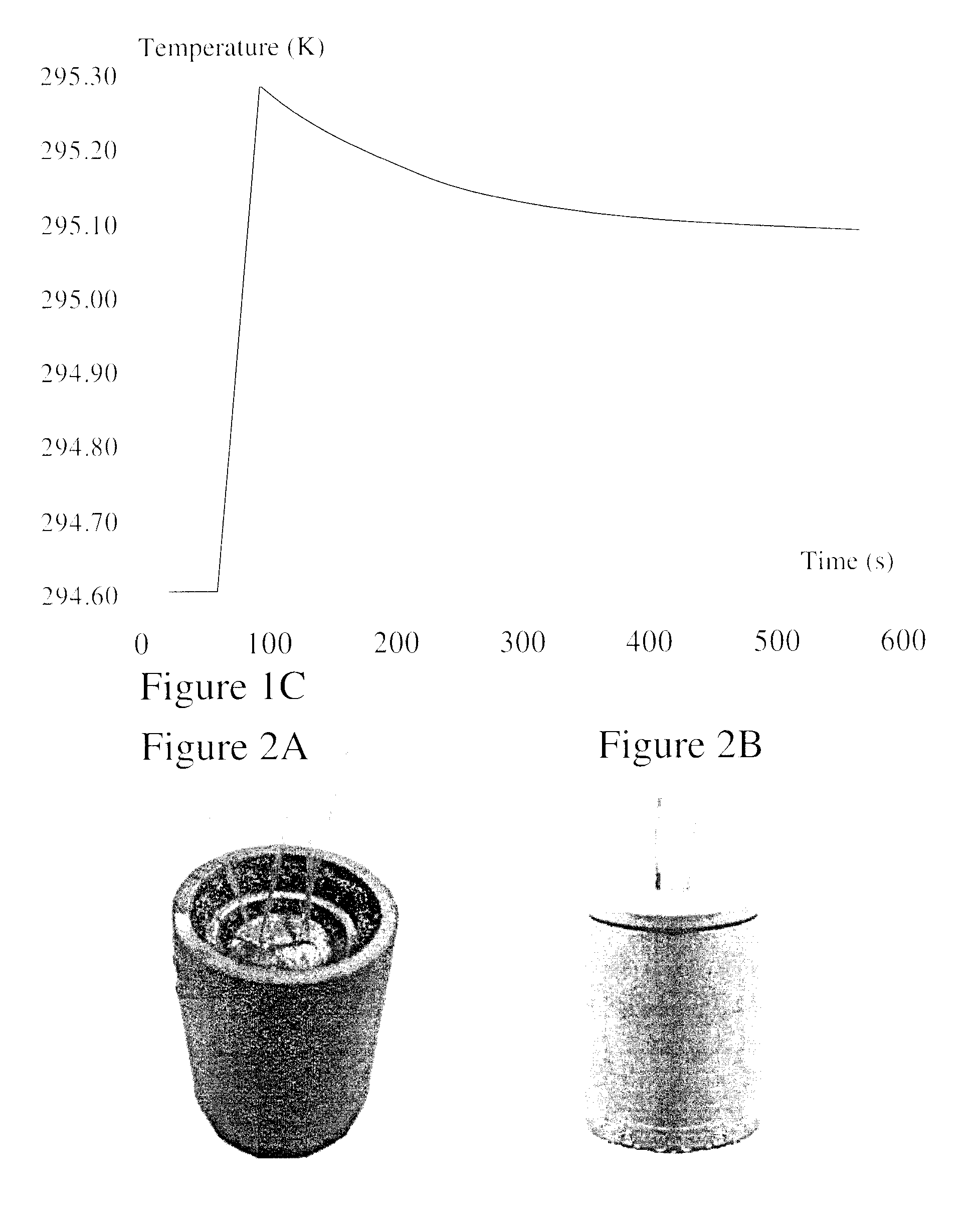
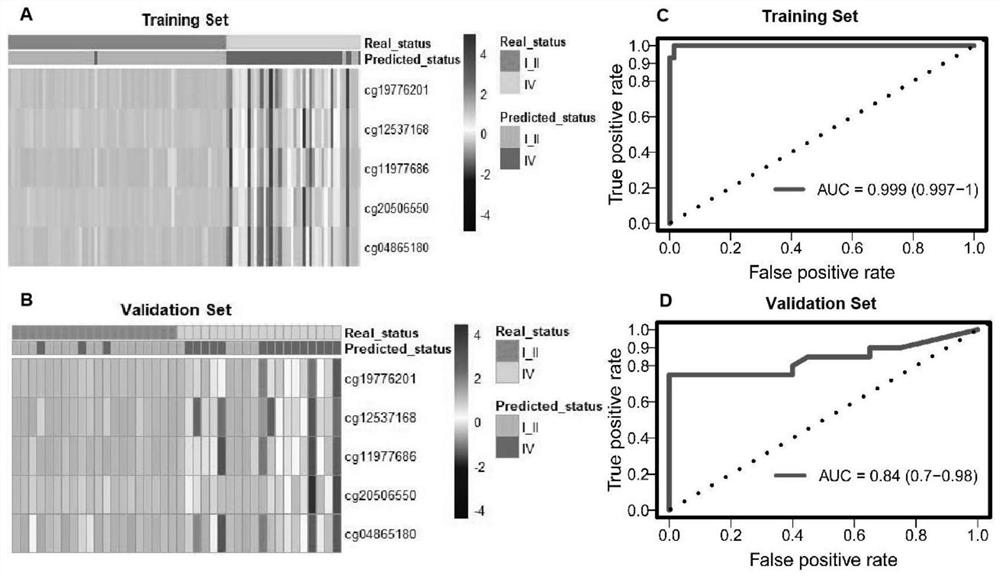
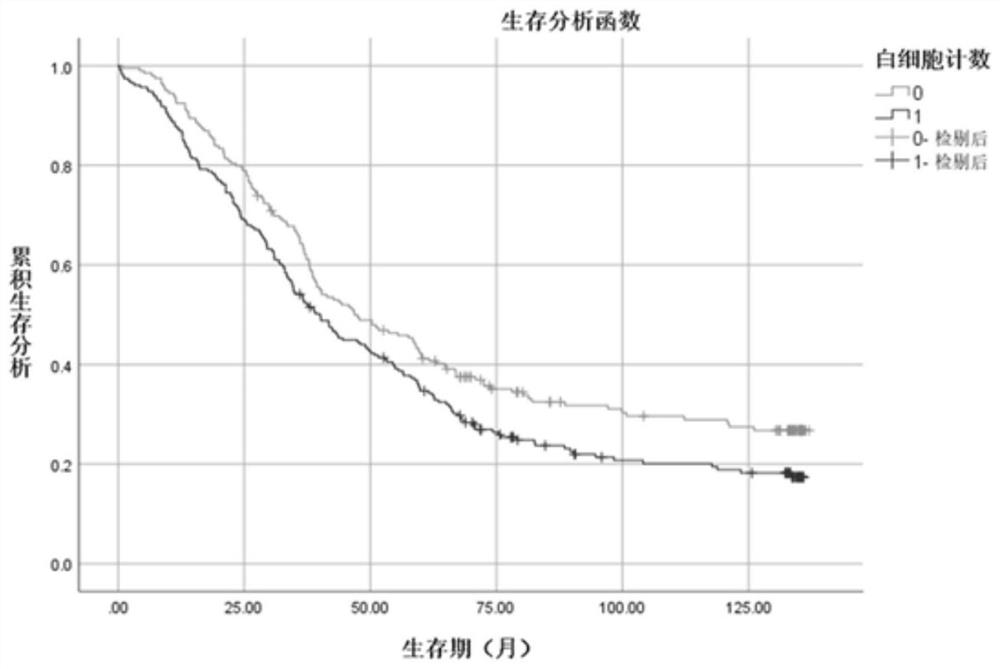
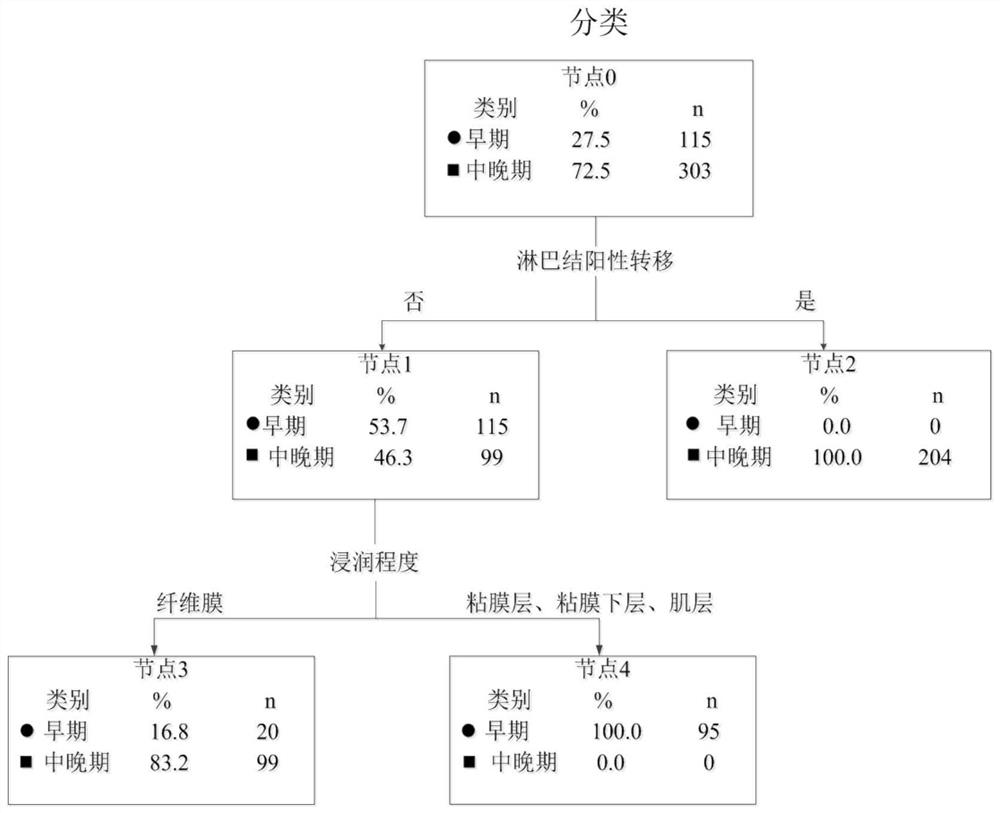
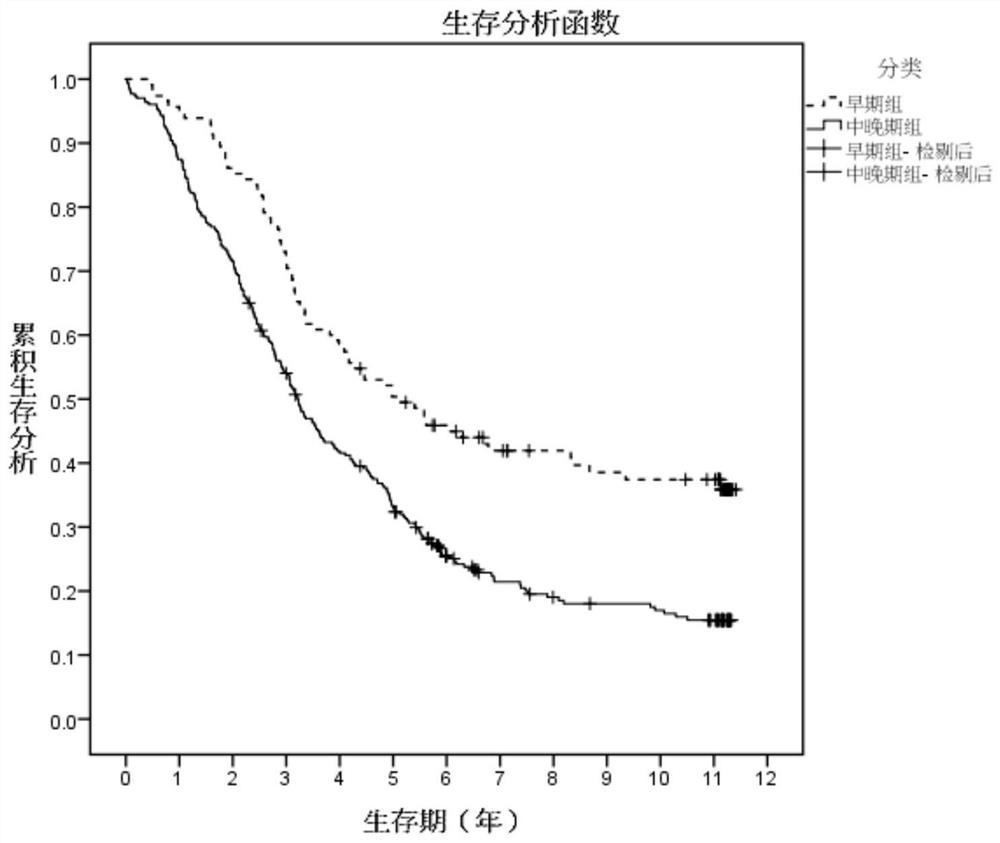
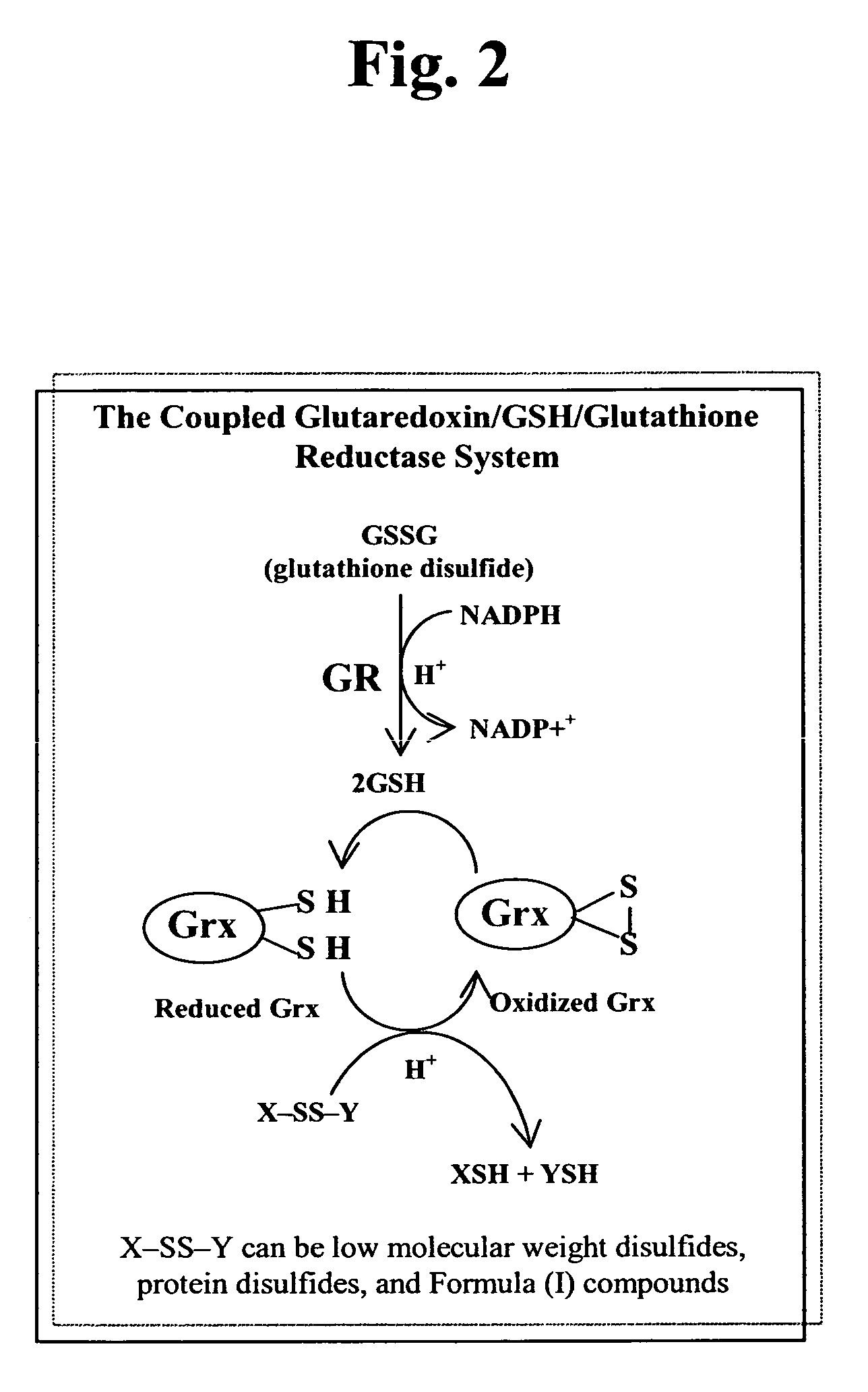
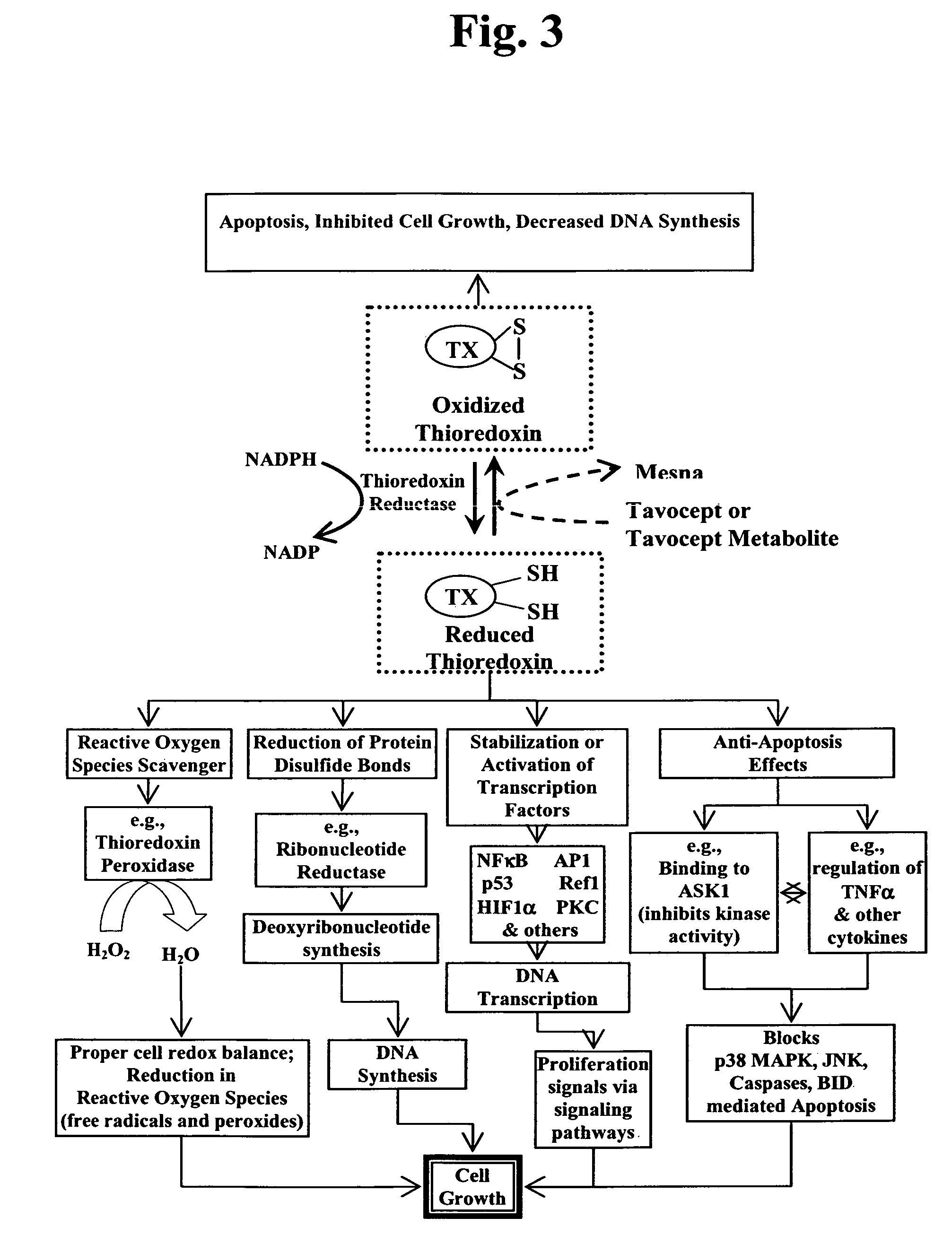
