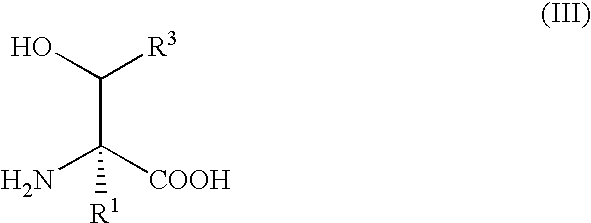Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Tetrahydrofolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
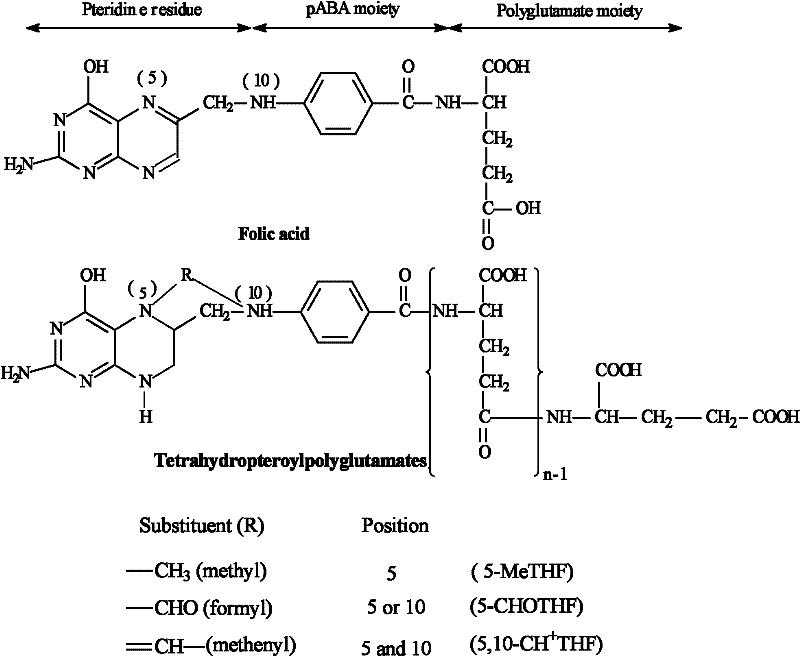
Tetrahydrofolic acid, or tetrahydrofolate, is a folic acid derivative.
Pharmaceutical composition containing a tetrahydrofolic acid
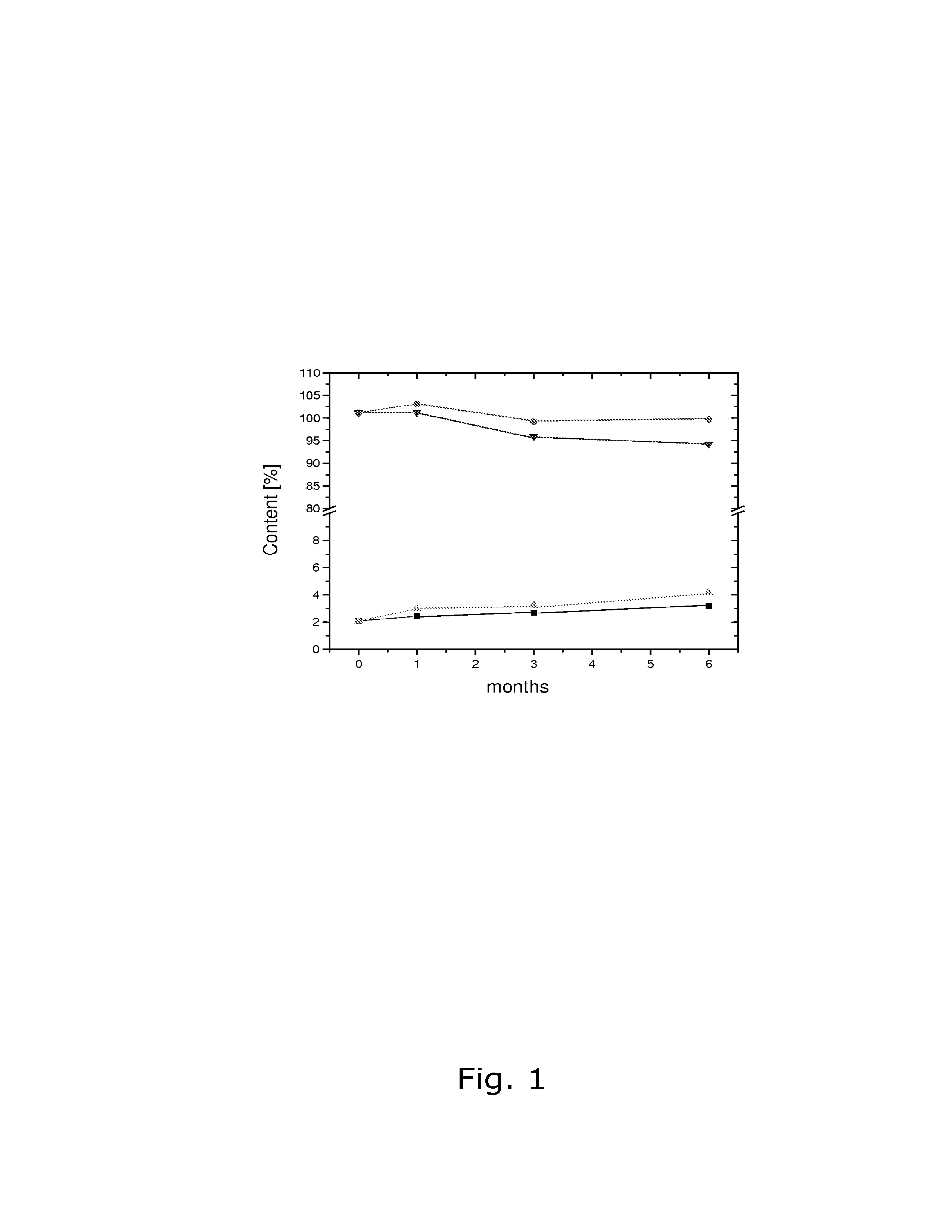
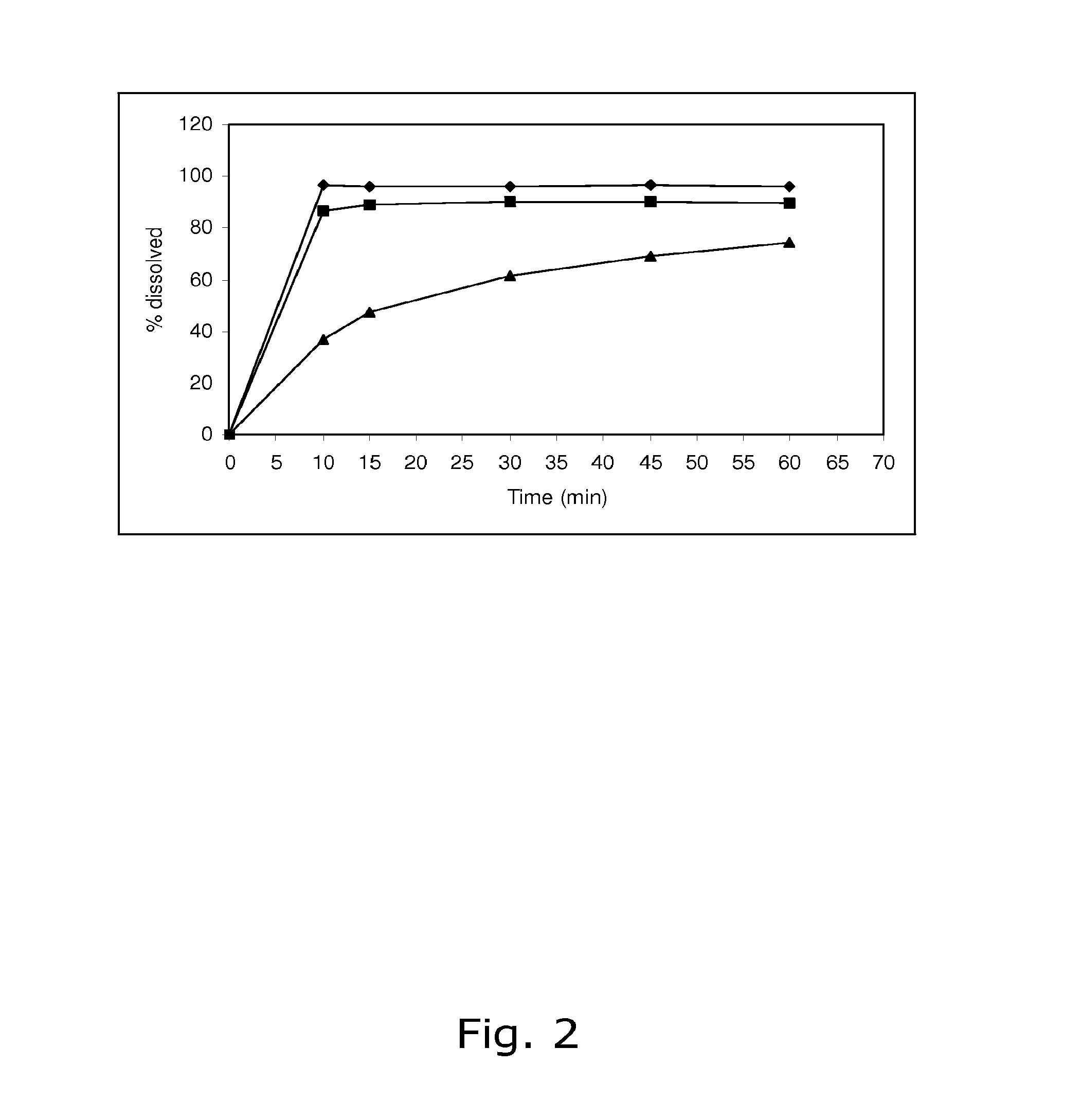
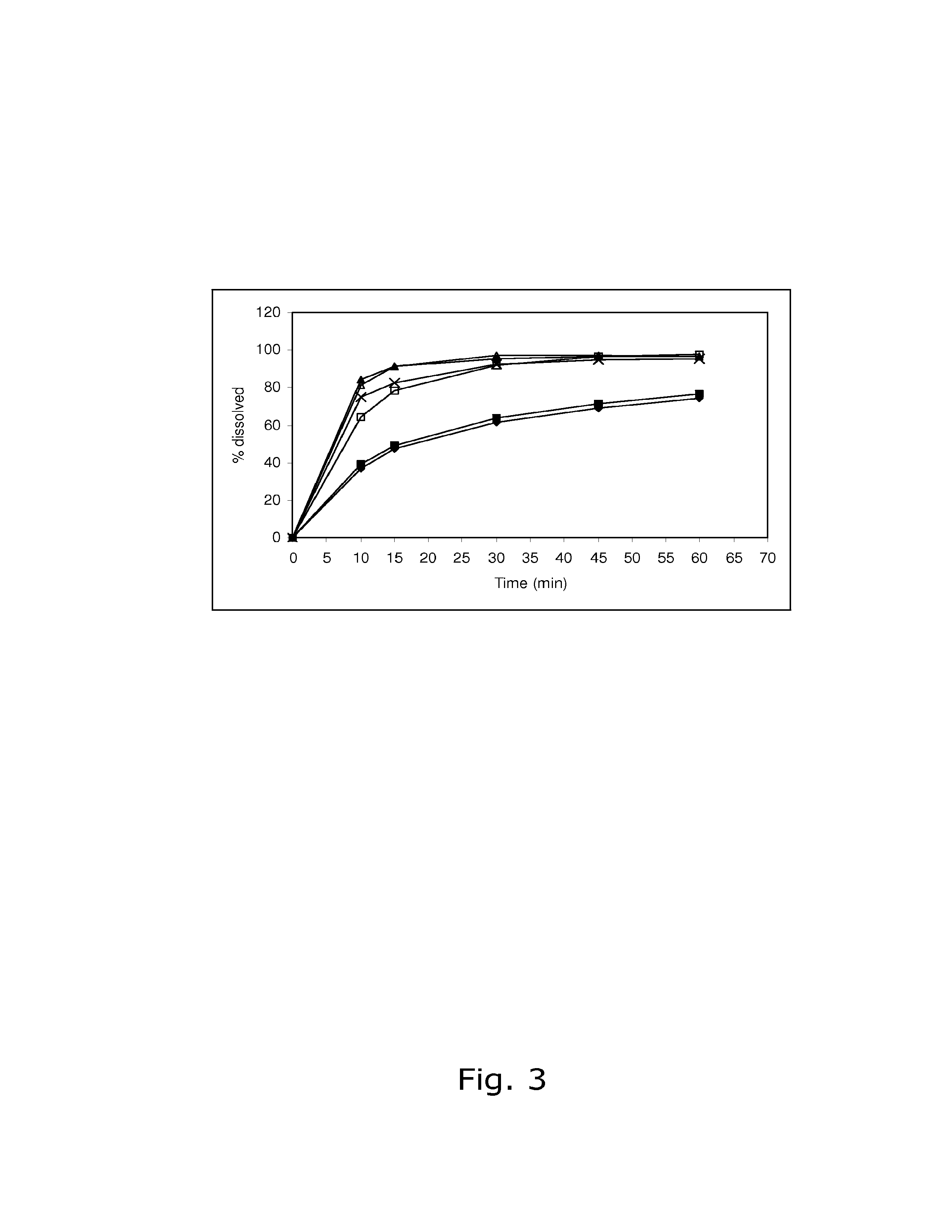
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Nutritional preparations
InactiveUS20060217385A1Promote maturityReduce and alleviate fetusBiocideAnimal repellantsPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives—all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Nutritional preparations
InactiveUS20060217386A1Promote maturityReduce and alleviate fetusBiocideSkeletal disorderPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives-all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Preparation method of D-pantoic acid inner ester
The invention relates to the technical field of biosynthesis, and concretely discloses a preparation method of D-pantoic acid inner ester. The preparation method at least comprises the following stepsthat 1, valine is subjected to enzymatic conversion under the effects of catalase and alpha-ketoisovaleric acid reductase to obtain alpha-ketoisovaleric acid; 2, the alpha-ketoisovaleric acid and tetrahydrofolic acid react under the effects of hydroxylmethyl transferase and magnesium chloride to prepare ketopantoic acid; 3, ketopantoic acid is subjected to enzymatic conversion under the effects of alpha-ketoisovaleric acid reductase to obtain pantoic acid; 4, pantoic acid is prepared into the pantoic acid inner ester. The operation process is simple; the conversion condition is mild; the production efficiency is high; the product quality is high; the enzyme used in the process can be cyclically used; the preparation method belongs to an energy-saving, environment-friendly, green and efficient production technology; the industrial production is convenient.
