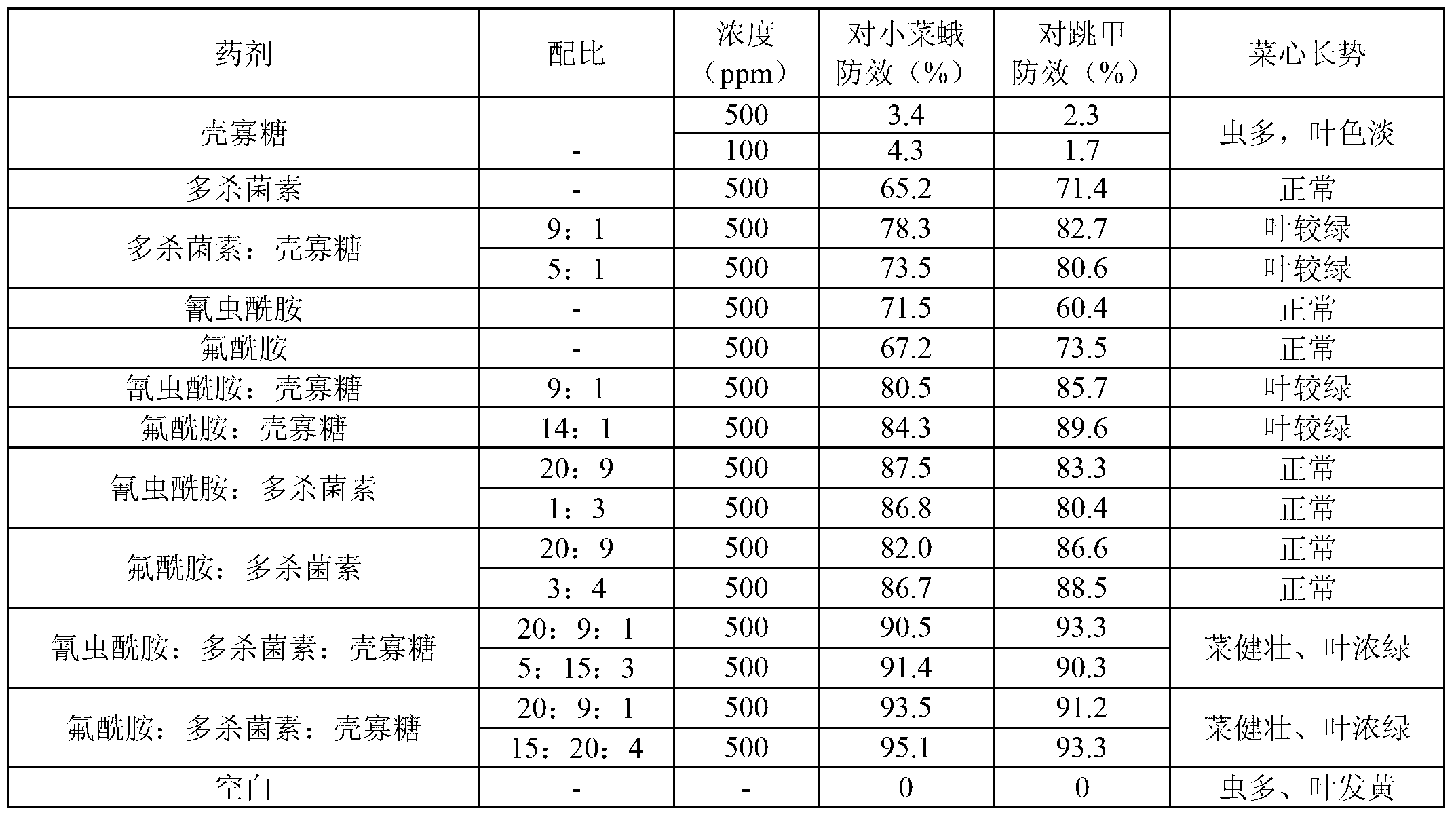
Insecticide acaricide taking oligose and macrolides composition as synergist and application of insecticide acaricide
The invention discloses an insecticide acaricide taking an oligose and macrolides composition as a synergist, and in particular relates to a synergism composition capable of enhancing the insecticiding effect of the insecticide acaricide. The composition comprises oligose and a macrolides insecticide; the macrolides insecticide is abamectin, pleocidin, emamectin benzoate or ivermectin; the oligose is pectic oligosaccharide, sodium alginate oligosaccharides or chitosan oligosaccharide; the mass part ratio of the oligose to the macrolides insecticide is (1-30):(1-10). The synergism composition can enhance the insecticidal efficacy of the insecticide acaricide and realize the synergism effect with other insecticide acaricides.
