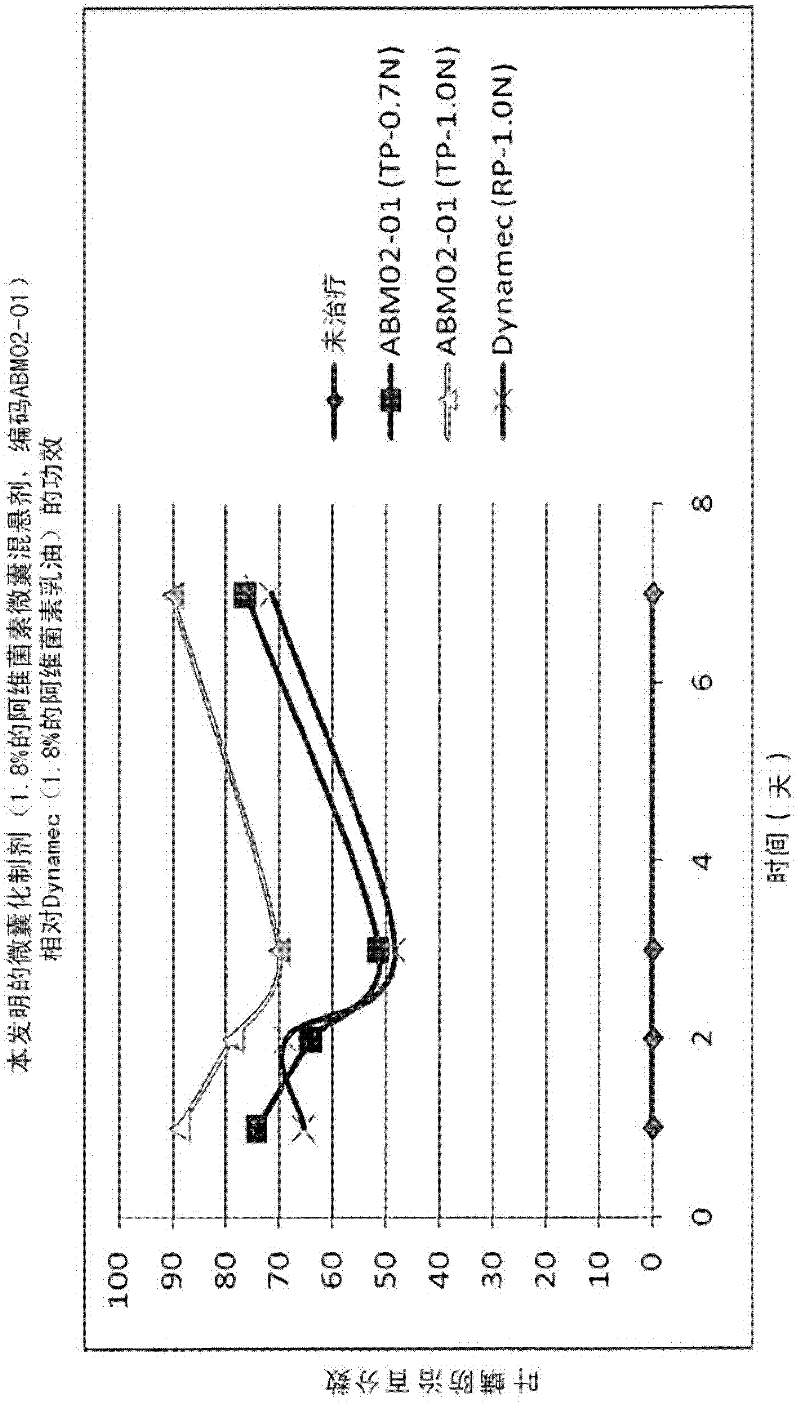
Microcapsules containing macrolide lactones abamectin, milbemectin, avermectins, milbemycins, emamectins, ivermectins and mectins in general
A technology of macrocyclic lactone and microcapsules, which is applied in the preservation of human or animal bodies, botany equipment and methods, animal husbandry, etc., and can solve the problems of no indication of emulsifiable activity of emulsifiable oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0076] Example 2 - Sustained Release Formulation
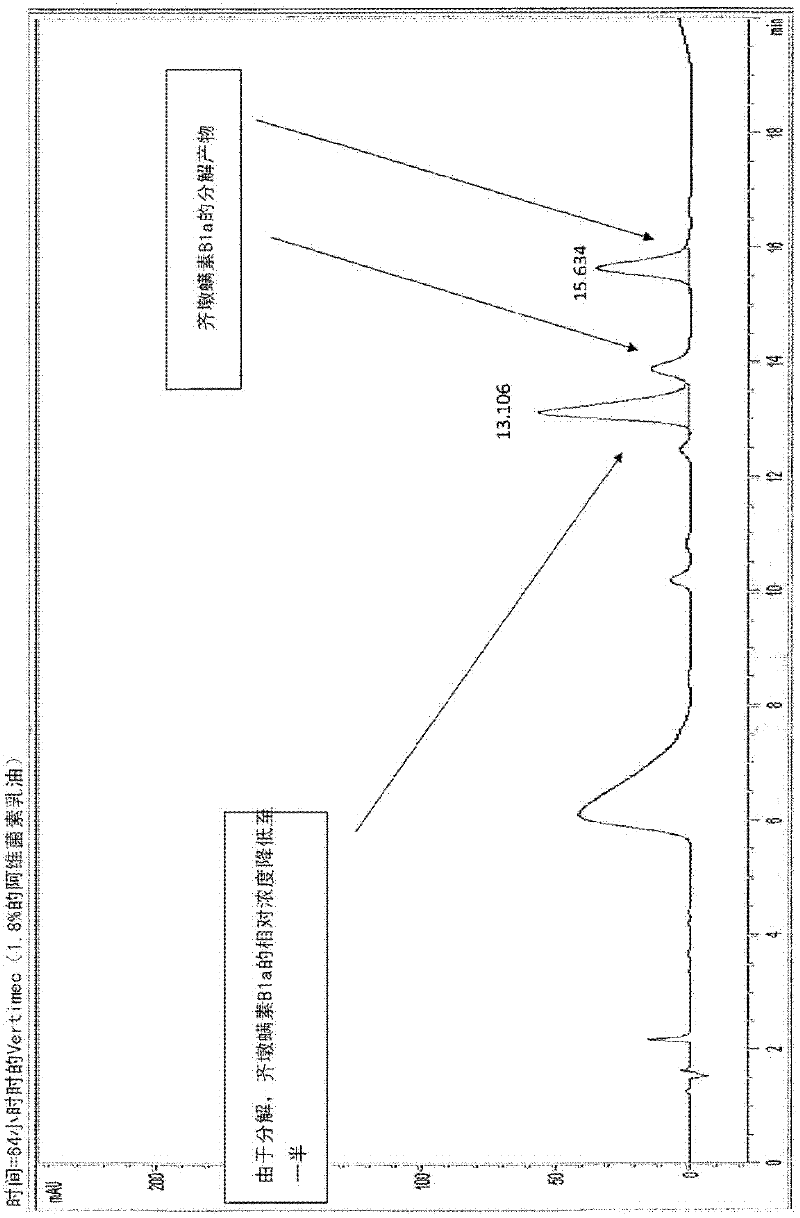
[0077] The present invention can take advantage of the UV protective synergistic activity of the formulation ingredients, their chemical compatibility with the mectins, their demonstrated sufficient biological activity and their good physicochemical properties for sustained release formulations of the mectins. This preparation is an embodiment of the present invention in terms of sustained release of macrolides, and the mibaycin (milbemycin A 3 and A 4 ) and Abamectin (Abamectin B 1a and B 1b ) microencapsulation is illustrated.
[0078] Formulation of Example 2.A
[0079]
[0080] The formulations according to the invention meet the stated requirements and show increased UV protection compared to the conventional EC formulations of Abamectin and Mibamectin detailed below (comparative formulations), and of course also compared to prior art formulations Improved particle size uniformity and physical stability of the for...
Embodiment 3
[0084] Example 3 - Encapsulation levels of mectins compared to similar methods of the prior art
[0085] A formulation very similar to the formulation claimed in the invention and method was prepared for comparison according to the method of the invention and using the following formulation.