Owner:JING JING PHARMA
Kit for estimating pre-pregnant nourishment metabolism inheritance capability
InactiveCN101240324AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNutrition
The invention discloses an agent box for assessing pre-pregnant nutrition metabolism hereditary ability. The agent box comprises specificity primer pair and specificity fluorescent detecting probe pair for detecting synchronously number rs1801133 and rs1801131 SNP site on 5,10-methano tetrahydrofolic acid reducing ferment gene, number rs1801394 SNP site on methilanin synthetase reducing ferment gene, number rs731236 and rs1544410 on vitamin D receptor gene, number rs5443 SNP site on G albumen Beta3 second unit gene, general component for detecting fluorescent definite quantity PCR etc.. The agent box of the invention assesses pre-pregnant nutrition metabolism hereditary ability by detecting synchronously mononucleotide polymorphism site gene type correlative closely to pre-pregnant nutrition metabolism hereditary potence.
Owner:HAINAN ZHUJIAN BIOTECH
Compositions and methods for the treatment of osteoporosis and inflammatory joint disease
InactiveUS20060216361A1Avoid problemsUseful in treatmentBiocideCarbohydrate active ingredientsAdditive ingredientVitamin B6 synthesis
Compositions and methods for the treatment of osteoporosis and / or inflammatory joint disease are provided herein. The compositions contain a folate, such as a reduced folate, and folic acid. The folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The folate and folic acid can be given in the same dosage unit or separate dosage units, and more than one dosage unit can be given per dose. The compositions may also contain one or more vitamins and minerals selected from vitamin B12, vitamin B6, vitamin D3, calcium, magnesium, and polyunsaturated fatty acids (PUFAs). These ingredients are optional, but preferable (especially the vitamins and minerals). The compositions may further contain one or more additional ingredients such as vitamins, minerals, and laxatives. The compositions are useful in the treatment of all forms of osteoporosis, including primary osteoporosis and secondary osteoporosis, and / or inflammatory joint diseases, especially in patients having a folic acid metabolism deficiency. The compositions are particularly useful in the treatment of inflammatory joint diseases, with complications that include bone loss, fracture, and osteoporosis. In addition, the compositions are beneficial for the prevention of osteoporosis in subjects who do not yet have the disease, but who are at risk for getting osteoporosis, such as post-menopausal women, subjects with osteopenia (mid thinning of the bone mass), subjects with an inflammatory joint disease, or people who are over the age of 70.
Owner:SCIELE PHARMA CAYMAN
Method for measuring content of total folic acid and derivatives thereof in vegetables synchronously and quantitatively
The invention discloses a method for measuring the content of total folic acid and derivatives thereof in vegetables synchronously and quantitatively, which comprises the following steps of: 1) extracting folic acid in the vegetables; 2) adding mouse serum conjugase into the obtained vegetable folic acid extract, incubating and centrifugating to obtain vegetable folic acid deconjugation extract; 3) performing solid phase extraction on the vegetable folic acid deconjugation extract and purifying; 4) separating four folic acid derivatives comprising tetrahydrofolic acid, 5-methyl-tetrahydrofolic acid, 5-formyl-tetrahydrofolic acid and the folic acid in the vegetables by using a chromatographic column; and 5) measuring the folic acid quantitatively. By the method, the content of the total folic acid and different folic acid derivatives can be measured synchronously.
Owner:ZHEJIANG UNIV
Biosynthesis method for increasing accumulation of L-5-methyltetrahydrofolate
InactiveCN102776217AHigh utilization rate of raw materialsReduce energy consumption and production costsBacteriaMicroorganism based processesMethylenetetrahydrofolate reductase5-Methyltetrahydrofolate
The invention provides a biosynthesis method for increasing accumulation of L-5-methyltetrahydrofolate, and an L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid and a construction method and application thereof. The L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid comprises DHFR (dihydrofolate reductase) gene folA and a MTHFR (methylenetetrahydrofolate reductase) gene metF sequence. The biosynthesis method for increasing accumulation of L-5-methyltetrahydrofolate includes converting the L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid to accumulate an original strain of the L-5-methyltetrahydrofolate so as to obtain a recombinant strain, and fermenting the recombinant strain. The accumulation of the L-5-methyltetrahydrofolate in final fermentation product is evidently higher than that of the L-5-methyltetrahydrofolate in the original strain. Utilization rate of raw materials is increased, production cost and energy consumption are reduced, and a foundation for industrial biosynthesis of the L-5-methyltetrahydrofolate is laid.
Owner:CHINA PHARM UNIV
Kit and method for determining mutation sites of genes of acetaldehyde dehydrogenase 2 and methylene tetrahydrofolic acid reductase by virtue of single tube at the same time
InactiveCN103834733AMicrobiological testing/measurementMultiplex pcrsMethylenetetrahydrofolate reductase
The invention provides a kit and method for determining mutation sites of genes of acetaldehyde dehydrogenase 2 and methylene tetrahydrofolic acid reductase by virtue of a single tube at the same time and belongs to the field of molecular biology test. The kit comprises a whole blood genome DNA (deoxyribonucleic acid) extraction reagent, a multiple PCR (polymerase chain reaction) amplification primer, a multiple PCR amplification reaction reagent, a single-strained DNA separation and purification reagent, a pyrosequencing primer, a pyrosequencing reagent and a kit body. The method comprises the following steps: extracting human whole blood genome DNA, carrying out multiple PCR amplification reaction, separating and purifying a single-strained DNA sample, pyrosequencing and analyzing results. The kit can be used by a person subjected to physical examination and hope to know individual ethyl alcohol and folic acid metabolic capability, can be used for predicting individual nitroglycerin metabolic capability and curative effect and guiding a patient suffering from myocardial infarction to reasonably select and prepare vasodilatation first-aid medicaments and can also be used for predicting the individual folic acid metabolic capability and guiding pregnant and lying-in women to supplement the right dosage of folic acid supplements.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Magnesium electrode for magnesium battery and preparation method thereof
InactiveCN101997107AImprove discharge performanceImprove bindingElectrode manufacturing processesElectrode carriers/collectorsSurface layerGrignard reagent
The invention relates to a magnesium electrode for a magnesium battery and a preparation method thereof, which belong to the technical field of magnesium batteries. The magnesium electrode preparation method comprises the following steps of: depositing an electrode material in a dual-layer structure on a base material, wherein the base layer is a copper layer, and the surface layer is a magnesiumor magnesium alloy layer; adopting an electroplating method for preparing a copper transition layer, and then electrodepositing magnesium in THF (Tetrahydrofolic Acid) solution of Grignard reagents. The dual-layer magnesium electrode prepared via the electrodepositing method in the invention has the advantages of firm and compact combination of the magnesium layer and the base material, good discharge performance and the like, in addition, the electrode surface does not have passive films, and magnesium plating layers have good quality.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER IP GMBH
Preparation method of medicinal calcium folinate
ActiveCN102399223AAvoid the risk of residual toxic substancesHigh yieldOrganic chemistryBulk chemical productionCarboxylic saltToxic material
The invention relates to the field of preparation of bulk pharmaceutical chemicals, in particular to a medicinal calcium folinate. The method comprises the following steps of: firstly reducing folic acid to generate tetrahydrofolic acid; then acylating tetrahydrofolic acid to generate leucovorin hydrochloride; and finally salifying leucovorin hydrochloride to generate calcium folinate. By the adoption of the method, the residual risk of toxic substances in medicines in the traditional process can be avoided completely; nontoxic organic acid zinc is used as a catalyst and nontoxic organic amine carboxylate servers as a reduzate and a protective group of aldehyde group, therefore the product is safer and the yield and purity of the product can be improved; a low-temperature and acidification condition is adopted in the method for keeping the leucovorin hydrochloride which serves as an intermediate stable, therefore the yield of the product can be improved, and impurities in the product can be reduced, and the yield of the finished calcium folinate product, based on calcium folinate, is improved to 30-38% from original 15-20%.
Owner:HUZHOU ZHANWANG PHARMA
Multiple folate formulation and use thereof
A multiple folate composition having at least 3 different forms of folate comprising at least one of folic acid (a salt or ester thereof); at least one of a folinic acid (a salt or ester thereof); and at least one of a 5-methyl-tetrahydrofolic acid (a salt or ester thereof) is disclosed. The composition is useful as a nutritional supplement or medication in the treatment of folate deficiency and the sequella thereof and / or in conditions responsive to administration of metabolically useful folate. The compositions are particularly of use in patients who have impaired or reduced ability to convert folic acid to its metabolically active forms, and in the treatment of depression.
Owner:JAYMAC PHARMA
Food and vitamin preparations containing the natural isomer of reduced folates
InactiveUS7172778B2HealthGood for healthOrganic active ingredientsVitamin food ingredientsDietary supplementVitamin Preparations
A composition for human or animal consumption for supplying folate which includes a natural isomer of reduced folate, such as (6S)-tetrahydrofolic acid, 5-methyl-(6S)-tetrahydrofolic acid, 5-formyl-(6S)-tetrahydrofolic acid, 10-formyl-(6R)-tetrahydrofolic acid, 5,10-methylene-(6R)-tetrahydrofolic acid, 5,10-methenyl-(6R)-tetrahydrofolic acid, 5-formimino-(6S)-tetrahydrofolic acid, and their polyglutamyl derivatives is disclosed. Such compositions include multivitamin preparations (with or without minerals and other nutrients); breakfast foods such as prepared cereals, toaster pastries and breakfast bars; infant formulas; dietary supplements and complete diet and weight-loss formulas and bars; animal feed (for example pet foods) and animal feed supplements (such as for poultry feed). The amount of the natural isomer of a reduced folate in a composition for human consumption can range between about 5% and about 200% of the daily requirement for folic acid per serving or dose.
Owner:SOUTH ALABAMA MEDICAL SCI FOUND
Stable pharmaceutical composition of (6S)-5-methyl-calcium tetrahydrofolate
InactiveCN104490887AQuick and reliable releaseImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMethyl groupClelands Reagent
The invention discloses a stable pharmaceutical composition, which contains (6S)-5-methyl-calcium tetrahydrofolate crystal and / or pharmaceutically acceptable reducing substances, and pharmaceutically acceptable auxiliary materials. Specifically, the reducing substances can protect (6S)-5-methyl-tetrahydrofolic acid and its salt from being oxidized, and can be selected from vitamin C and its salt, isovitamin C and its salt, mercaptoethanol, cysteine, mercaptoethyl sulfonic acid, dithiothreitol, reduced glutathione, and lipoic acid. The stable pharmaceutical composition provided by the invention has good stability during processing and storage, the drug risk caused by degradation can be avoided, and at the same time fast and reliable release of (6S)-5-methyl-tetrahydrofolic acid in the composition can be ensured.