Owner:HAINAN ZHENGYE ZHONGNONG HIGH TECH
Macrolide compound ultralow volume formulation
The invention relates to a macrolide compound ultralow volume formulation. An active component I and an active component II are used as active components with the addition of a solvent and an auxiliary agent to prepare the ultralow volume liquid formulation, Wherein the active component I contains one or more ingredients selected from the group consisting of a macrolide compound avermectin, emamectin benzoate and ivermectin; the active component II contains one or more ingredients selected from the group consisting of chlorantraniliprole, flubendiamide, metaflumizone, ethiprole, indoxacarb, spinosad, spinetoram, guinalphos and basudin; the content of the active component I is 0.1-5% and the content of the active component II is 0.1-20%; and the cotoxicity coefficient calculated according to Sunyunpei method. The invention is suitable for ultralow volume spraying, low volume spraying and electrostatic spraying, and has advantages of high work efficiency, fast effectiveness, long persistent period, water saving, cooperative synergism and the like.
Owner:GAUNGXI TIANYUAN BIOCHEM
Method of feeding the cattle with feed additives that increases beef production and reduces liver abscess
The present inventions relates to a method of increasing beef production in cattle with feed additives, comprising feeding cattle with feed, comprising an effective amount of an ionophore in combination with a macrolide antibiotic, and thereafter feeding cattle with feed, comprising zilpaterol and essentially no ionophore or macrolide antibiotic for the succeeding about 20 to 40 days.
Owner:INTERVET INT BV
Preparation method of macrolide antibiotics molecular engram polymer microsphere
InactiveCN101507916AOther chemical processesAlkali metal oxides/hydroxidesWater bathsFunctional monomer
The invention discloses a method for preparing macrolide antibiotics molecular engram polymer microspheres, which is characterized by comprising the following steps: dissolving a dispersant, namely polyvinyl alcohol or hydroxyethyl cellulose into secondary distilled water to prepare a water-phase dispersion liquid; dissolving engram molecules and functional monomers into an organic solvent to prepare an oil-phase mixture; adding the oil-phase mixture into the water-phase dispersion liquid under the action of stirring, adding an initiator, namely azo-bis-iso-butyrynitrile into the mixture, performing thermal initiation polymerization on the mixture in water bath, and obtaining polymer microspheres; and adopting an ultrasonic extraction method to elute the engram molecules in a butyl acetate aqueous solution or a methanol solution of acetic acid, using distilled water to wash the engram molecules, and performing vacuum drying on the engram molecules to obtain the macrolide antibiotics molecular engram polymer microspheres. Through the method, the macrolide antibiotics molecular engram polymer microspheres are prepared in water phases and are recognized in the water phases; the reorganization result is close to that obtained by a natural biological molecular recognition system; and the invention provides a method for recognizing, separating and analyzing hydrophilic medicaments .
