Clinical-grade human mesenchymal stem cell serum-free complete medium
A complete culture medium and mesenchymal stem cell technology, applied in animal cells, vertebrate cells, bone/connective tissue cells, etc., can solve the problems of cell pollution, increase of pathogens, high price, etc., to promote cell growth, promote cell proliferation, Effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 A clinical-grade human mesenchymal stem cell serum-free complete medium provided by the present invention. The composition and concentration are shown in Table 1. The following components are dissolved and mixed according to their respective characteristics, and the volume of HEPES is fixed, 0.22μm Sterilize through filtration membrane and store at 4°C.
[0035] Table 1 Composition and concentration of the clinical grade human mesenchymal stem cell serum-free complete medium of the present invention
[0036]
[0037]
Embodiment 2
[0038] Example 2 The clinical grade human mesenchymal stem cell serum-free complete medium of the present invention has been tested by a third-party, and all indicators have reached the standard, as shown in Table 2:
[0039] Table 2 The third-party quality test items and results of the clinical grade human mesenchymal stem cell serum-free complete medium of the present invention
[0040]
[0041] It can be seen from Table 2 that the clinical grade human mesenchymal stem cell serum-free complete medium of the present invention has all the test indicators qualified. Only qualified media can be used in clinical practice.
Embodiment 3
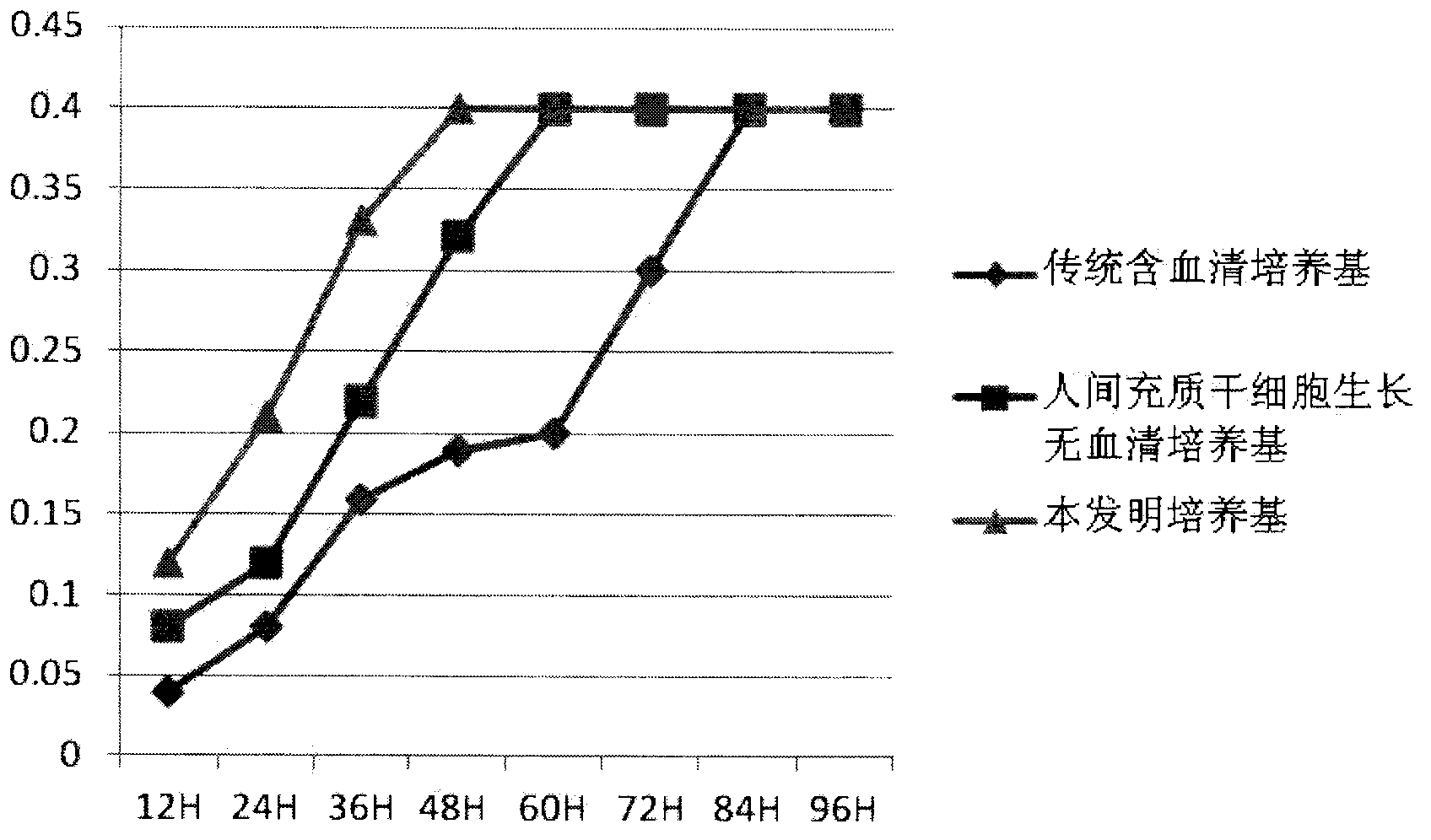
[0042] Example 3. Experiment of culturing human mesenchymal stem cells using the clinical grade human mesenchymal stem cell serum-free complete medium of the present invention (taking adipose stem cells as an example).
[0043] (1) Preparation of adipose stem cells (same as described in most documents): The fat used in the experiment was obtained from abdominal liposuction patients. Under aseptic conditions, 50 mL of a fat-physiological saline mixture was obtained, centrifuged, and washed twice with PBS to remove anesthetics and blood cells to obtain fat particles with higher purity. 0.1% collagenase digestion in a constant temperature shaker at 37°C for 60min, 1500r / min, centrifugation for 10min, remove the upper undigested adipose tissue and grease, resuspend the precipitate and filter on a 100um filter, centrifuge again, red blood cell lysate lysis red blood cells for 5min, phosphate buffer Wash twice and use the following three kinds of culture media:
[0044] 1. Traditional s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com