Owner:LIANYUNGANG JINKANG HEXIN PHARMA CO LTD
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Epithelial tissue staining reagent and preparation method thereof
ActiveCN105199430ANon-irritatingImprove reducibilityPreparing sample for investigationOrganic dyesCysteamineSolubility
The invention discloses an epithelial tissue staining reagent. The epithelial tissue staining reagent comprises components in percentage by weight as follows: 0.5%-15% of folic acid derivatives, 0.02%-10% of methylene blue, 0.1%-5% of horseradish peroxidase, 2%-10% of aminogalactose, 2%-10% of N-phthaloyl chitosan oligosaccharide, 2%-6% of medical glacial acetic acid and the balance of deionized water, wherein the folic acid derivatives preferably adopt one or more of 5,6,7,8-tetrahydrofolic acid, histidine N-acetyl-2-ethanediamide folic acid and cysteamine modified folic acid. The epithelial tissue staining reagent has the advantages as follows: the raw materials don't have irritation to skin and tract mucosa; the specificity is better; the selected folic acid derivatives are more easily combined with a folic acid receptor; saccharide derivatives have good reducibility and good solubility and are easy to prepare.
Owner:济宁博联生物科技有限公司
Crystal form A of 5-methyl-(6S)-tetrahydrofolic acid and preparation method thereof
The invention relates to a crystal form A of 5-methyl-(6S)-tetrahydrofolic acid and a preparation method thereof. The crystal form A of the 5-methyl-(6S)-tetrahydrofolic acid has characteristic peaks at about 7.3, 9.3, 11.6, 12.1, 14.0, 14.4, 15.6, 16.7, 17.2, 18.2, 18.5, 18.9, 19.9, 20.8, 21.5, 21.8, 22.1, 22.6, 23.2, 23.5, 23.9, 24.4, 24.7, 25.6, 26.5, 27.1, 27.8, 28.4 and 29.1 in X-ray powder diffraction expressed