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Macrolide antibiotic bacterium dregs innocent treatment method
InactiveCN101380509ASolve pollutionRealize resource utilizationClimate change adaptationBioloigcal waste fertilisersEconomic benefitsMacrolide resistance
The invention discloses a harmless treatment method of macrolide antibiotic residues, which belongs to the technical field of solid waste treatment. The method comprises the following steps: firstly, inversely and mechanically stirring effectively broken sclertium, and then harmlessly treating the macrolide antibiotic residues with selectomycin harmless treatment microbial inoculum. The treated antibiotic residues can be used as vegetable fertilizer additive to promote the vegetable growth and solve the problem of environmental pollution caused by macrolide antibiotic residues, thus the method realizes the comprehensive utilization of resources and has better economic benefits.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
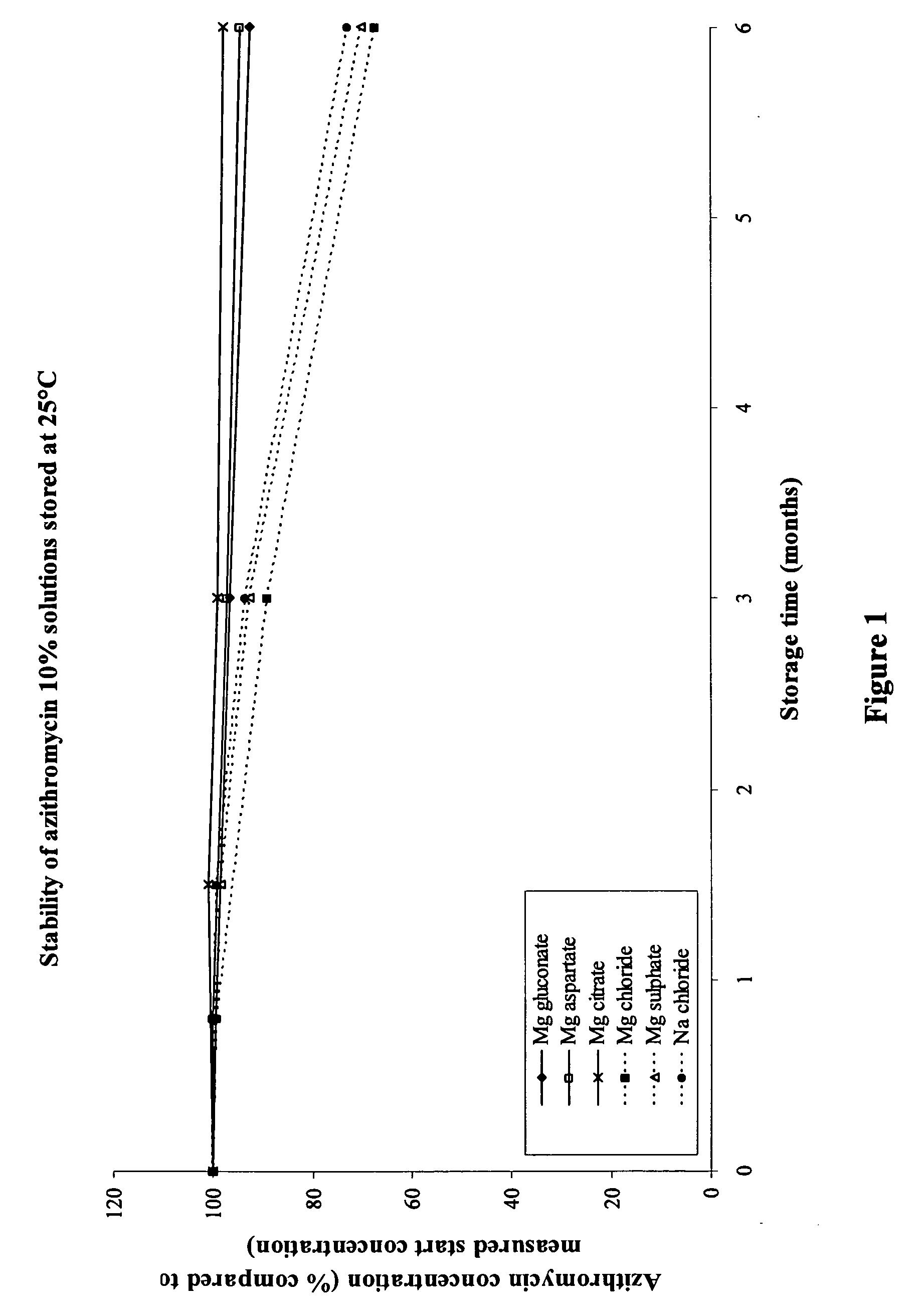
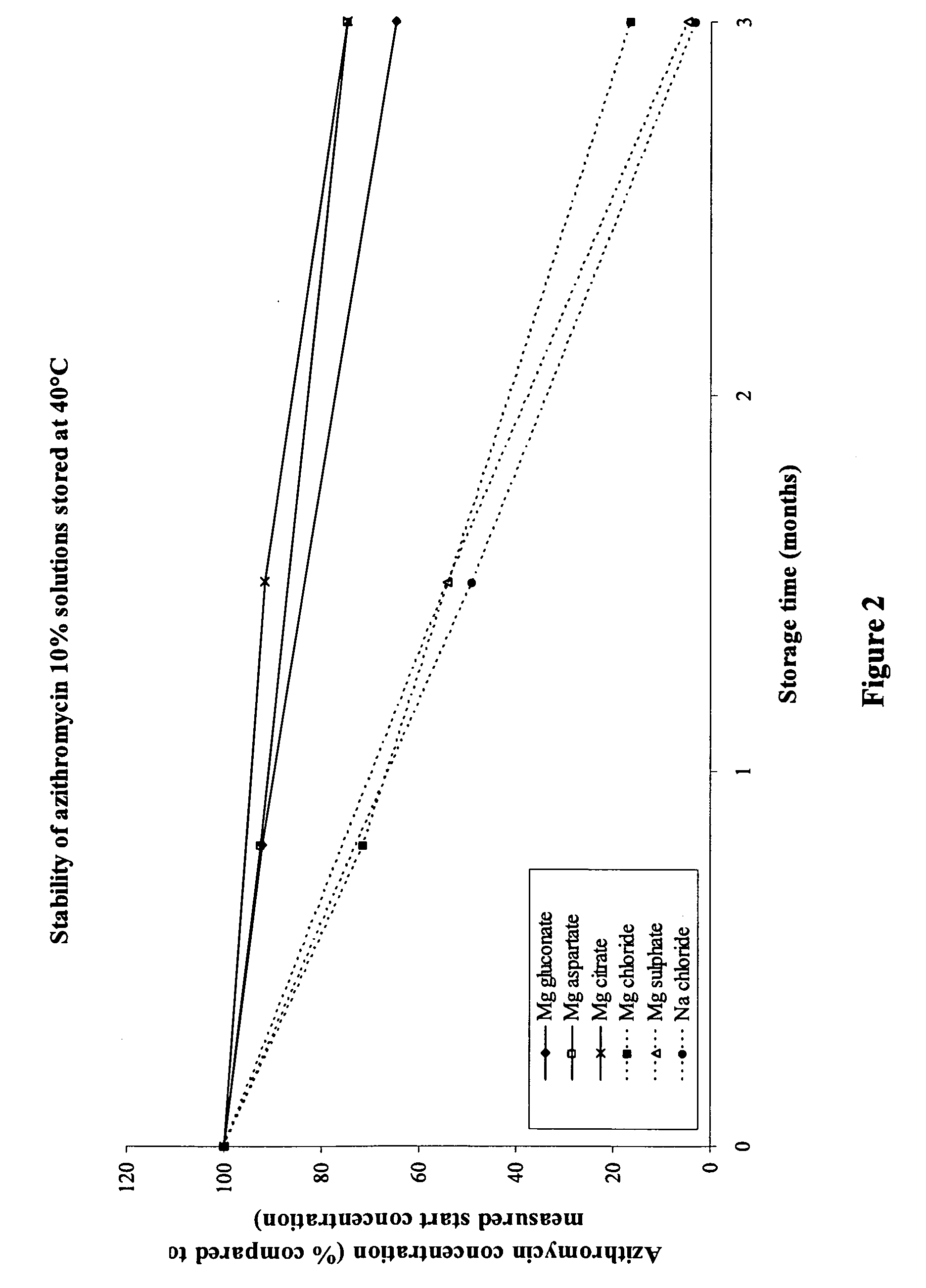
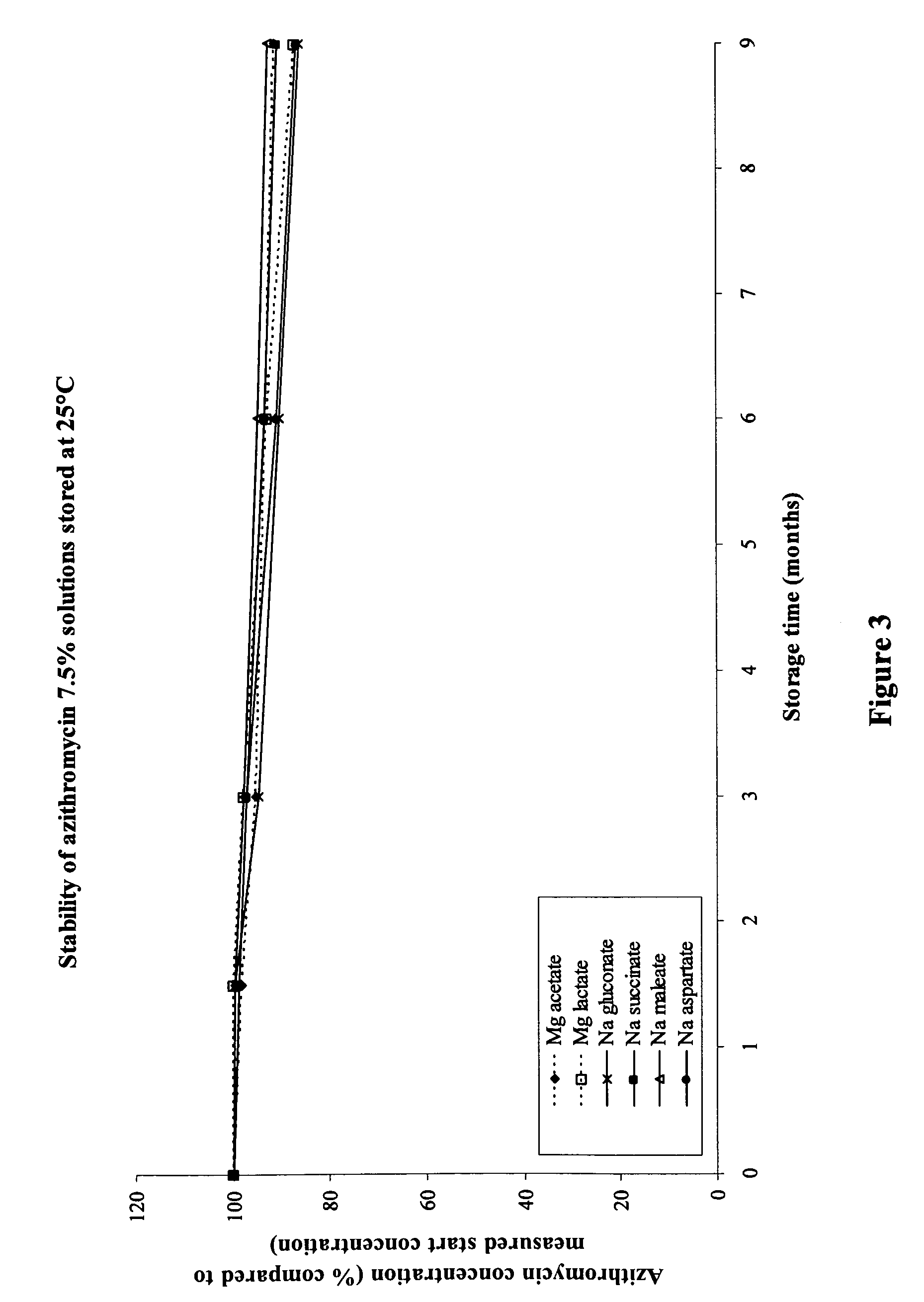
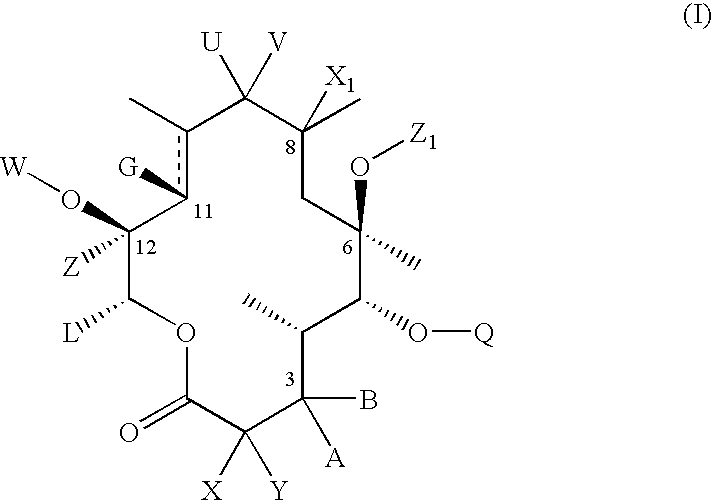
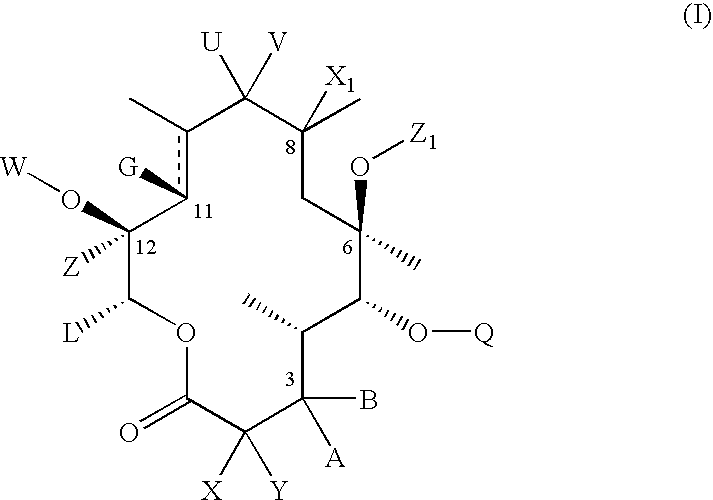