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Patient survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
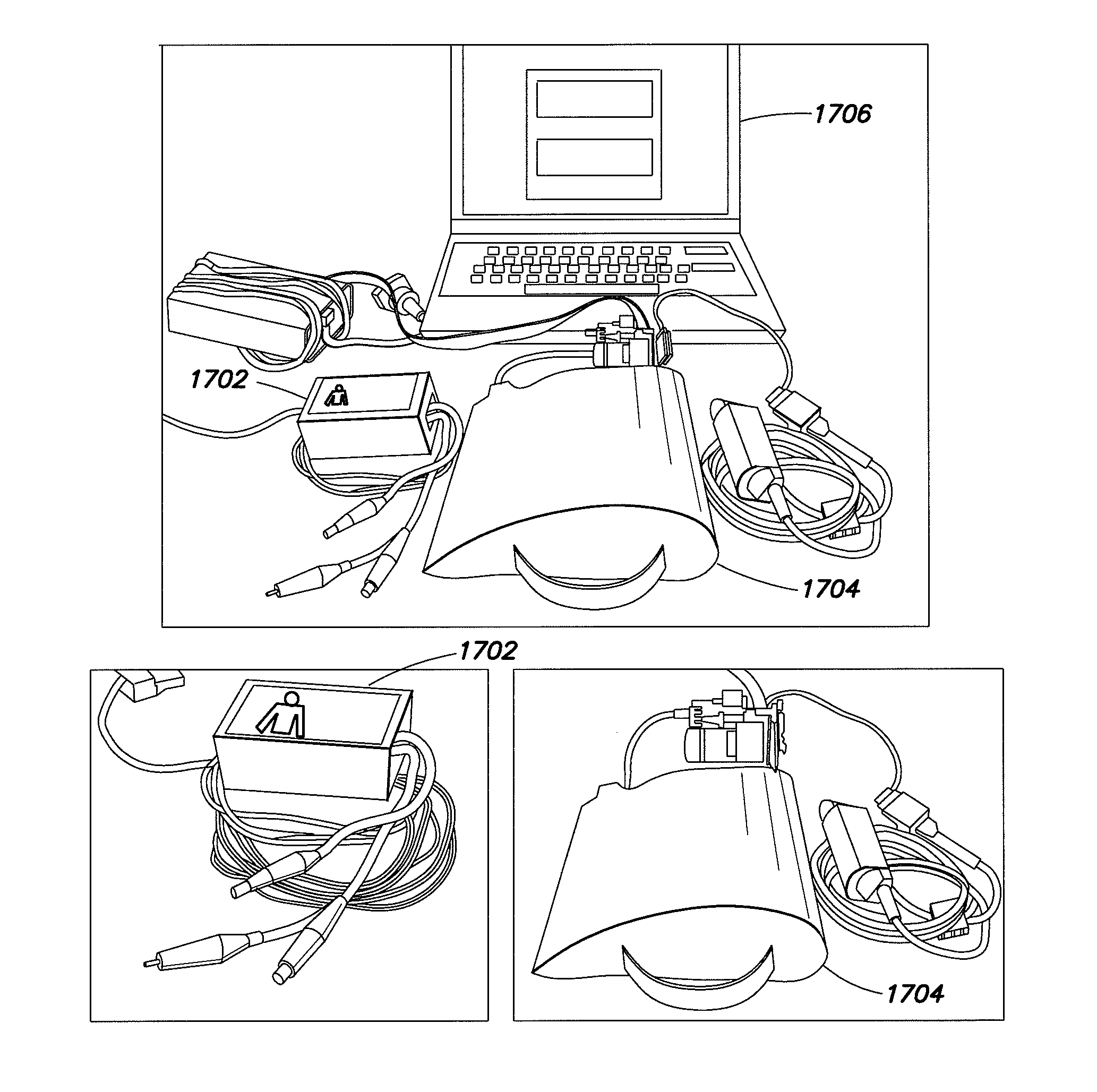
Method of predicting acute cardiopulmonary events and survivability of a patient