by 2[theta] by utilization of Cu-K[alpha] radiation. The crystal form A of the 5-methyl-(6S)-tetrahydrofolic acid is good in stability, high in purity, good in reproducibility and good in dissolvability. The preparation method is simple and is suitable for industrial production.
Owner:SHANGHAI SYNCORES TECH INC
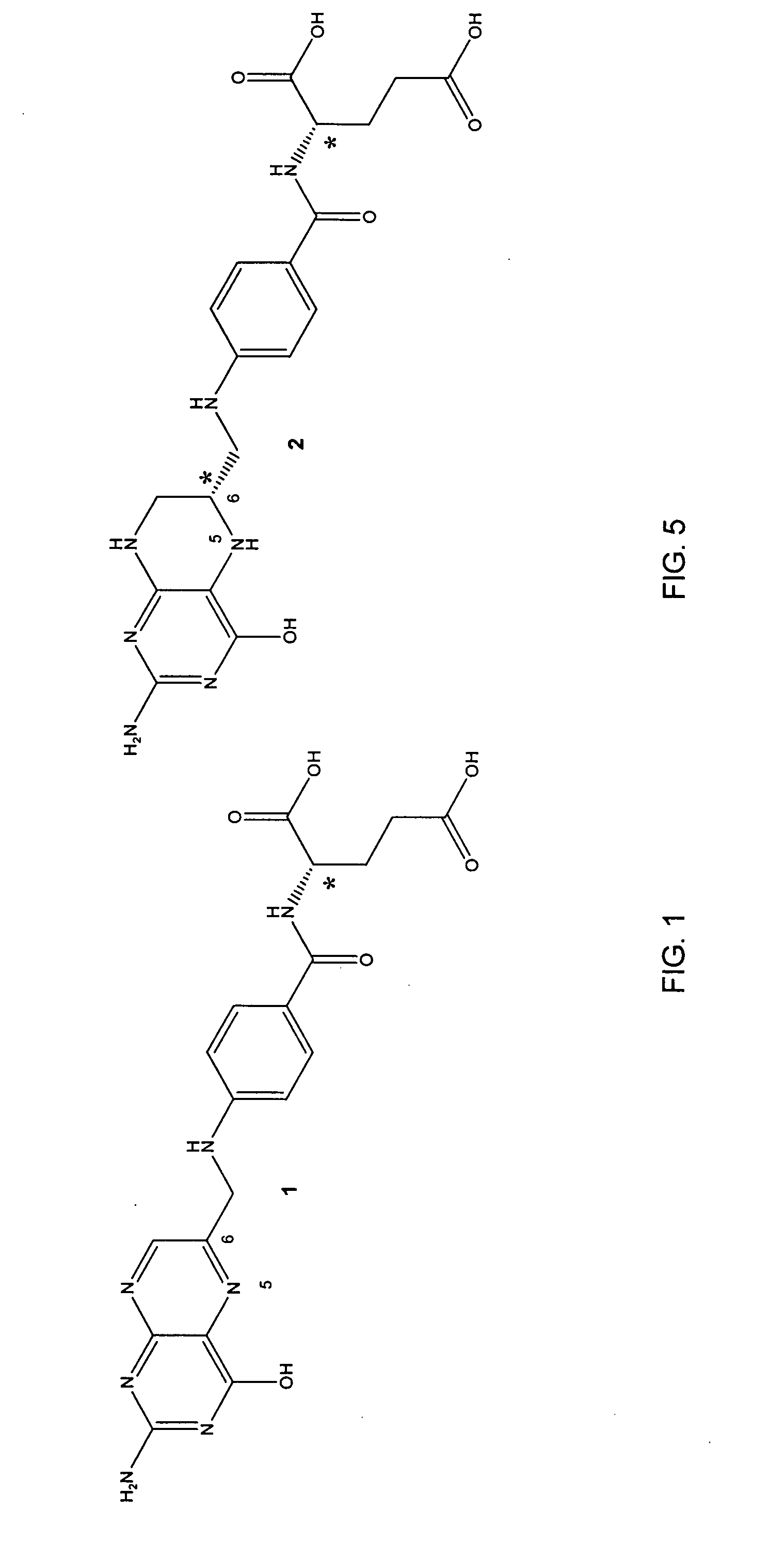
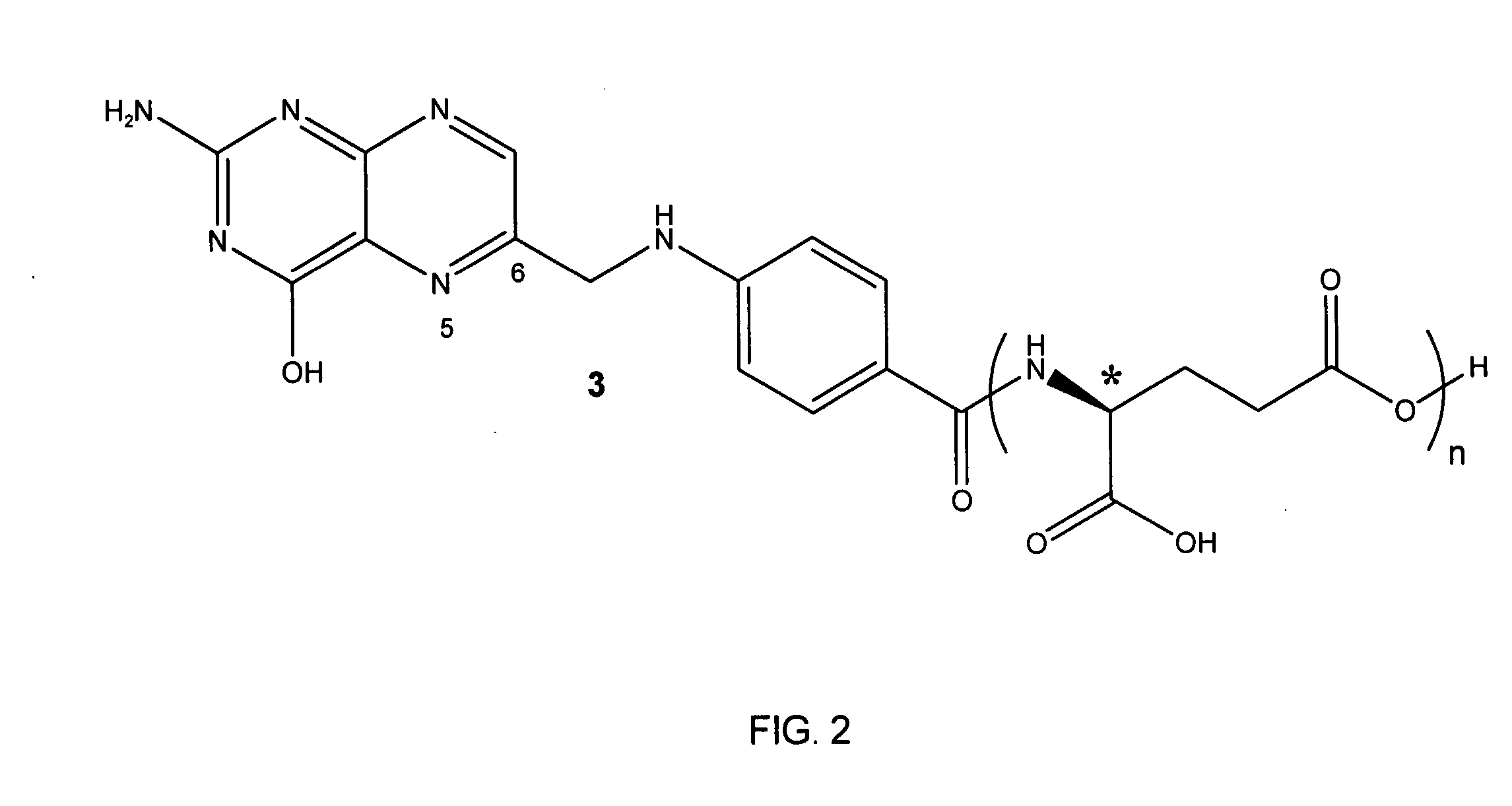
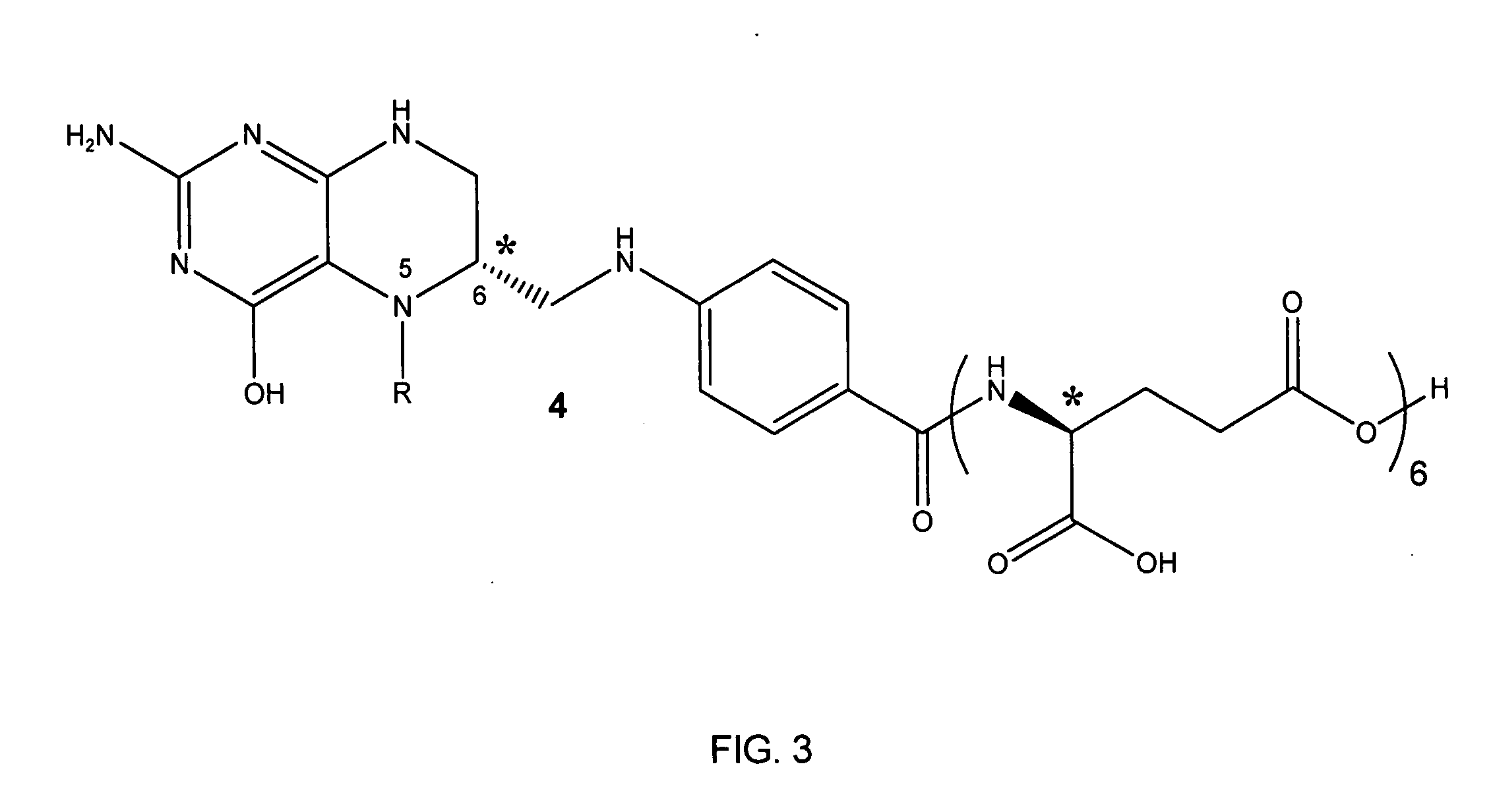
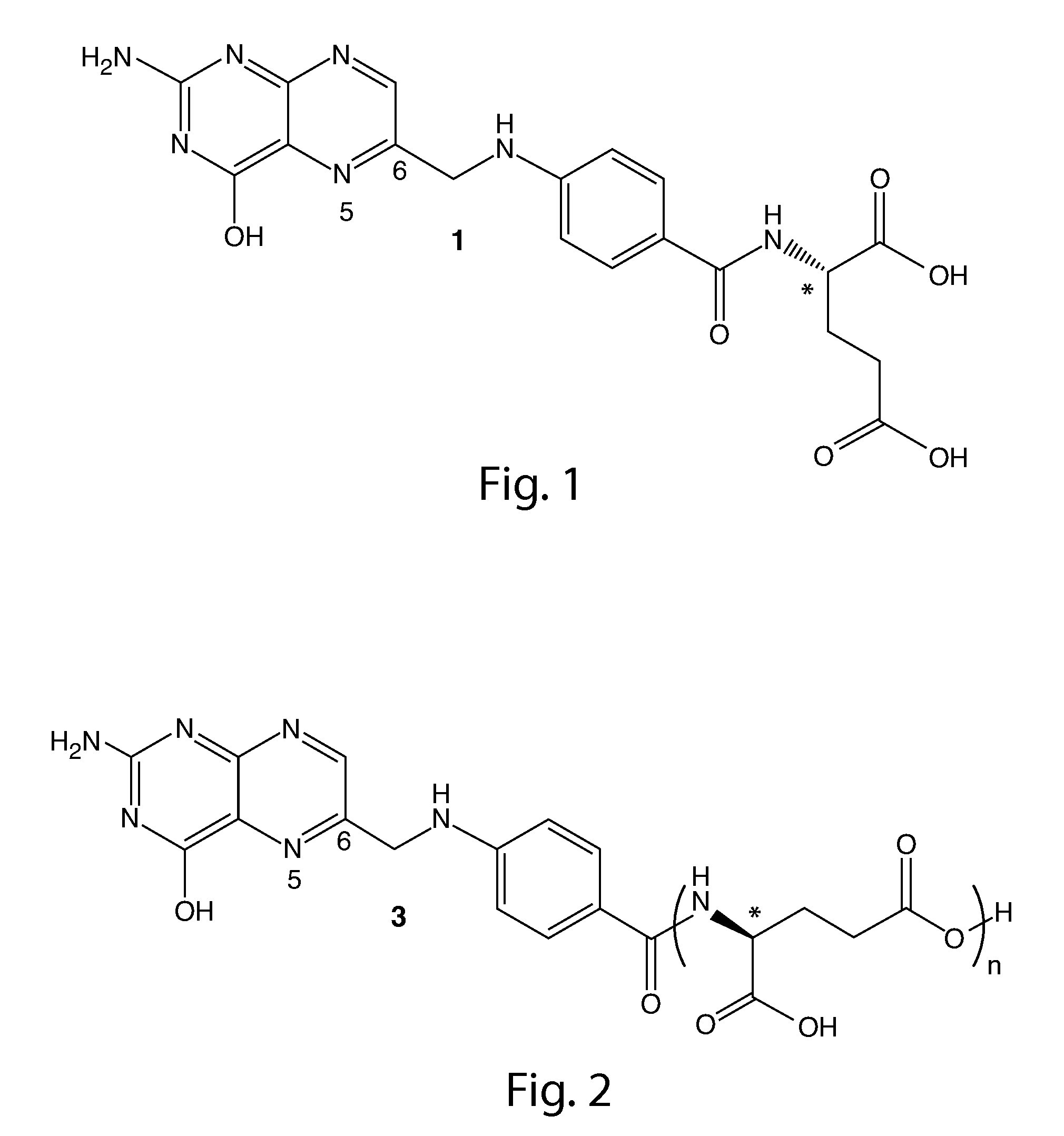
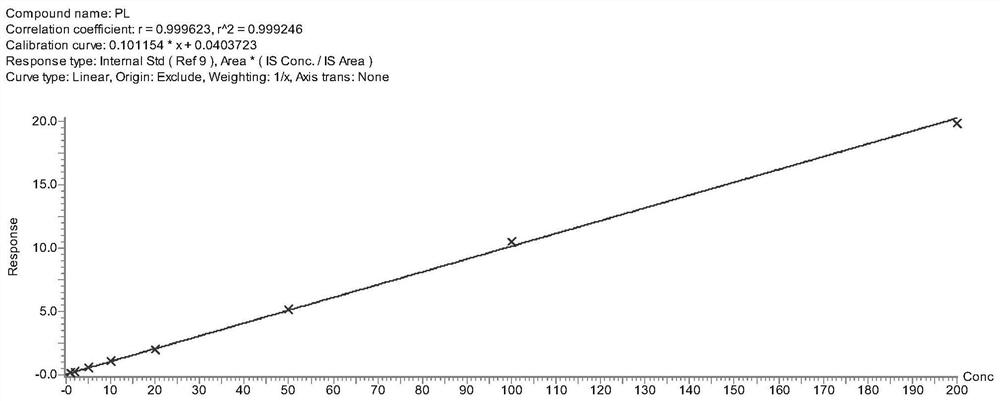
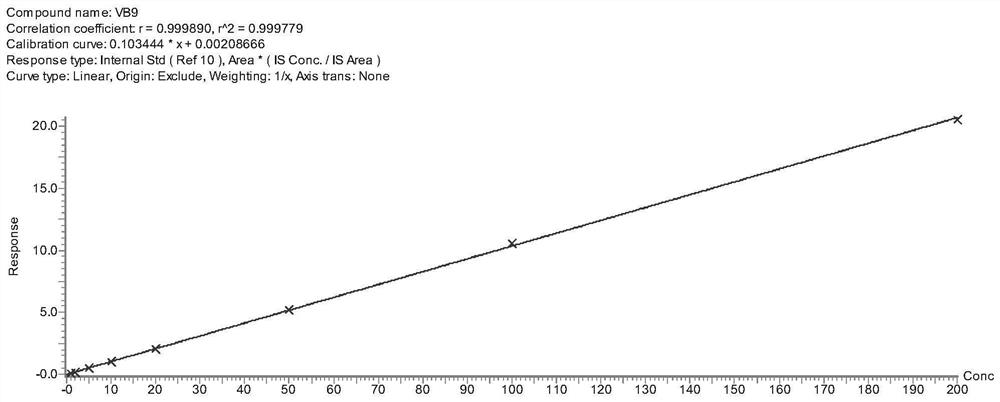
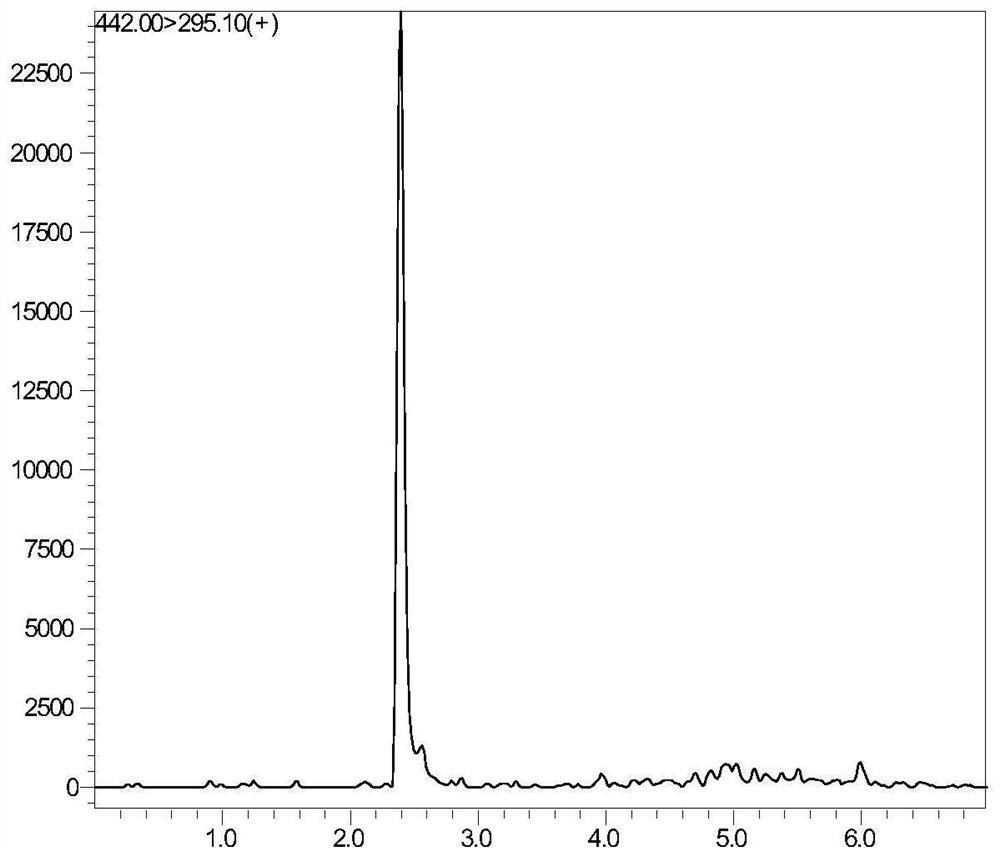
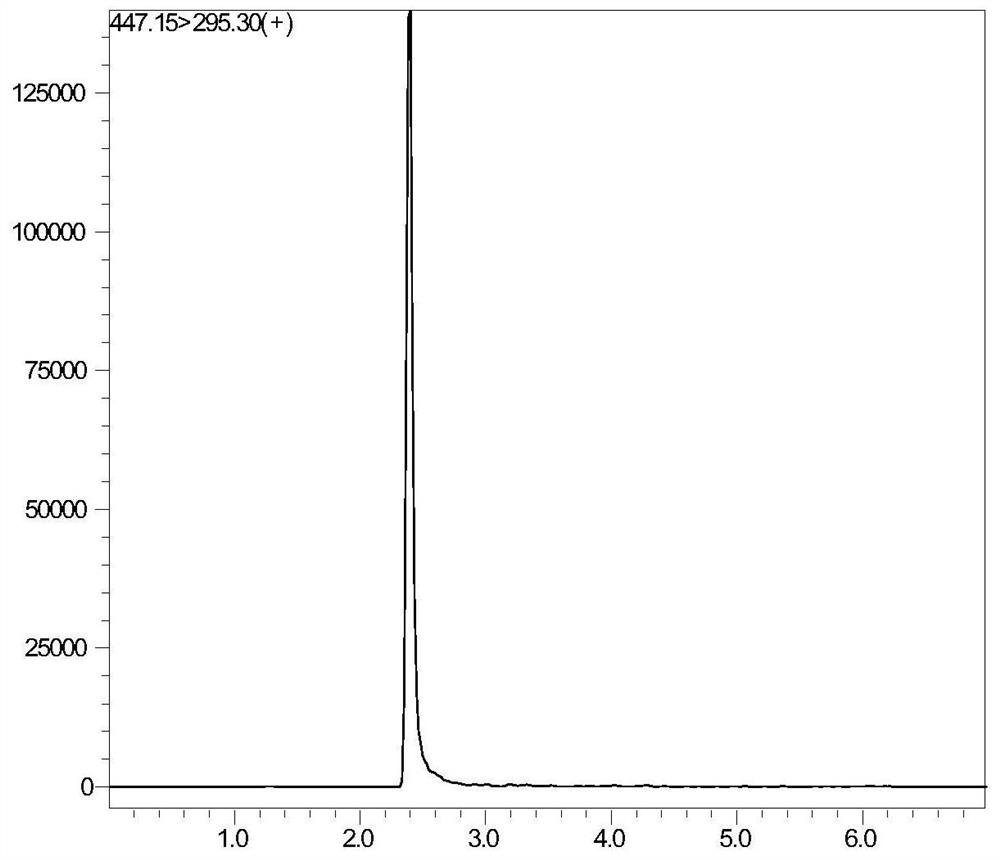
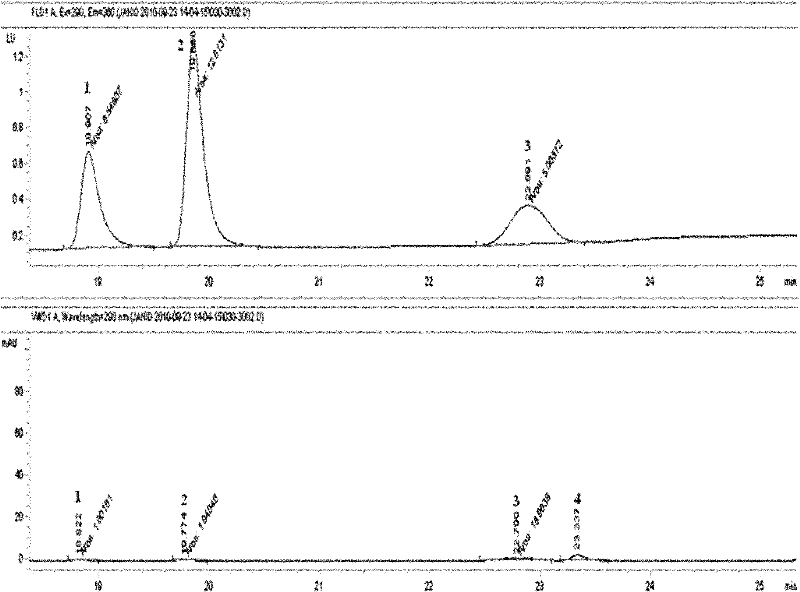
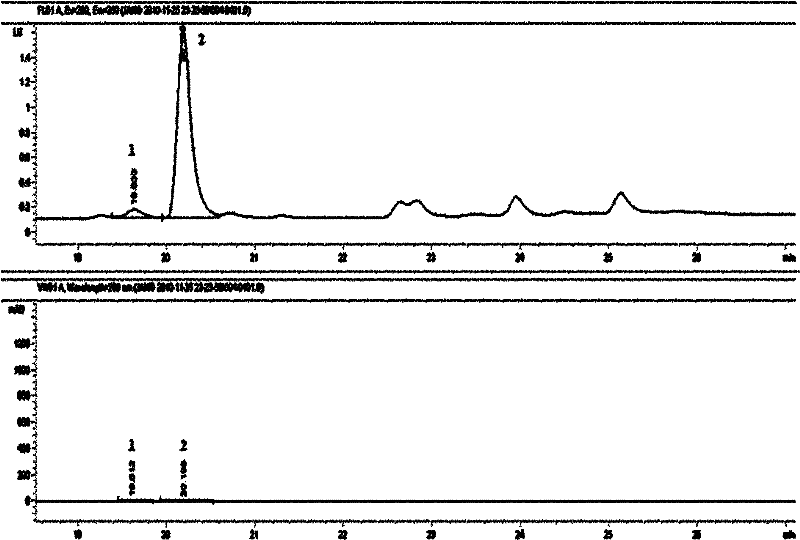
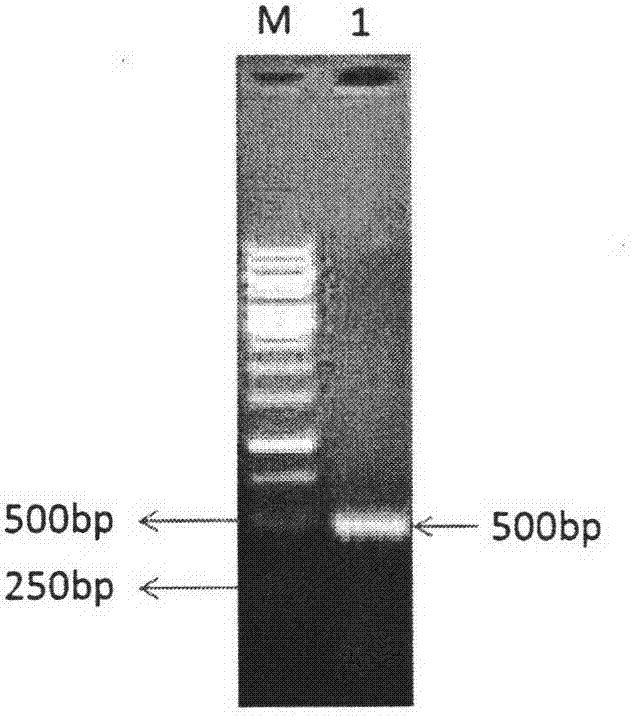
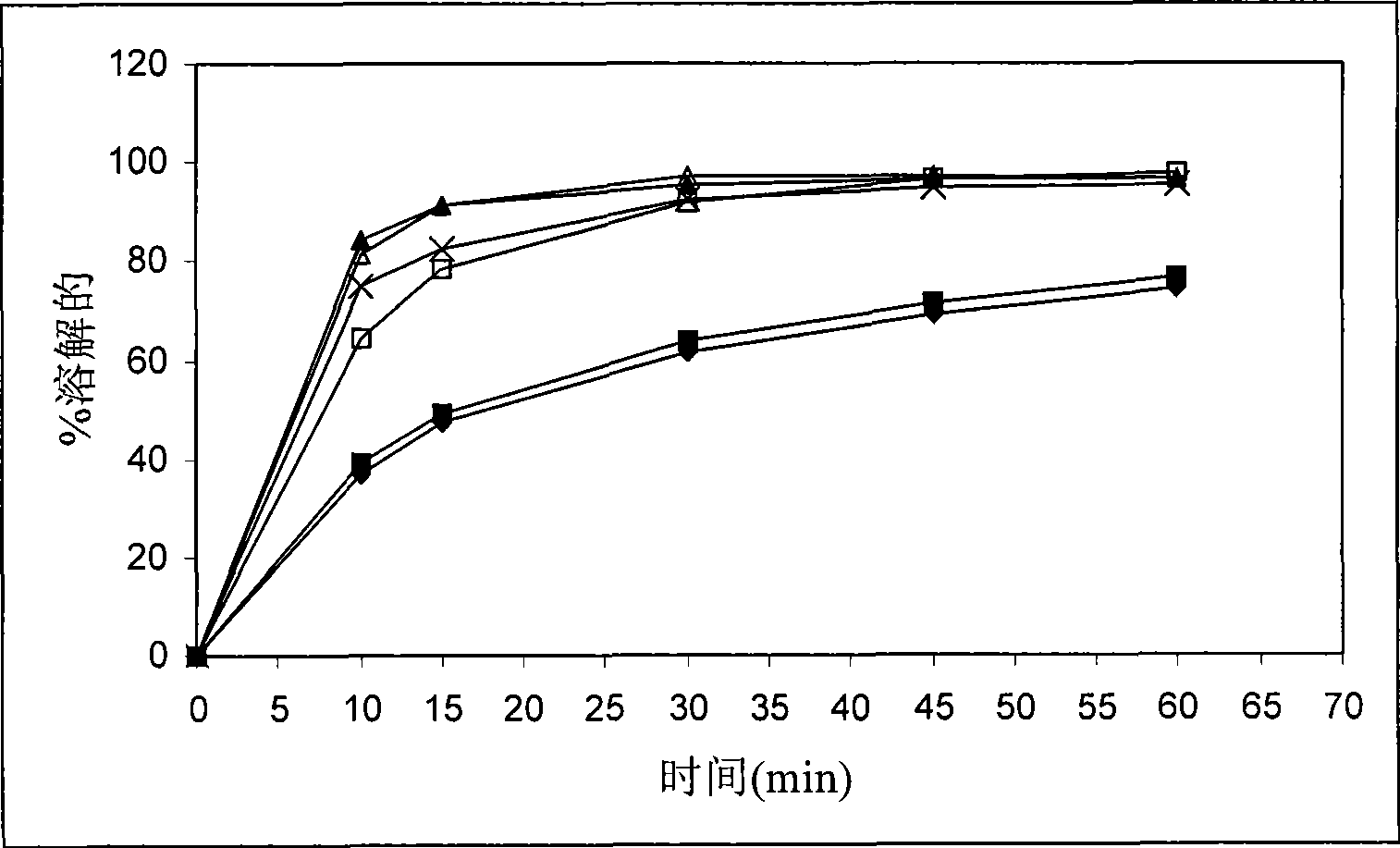
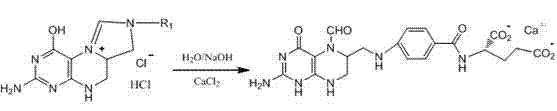
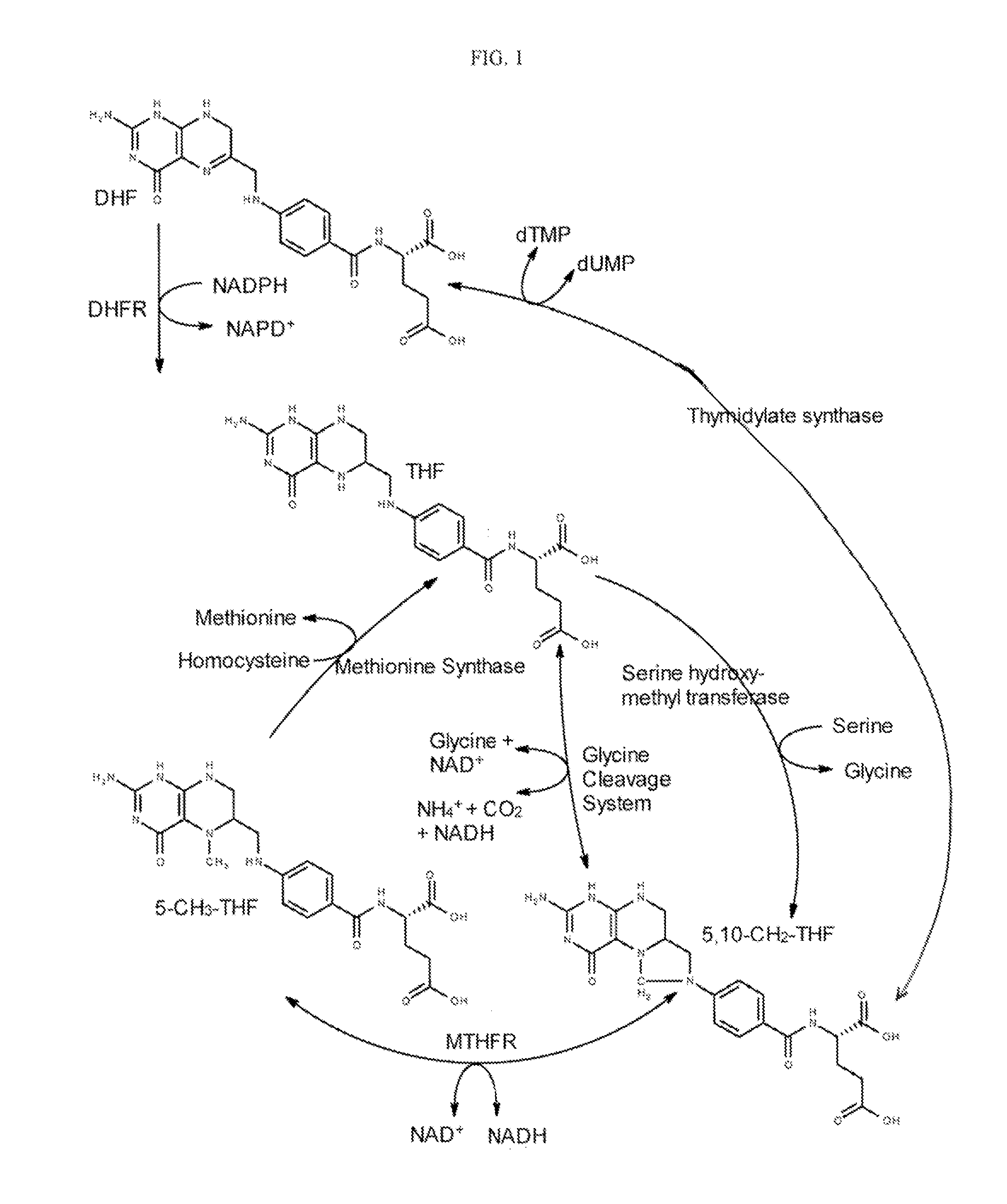