Macrolide compositions having improved taste and stability
InactiveUS20090232744A1Improve stabilityBad tasteBiocideDispersion deliverySodium acetateSodium lactate
The invention provides an aqueous pharmaceutical composition for administration as an aerosol to the respiratory tract, nose or oropharyngeal region comprising (i) a macrolide having a poor taste and poor chemical stability in aqueous solution; (ii) at least one salt selected from the group consisting of sodium gluconate, sodium aspartate, sodium acetate, sodium lactate, sodium succinate, sodium maleate, magnesium gluconate, magnesium aspartate, magnesium citrate, magnesium acetate, magnesium lactate, magnesium succinate, and magnesium maleate; or mixtures thereof and (iii) a taste-masking agent different from said salt; wherein (a) the concentration of said macrolide in the composition is in the range of about 0.25 wt.-% to about 15 wt.-%; (b) the molar ratio of said macrolide:said salt is in the range from about 1:0.5 to about 1:100; (c) the pH of the composition is in the range of about 3 to 9; and (d) the osmolality of the composition is in the range of about 150 mOsmol / kg to about 1500 mOsmol / kg. The invention further provides a method of generating an aerosol, preferably by means of a nebuliser, which uses such an aqueous pharmaceutical composition. The macrolide may be used alone or in combination with other drugs. The composition is suitable to treat inflammatory disorders and / or infections of the respiratory tract. It has an improved taste and stability.
Owner:PARI PHARMA GMBH
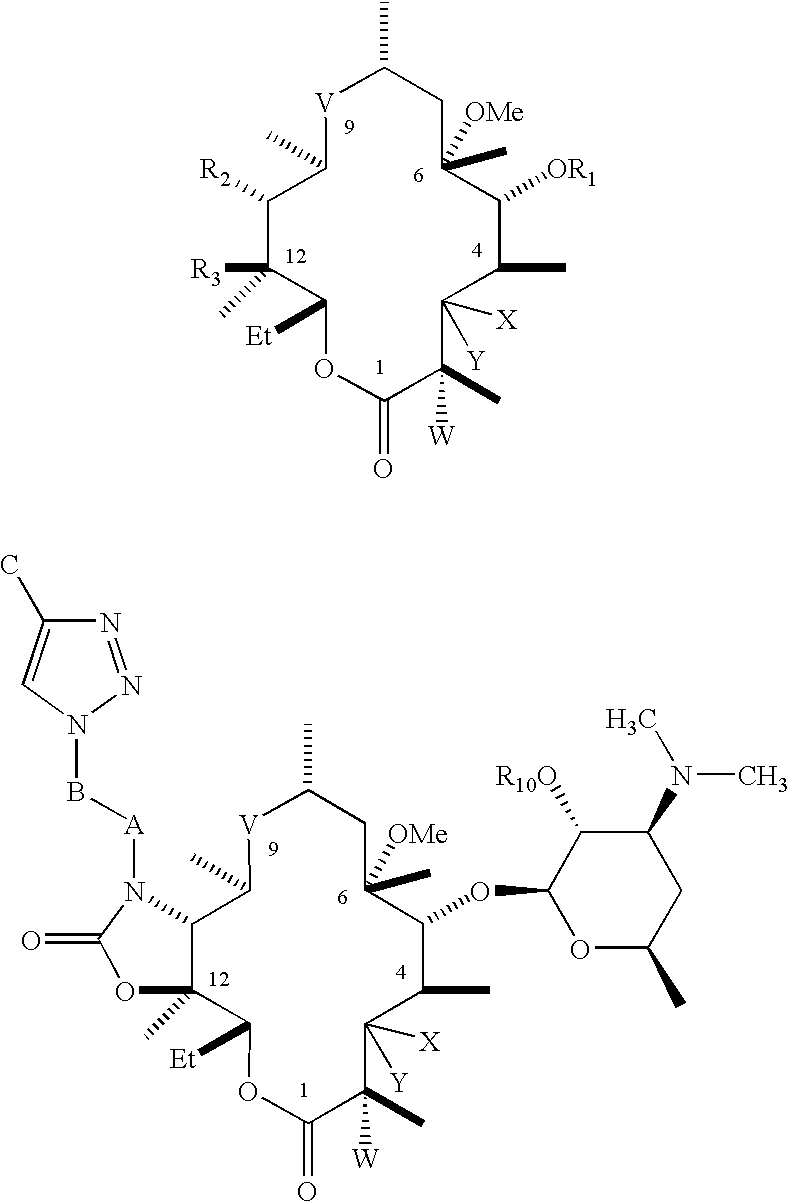
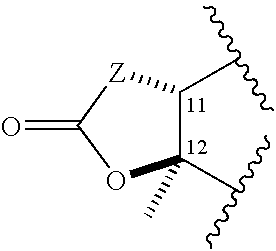
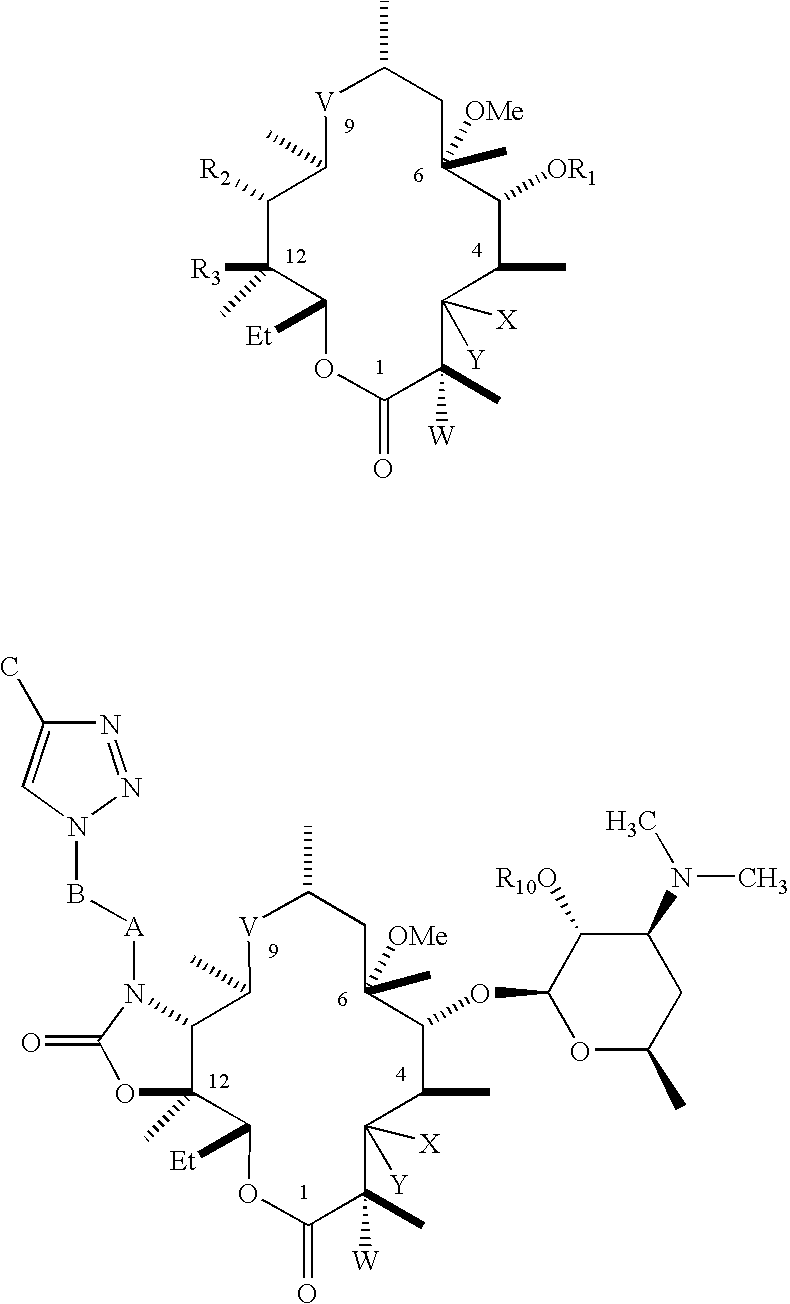
C-8 halogenated macrolides
Owner:ENANTA PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com