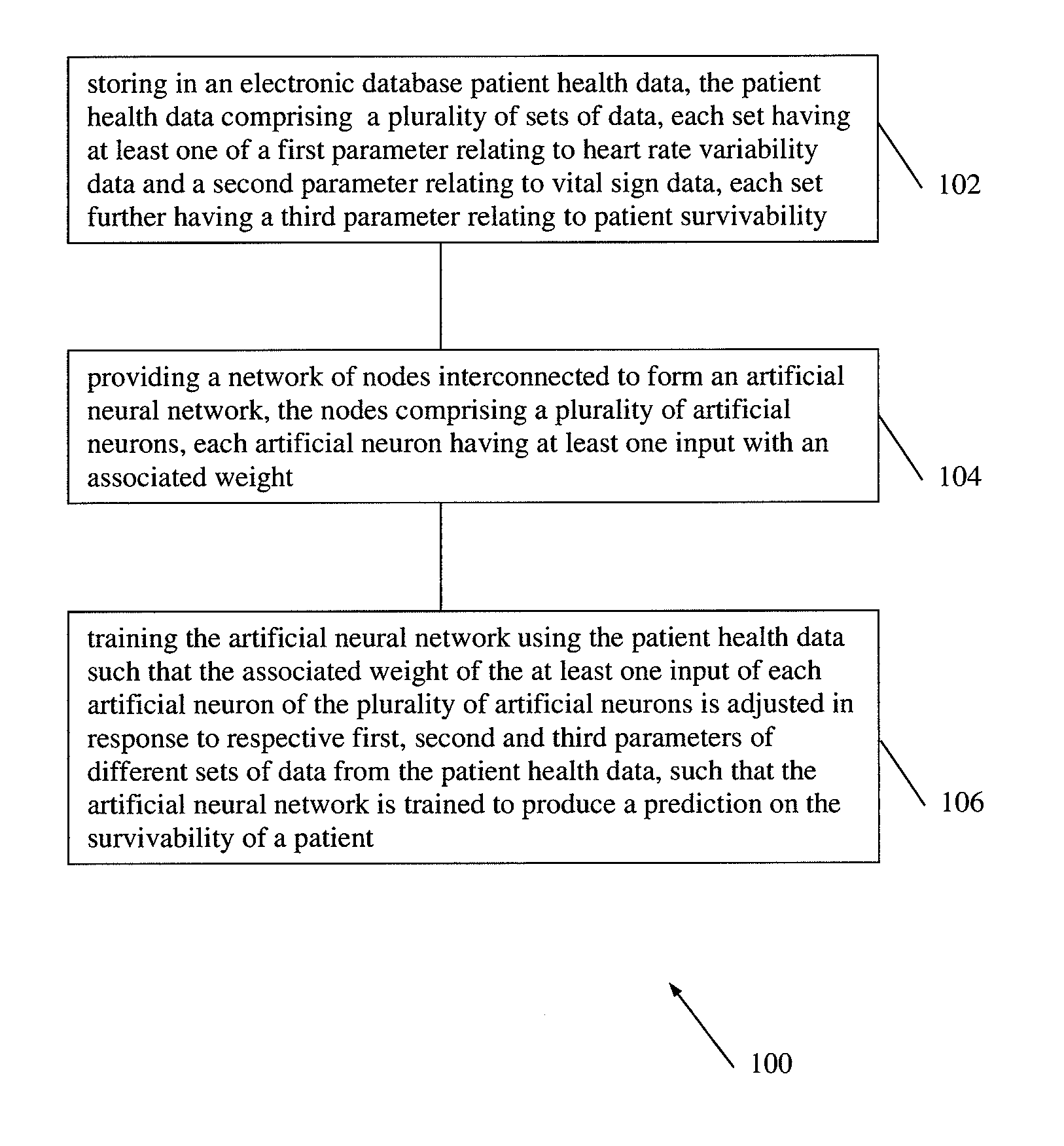
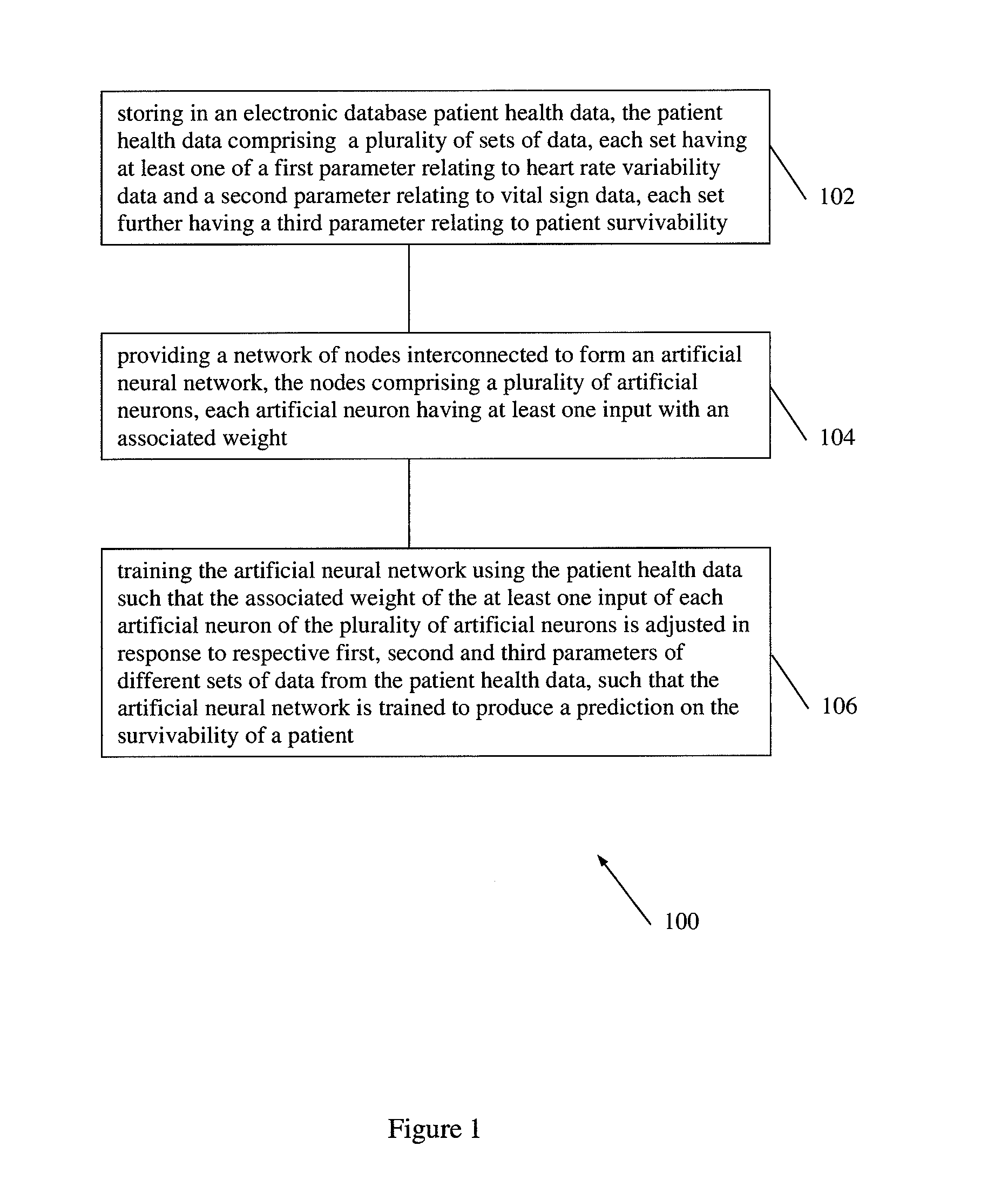
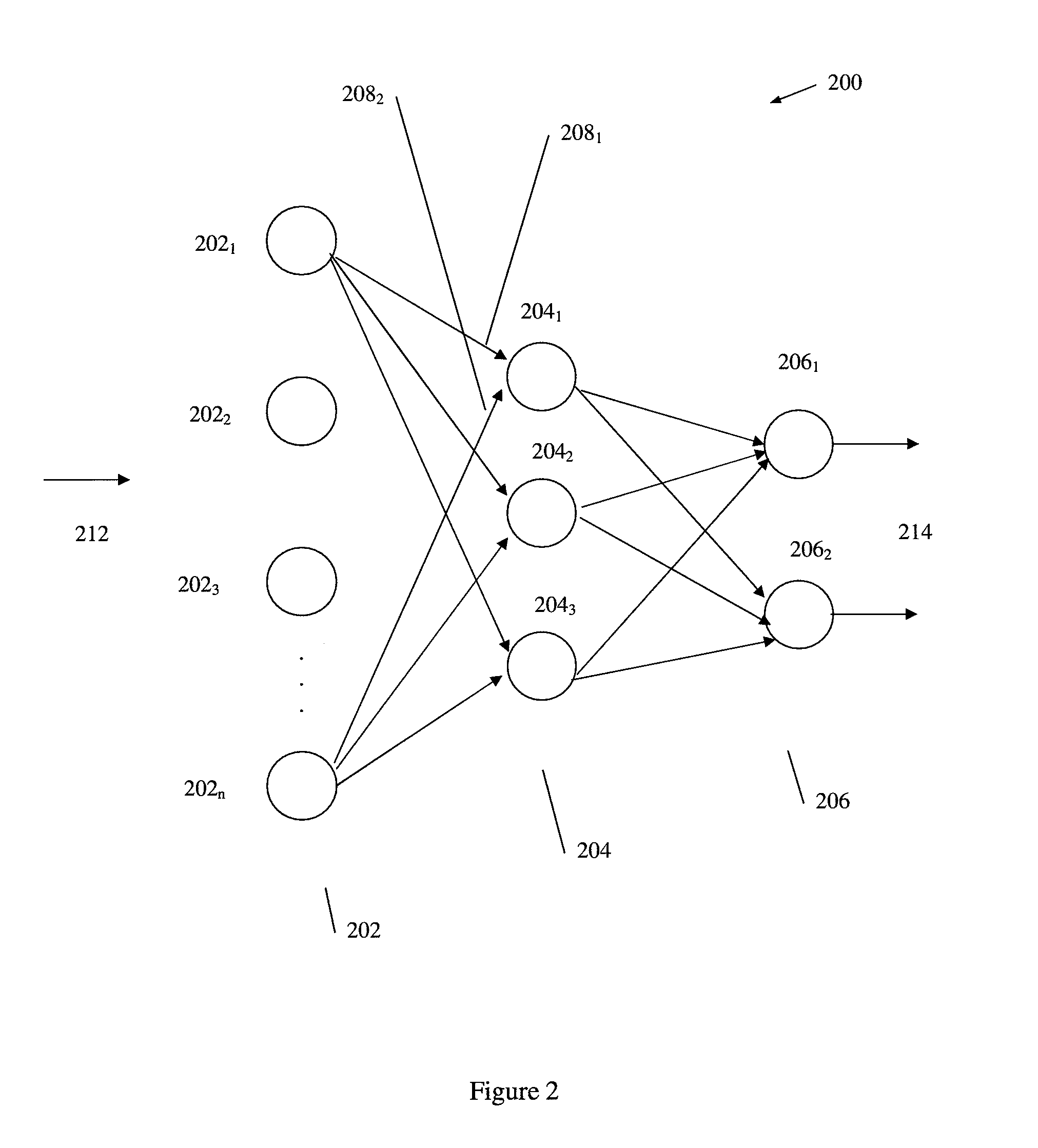
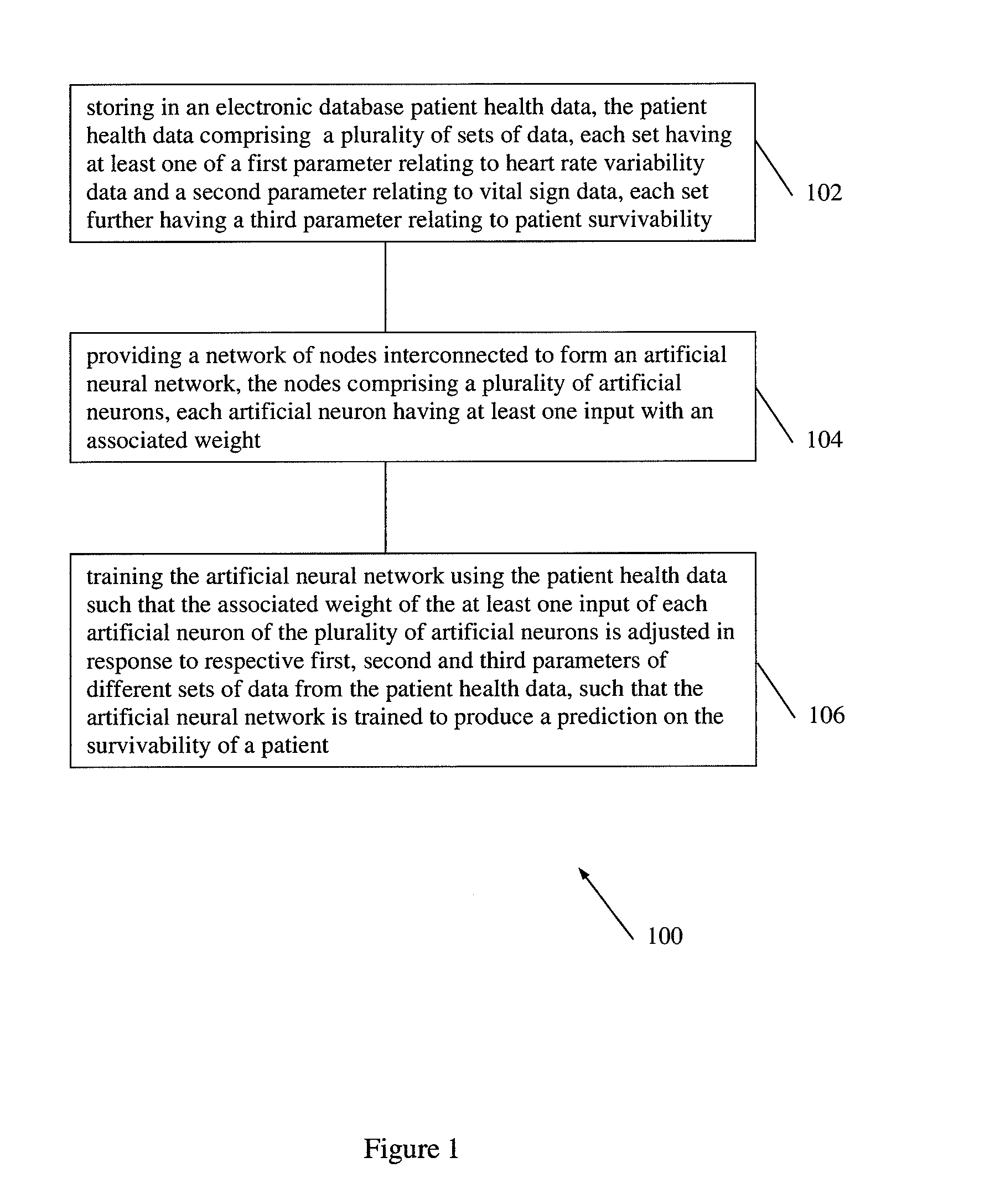
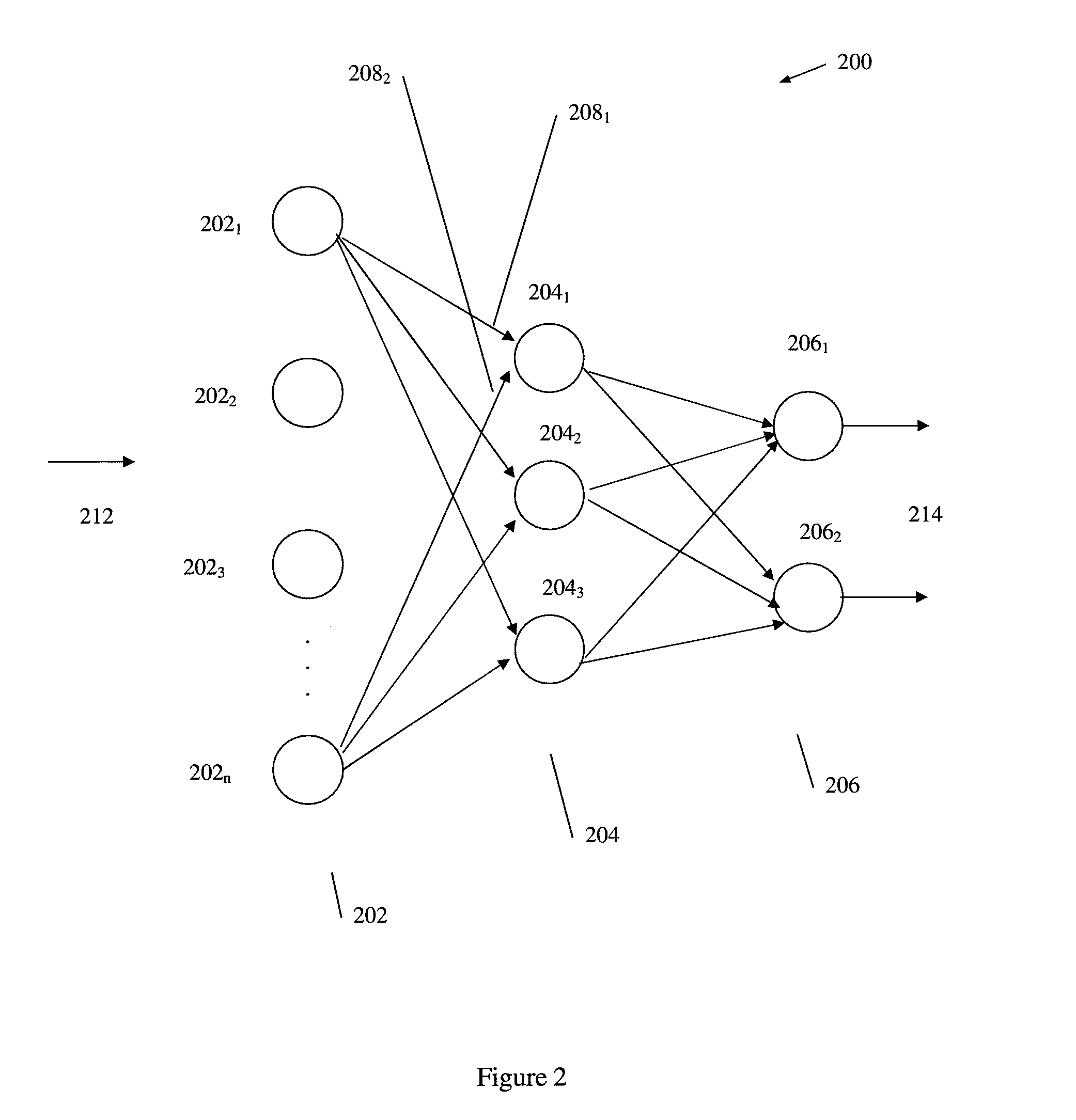
According to embodiments of the invention, there is provided a method of producing an artificial neural network capable of predicting the survivability of a patient, the method including: storing in an electronic database patient health data, the patient health data comprising a plurality of sets of data, each set having at least one of a first parameter relating to heart rate variability data and a second parameter relating to vital sign data, each set further having a third parameter relating to patient survivability; providing a network of nodes interconnected to form an artificial neural network, the nodes comprising a plurality of artificial neurons, each artificial neuron having at least one input with an associated weight; and training the artificial neural network using the patient health data such that the associated weight of the at least one input of each artificial neuron of the plurality of artificial neurons is adjusted in response to respective first, second and third parameters of different sets of data from the patient health data, such that the artificial neural network is trained to produce a prediction on the survivability of a patient.