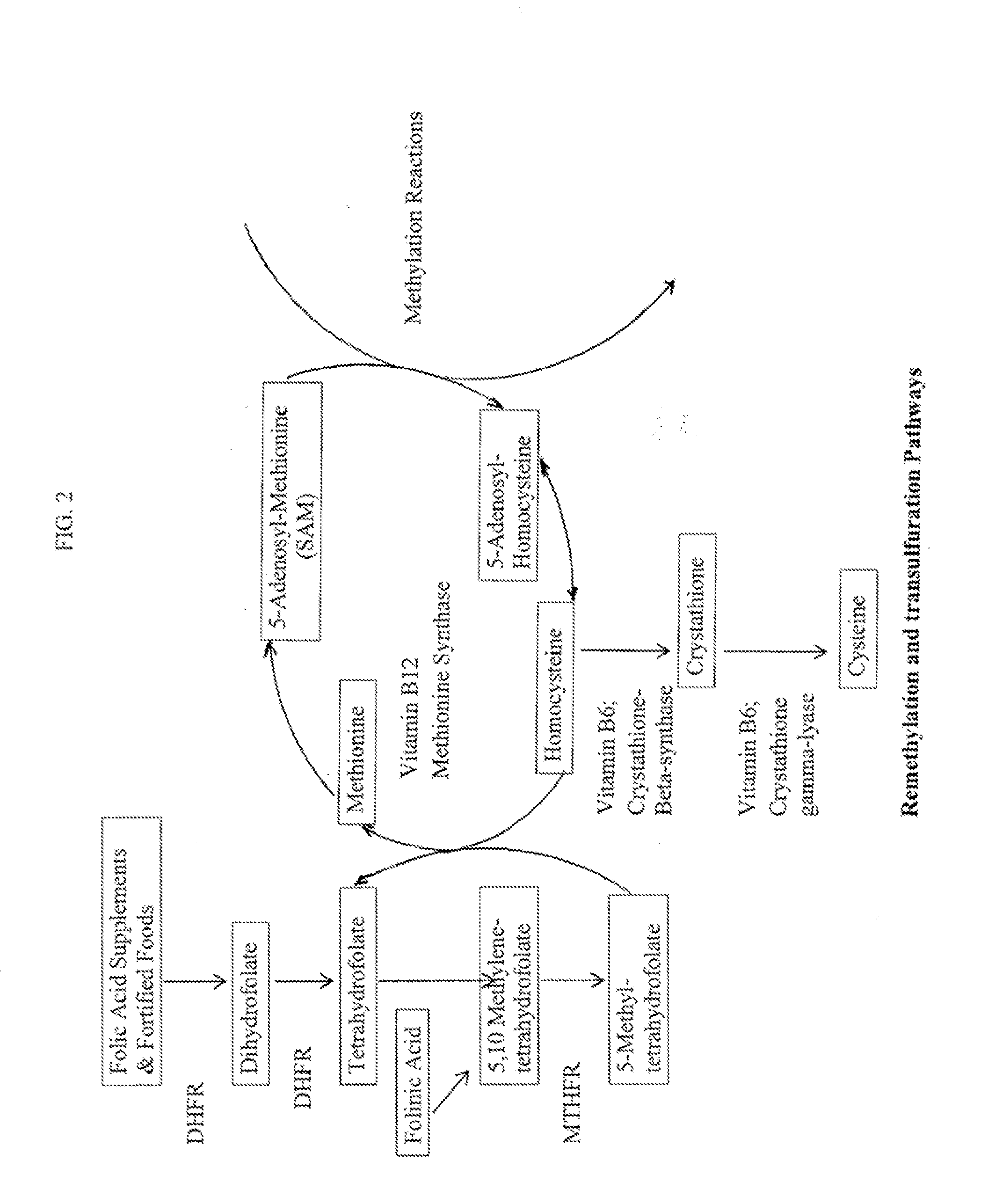
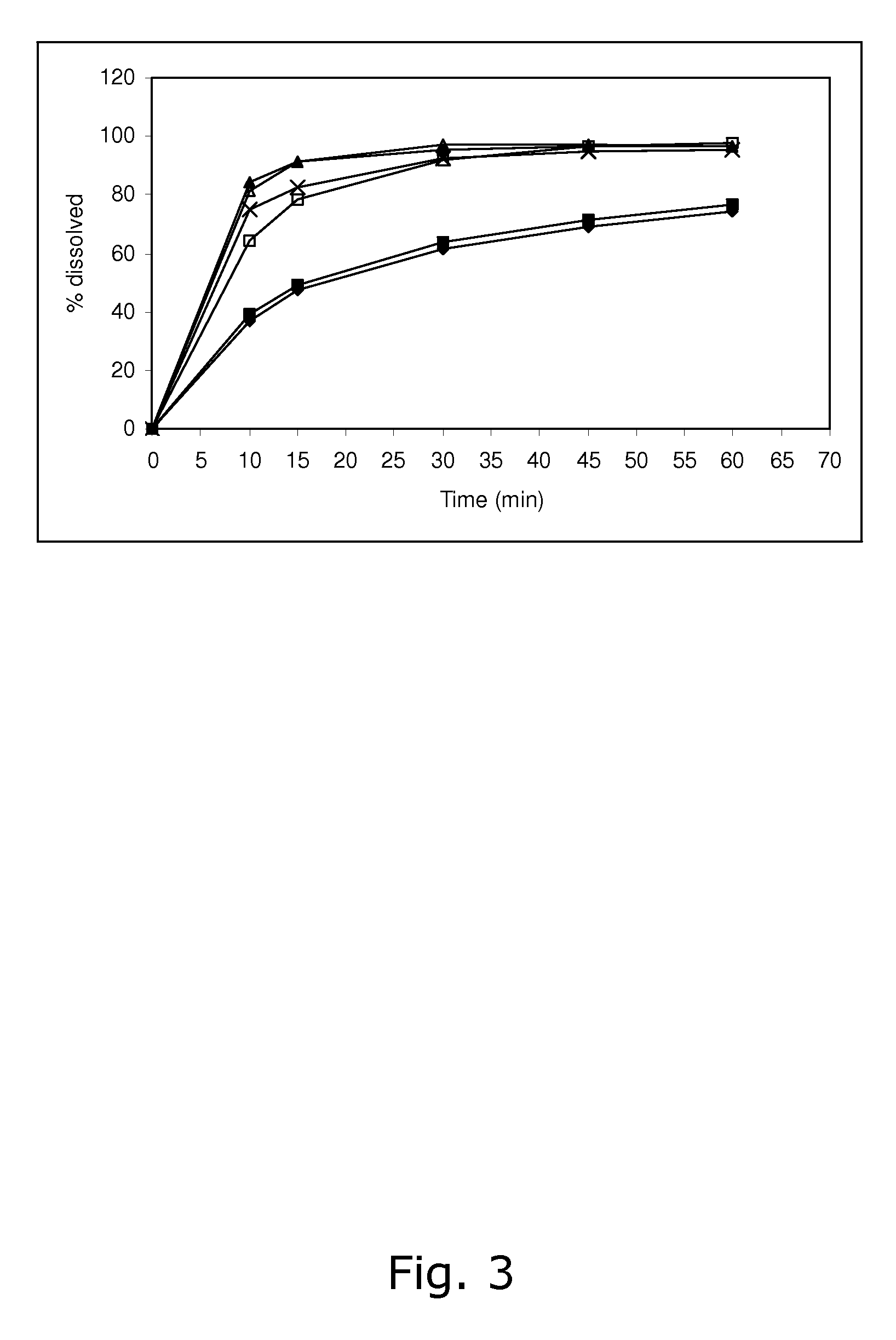
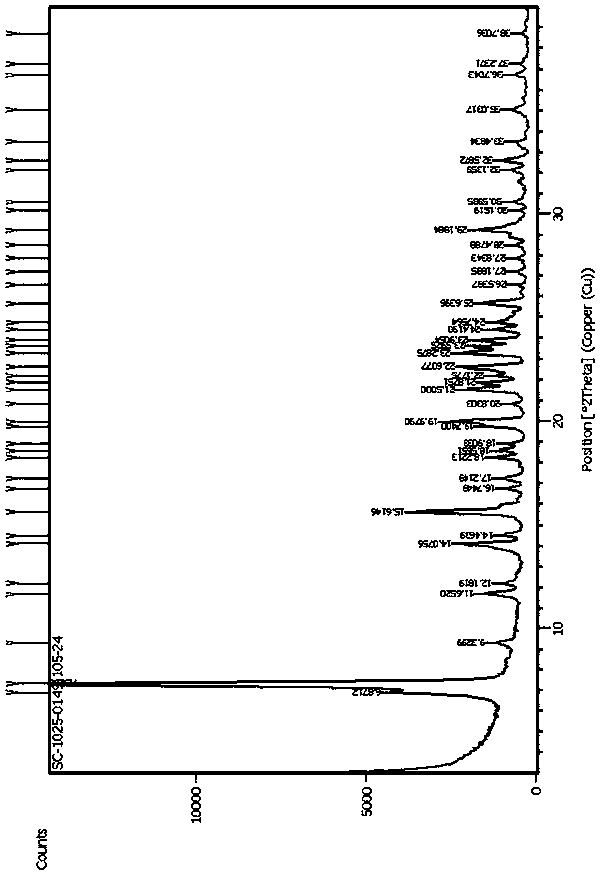
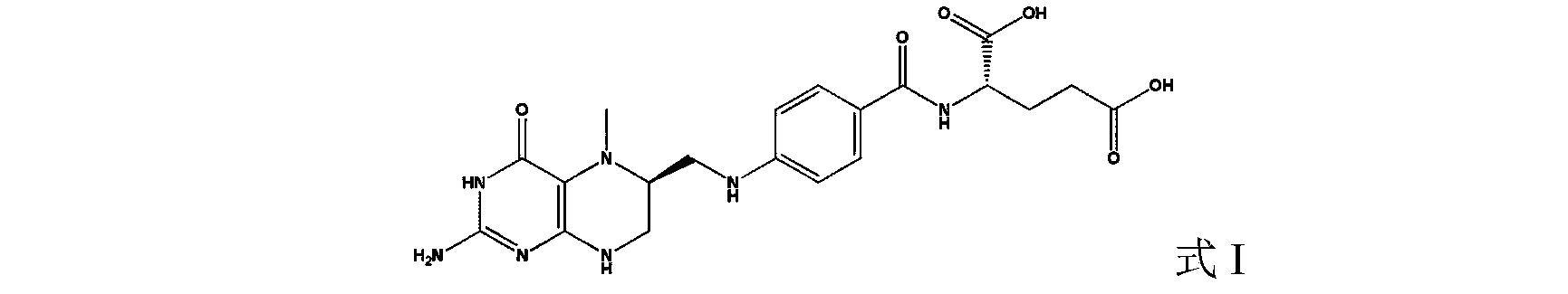
Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug
Owner:PEKING UNIV
Synthesis of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid
InactiveUS20070190596A1Reducing DHFAOrganic chemistryFermentationTetrahydrofolic acidFolate supplement
A process for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid, also known as (6S)-5-methylTHFA, the process comprising the steps of: (1) reducing folic acid (FA) so as to yield dihydrofolic acid (DHFA); (2) stereoselectively reducing DHFA with dihydrofolate reductase (DHFR) in the presence of NADP / NADPH, glucose and GluDH so as to yield (6S)-THFA; (3) converting (6S)-THFA to (6S)-5-methylTHFA; and (4) isolating (6S)-5-methylTHFA.