[0086] Formulation 3.A
[0087]
[0088] Formulation 3.B
[0089] Preparation 3.B of the present invention is the same as Example 1.A, except that abamectin is replaced by ivermectin.
[0090] The encapsulation level of pure ivermectin in formulation 3.A (microencapsulated based on the same reaction as our invention) was only 61%, while that of 3.B was 94.7%. This demonstrates that the degree of microencapsulation of the present invention is superior to that of conventional polyurea / polyurethane microencapsulation. We purposefully chose 3A because it is a close modification of the published microencapsulation method for the insecticide clomazone (which has been shown to control vol...
Embodiment 4
[0091] Example 4 - Mixed formulation of microencapsulated macrolides and suspended neonicotinoids
[0092]As detailed in the description, the present invention provides for any possible combination of active ingredients as long as there is no incompatibility (chemical and / or physicochemical). A representative example is the combination of 15% suspension concentrate of imidacloprid and thiamethoxam (ratio 1:1 wt.-%) and 3% microencapsulated abamectin.
[0093] To carry out this combination, a suspension concentrate of imidacloprid and thiamethoxam with the target concentrations is first prepared by any prior art method. Then prepare the microencapsulated formulation of Abamectin according to embodiment 1.A, wherein the active ingredient is increased accordingly and the same amount of water is reduced. In the microencapsulation preparation of avermectin, add any necessary surfactant in addition to make the microcapsules well dispersed in the suspending agent (for example, 1:1:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com