Owner:CHEM LAB INC
Synthesis of (6S)-5methyl-5,6,7,8-tetrahydrofolic acid
A process for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid, also known as (6S)-5-methylTHFA, the process comprising the steps of: (1) reducing folic acid (FA) so as to yield dihydrofolic acid (DHFA); (2) stereoselectively reducing DHFA with dihydrofolate reductase (DHFR) in the presence of NADP / NADPH, glucose and GluDH so as to yield (6S)-THFA; (3) converting (6S)-THFA to (6S)-5-methylTHFA; and (4) isolating (6S)-5-methylTHFA.
Owner:CHEM LAB INC
Method and kit for accurately detecting folic acid in blood
ActiveCN112083108ASpecific enrichment and purificationEfficient removalComponent separationBlood specimenInternal standard
The invention discloses a method and a kit for accurately detecting folic acid in blood. 1) The method for accurately detecting folic acid in blood comprises the steps of 1) adding an internal standard into a blood sample, enriching and purifying folic acid by using a specific solid-phase extraction column, and analyzing the purified liquid by using tandem mass spectrometry, and establishing a calibration curve through an internal standard method for quantification by taking the retention time and the ion pair abundance ratio as a qualitative basis, thereby being accurately detecting folic acid, 5-methyltetrahydrofolic acid and 5-formyl tetrahydrofolic acid in blood. 2) The invention also discloses a kit for accurately detecting folic acid in blood, which comprises a specific solid-phase extraction column, an internal standard mixed solution, a calibration solution, an activation solution, an equilibrium solution, leacheate 1 and leacheate 2, an extraction solution and a quality control sample. According to the invention, accurate quantitative detection of folic acid, 5-methyltetrahydrofolic acid and 5-formyl tetrahydrofolic acid in blood can be realized, and good precision and accuracy are achieved.
Owner:大连博源医学检验实验室有限公司
Target composition capable of improving light cognition impairment and preparation method thereof
ActiveCN109498771AHigh activityReduce resistanceOrganic active ingredientsNervous disorderGinkgo bilobaTetrahydrofolic acid
The invention belongs to the technical field of medicine, and particularly relates to a target composition capable of improving light cognition impairment and a preparation method thereof. The targetcomposition is prepared from the following components in percentage by weight: 20 to 30 percent of L-carnitine, 15 to 30 percent of fructus alpiniae oxyphyllae extract, 10 to 30 percent of folium ginkgo extract, 10 to 30 percent of boxthorn leaf extract, 0.005 to 0.02 percent of cyanocobalamin, 0.01 to 0.03 percent of L-5-methyl-tetrahydrofolic acid and 10 to 30 percent of auxiliary material. Thetarget composition has a remarkable effect when used for improving light cognitive impairment.
Owner:江西恒康药业有限公司
Reagent (kit) for measuring formaldehyde and method for measuring concentration of formaldehyde
InactiveCN101793788AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdditive ingredientPhosphoric acid
The invention relates to a reagent (kit) for measuring formaldehyde by using an enzyme-multiplied method, an enzyme colorimetric method and an enzyme coupling method as well as a method for measuring the concentration of the formaldehyde, and composition and ingredients of the reagent, belonging to the technical field of food / environmental test. The reagent (kit) comprises the main ingredients of buffer solution, coenzyme, adenosine triphosphoric acid, tetrahydrofolic acid, glyceric aldehyde-3-phosphoric acid, formaldehyde dehydrogenase, formic acid-dihydrofolic acid ligase, glyceric aldehyde-3-glycerol phosphate dehydrogenase and stabilizing agent. The concentration of the formaldehyde is measured by mixing a sample and the reagent according to a certain volume ratio to carry out a series of enzymatic reaction, placing the reactant under an ultraviolet / visible light analyzer and detecting the ascending degree of absorbance at a dominant wavelength of 340nm.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
New stable salt of 5,10-methylene-(6R)-tetrahydrofolic acid
The present invention is directed towards the hemisulfate salt of 5,10-methylene-(6R)-tetrahydrofolic acid, preferably in substantially crystalline form, as well as pharmaceutical compositions and uses thereof in therapy, preferably chemotherapy.
Owner:MERCK & CIE KG
Ultra-high performance liquid chromatography-mass spectrometry method for determining isotope dilution
The invention discloses an ultra-high performance liquid chromatography-mass spectrometry method for determining isotope dilution. The method comprises the following steps of 1) selecting three targetcompounds: pyridoxal, 5-methyltetrahydrofolic acid, and mecobalamine, 2) establishing a correction curve by taking the concentration ratio of the target compound to the isotope internal standard thereof as an X axis and the peak area ratio of the target compound to the isotope internal standard thereof as a Y axis, and calculating the contents of the three water-soluble vitamins, 3) preparing a protein precipitant, 4) pretreating a serum sample, and 5) preparing and processing a standard curve. A serum sample is treated in a pretreatment mode of combining protein precipitation and liquid-liquid extraction, on one hand, protein denaturation sedimentation in the sample is removed, and on the other hand, lipid impurities are removed, so that the treated sample is less interfered by a matrixin the detection process; a relatively simple pretreatment mode and a rapid detection method are applied to establish a method capable of simultaneously detecting multiple water-soluble vitamin metabolites.
Owner:CHONGQING UNIV CANCER HOSPITAL
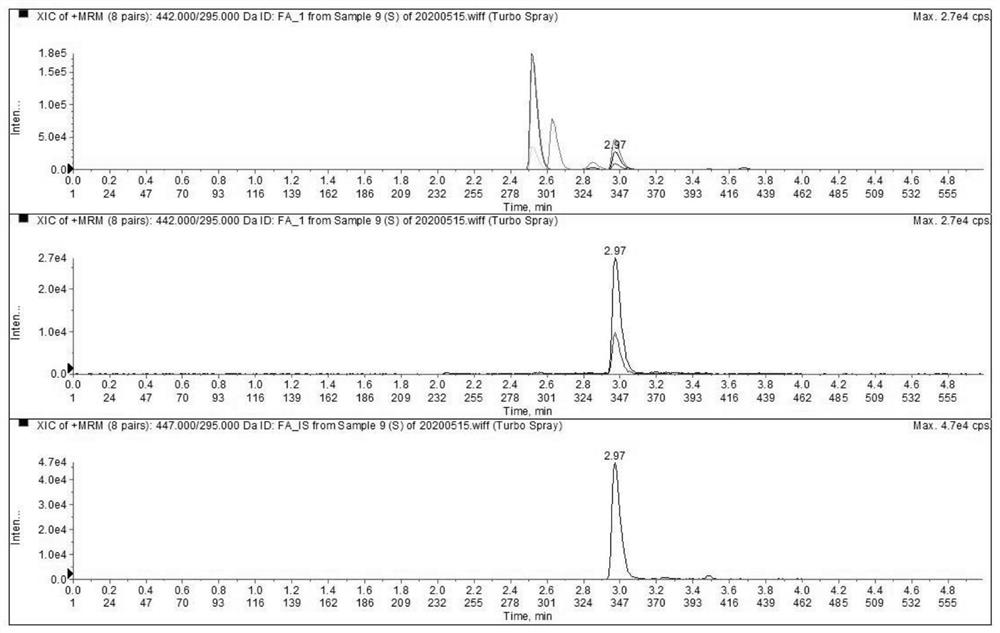
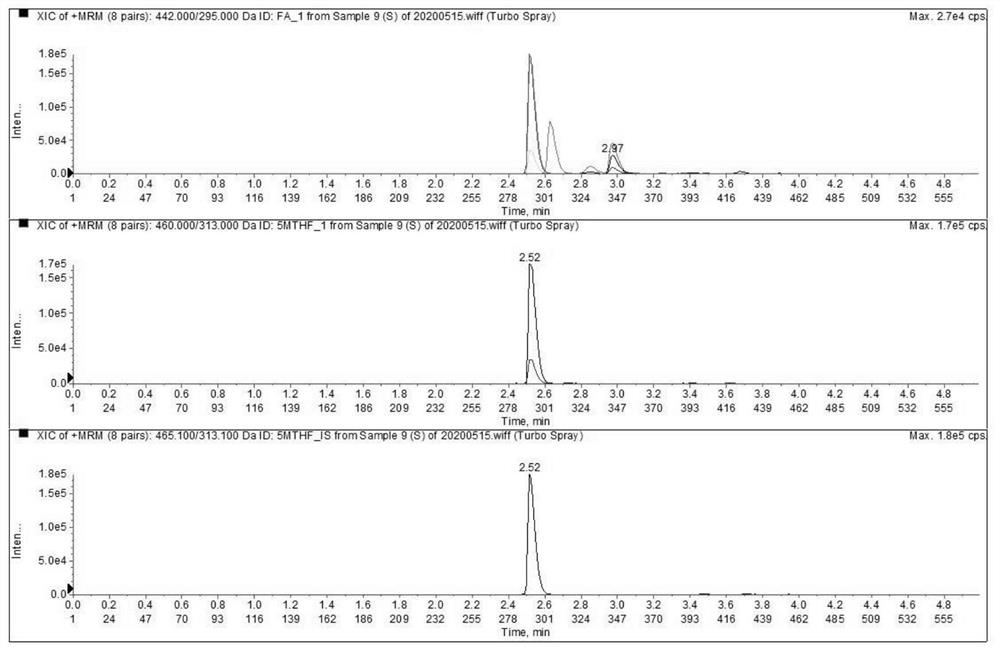
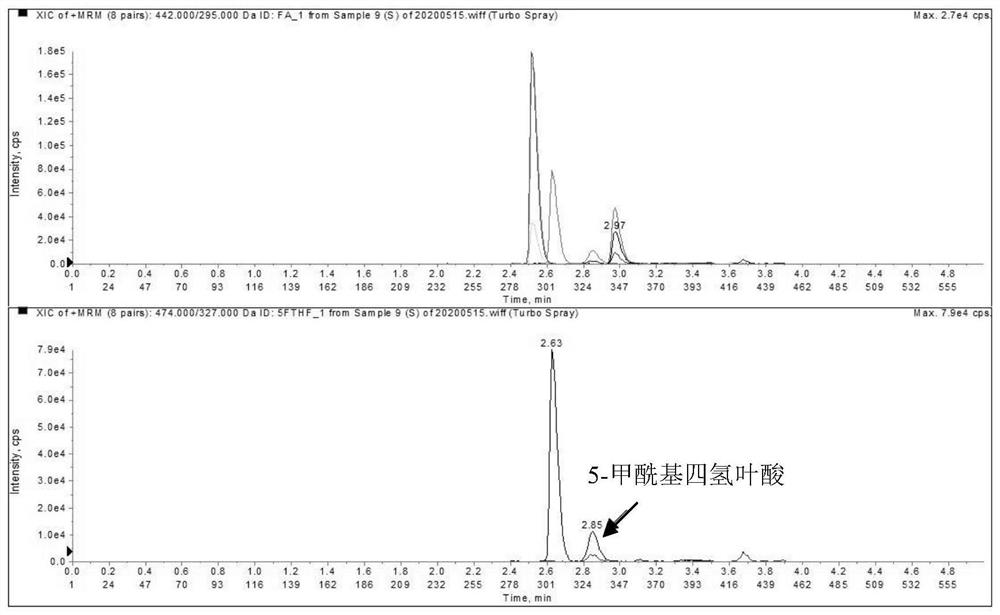
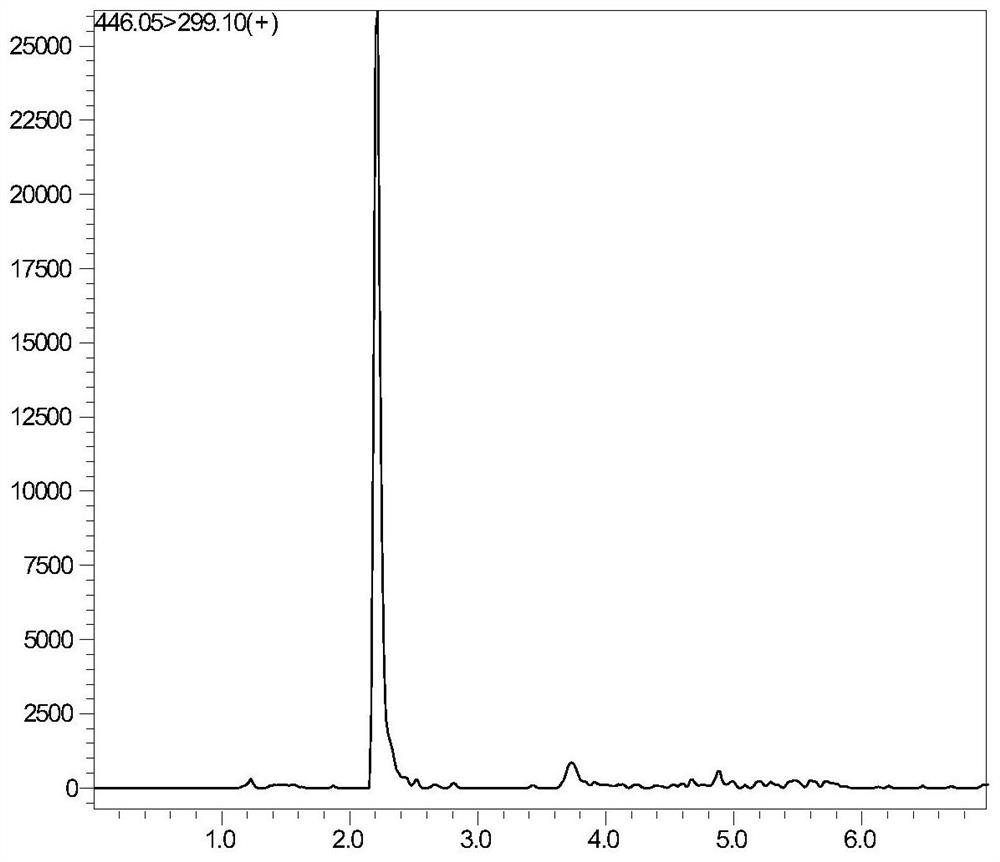
Liquid chromatography tandem mass spectrometry detection kit and detection method for testing folic acid metabolism derivatives of human body
The invention discloses a liquid chromatography tandem mass spectrometry detection kit and a detection method for testing folic acid metabolism derivatives of a human body, and belongs to the technical field of analysis and detection. The liquid chromatography tandem mass spectrometry detection kit for testing the folic acid metabolism derivatives of the human body can be used for detecting folic acid, tetrahydrofolic acid, 5-methyltetrahydrofolic acid, 5-formyltetrahydrofolic acid, 5,10-methylenetetrahydrofolate, S-adenosylmethionine, S-adenosylhomocysteine and homocysteine serum (plasma). The method has the characteristics of simplicity, rapidness, strong specificity, high sensitivity, comprehensive detection indexes and the like; the method can provide a reference basis for knowing the folic acid metabolism condition in clinical application.
Owner:广东南芯医疗科技有限公司 +1
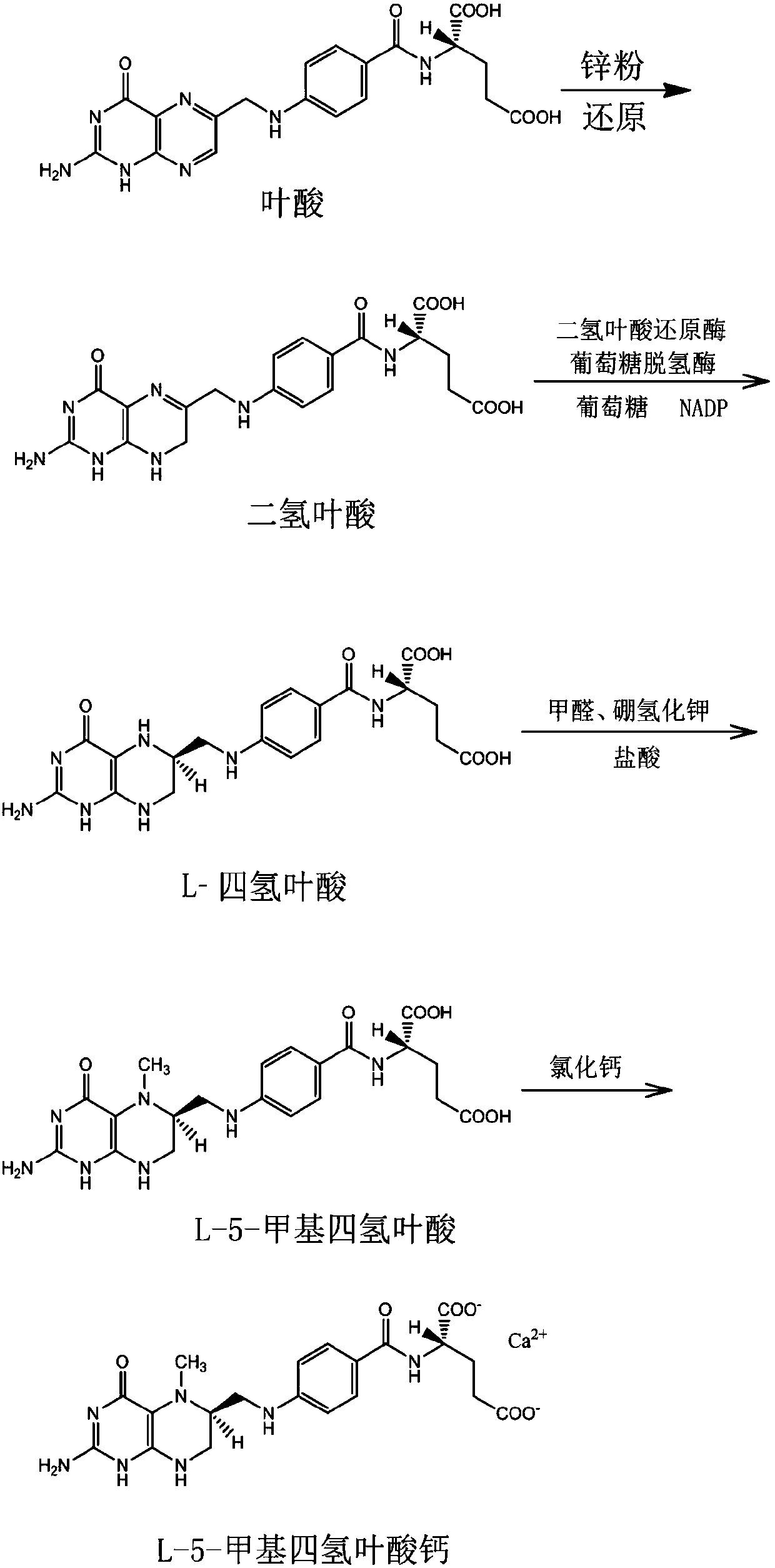
Method for preparing L-5-calcium methyl tetrahydrofolate through enzymic method
InactiveCN109627244AMild responseHigh yieldOrganic chemistryFermentationEnzymatic synthesisDihydrofolic acid
The invention belongs to the technical field of enzymatic synthesis, and relates to a method for preparing L-5-calcium methyl tetrahydrofolate through an enzymic method. The method comprises the following steps that 1, folic acid serves as the raw material, and zinc powder is used for making zinc powder reduced to be dihydrofolic acid; 2, dihydrofolic acid reductase and glucose dehydrogenase are added in dihydrofolic acid, and a reaction is conducted to obtain L-tetrahydrofolic acid; 3, formaldehyde and hydroboron are added in L-tetrahydrofolic acid, and a reaction is conducted for obtaining L-5-methyltetrahydrofolate; 4, L-5-methyltetrahydrofolate is salified, and L-5-calcium methyl tetrahydrofolate is obtained. Accordingly, the effective method for preparing L-5-calcium methyl tetrahydrofolate through the enzymic method is provided, the preparation method is free of splitting, mild in reaction, high in yield, high in product purity and friendly to environment, and has a very good industrial prospect.
Owner:ZHEJIANG SHENGDA BIO PHARM
Stabilized aqueous preparation for injection
InactiveUS20060058312A1Good storage stabilityImprove usabilityBiocideInorganic non-active ingredientsAntioxidantTetrahydrofolic acid
The present invention provides a stabilized aqueous preparation for injection containing 5-formyl-(6S)-tetrahydrofolic acid or a pharmacologically acceptable salt thereof as an active ingredient and a method of stabilizing 5-formyl-(6S)-tetrahydrofolic acid or a pharmacologically acceptable salt thereof. An aqueous preparation containing 5-formyl-(6S)-tetrahydrofolic acid or a pharmacologically acceptable salt thereof shows extremely excellent preservation stability in the presence of a pH regulator and an antioxidant.
Owner:NIPRO CORP
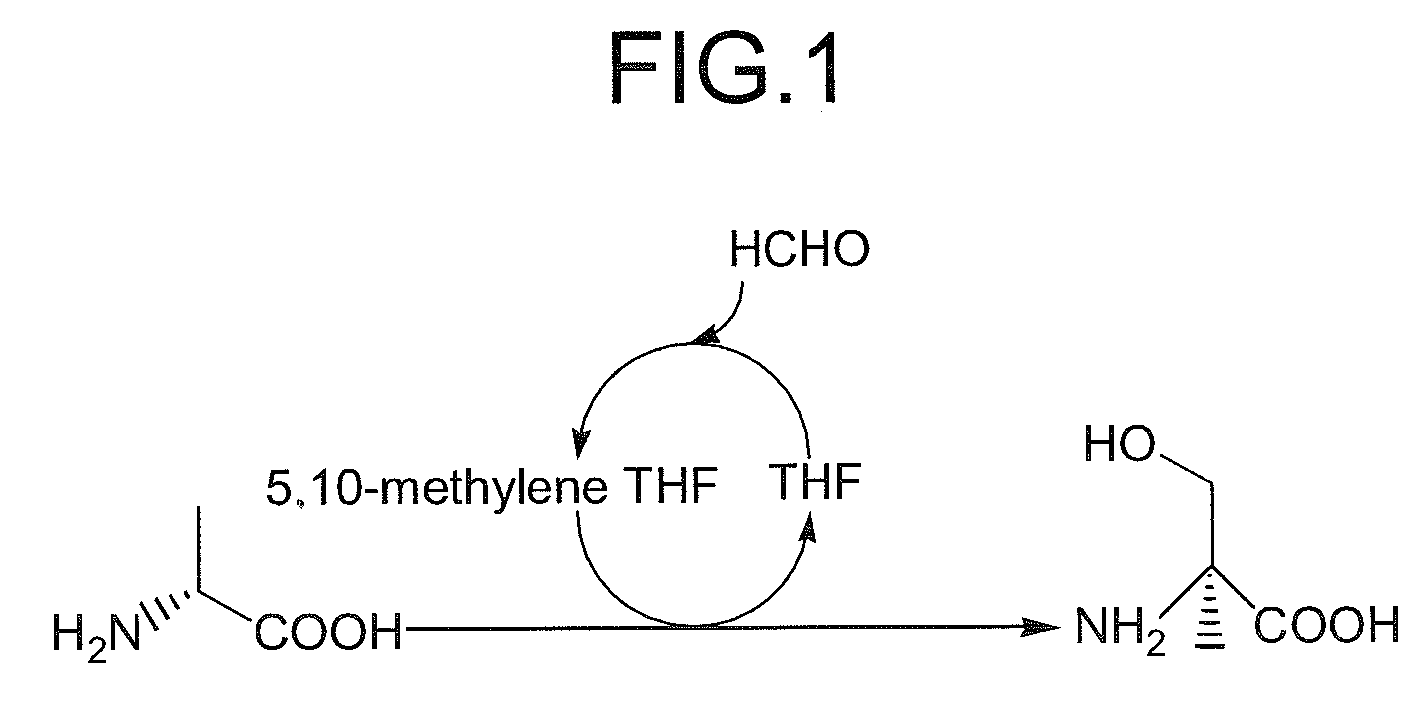
Method for producing beta-hydroxy amino acid and enzyme used therefor
InactiveUS7507559B2The process is simple and effectiveSimple procedureBacteriaSugar derivativesMicroorganismAminobacter
A method for producing β-hydroxy amino acid and its optically-active isomer is provided. The β-hydroxy amino acid is produced by reacting a predetermined D-α-amino acid and 5,10-methylene tetrahydrofolic acid in the presence of an enzyme derived from a microorganism belonging to the genera Paracoccus, Aminobacter, or Ensifer.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af150370-d7d8-4afa-8e6b-744a90b5acfd/A2005101177450002C1.PNG)
![Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af150370-d7d8-4afa-8e6b-744a90b5acfd/A2005101177450003C1.PNG)
![Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug Tetrahydropyridine [3,2-d] pyridine compound and its uses for preparing antineoplastic drug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af150370-d7d8-4afa-8e6b-744a90b5acfd/A2005101177450005C1.PNG)