Owner:SINGAPORE HEALTH SERVICES PTE +1
Method for Diagnosing, Prognosing and Treating Glioma
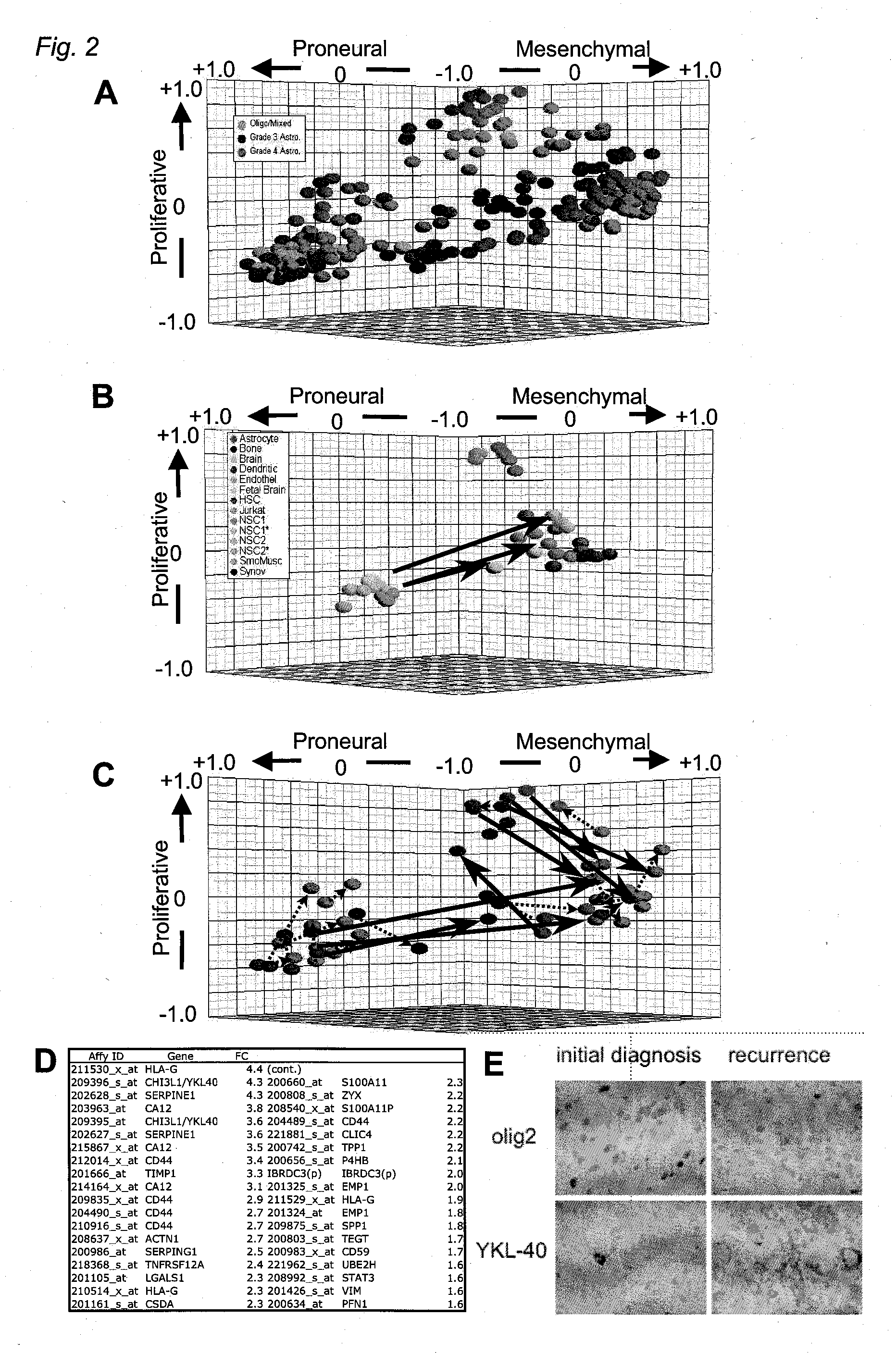
InactiveUS20070141066A1Long median patient survivalShort overall survivalHeavy metal active ingredientsCompound screeningAbnormal tissue growthAnti mitotic
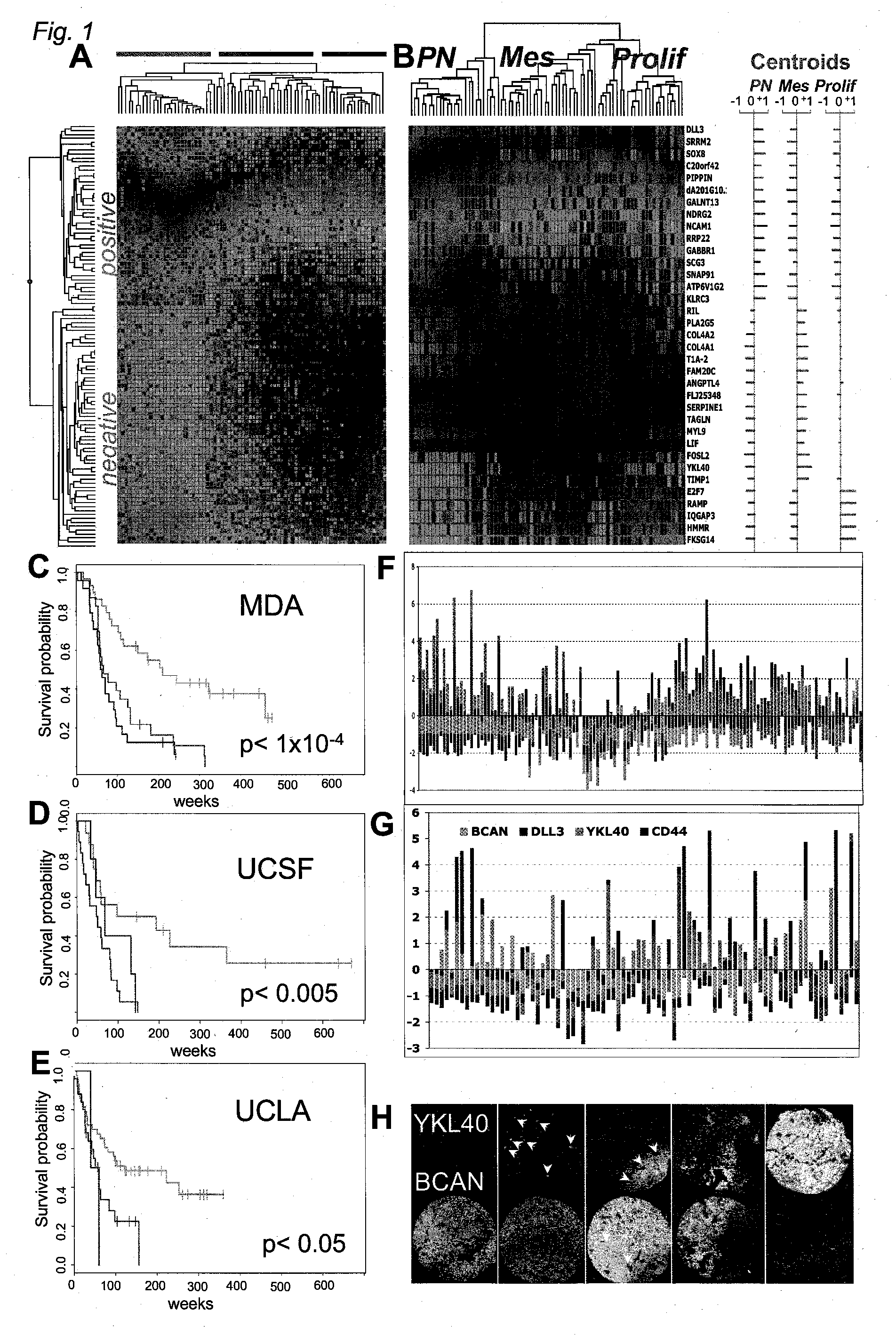
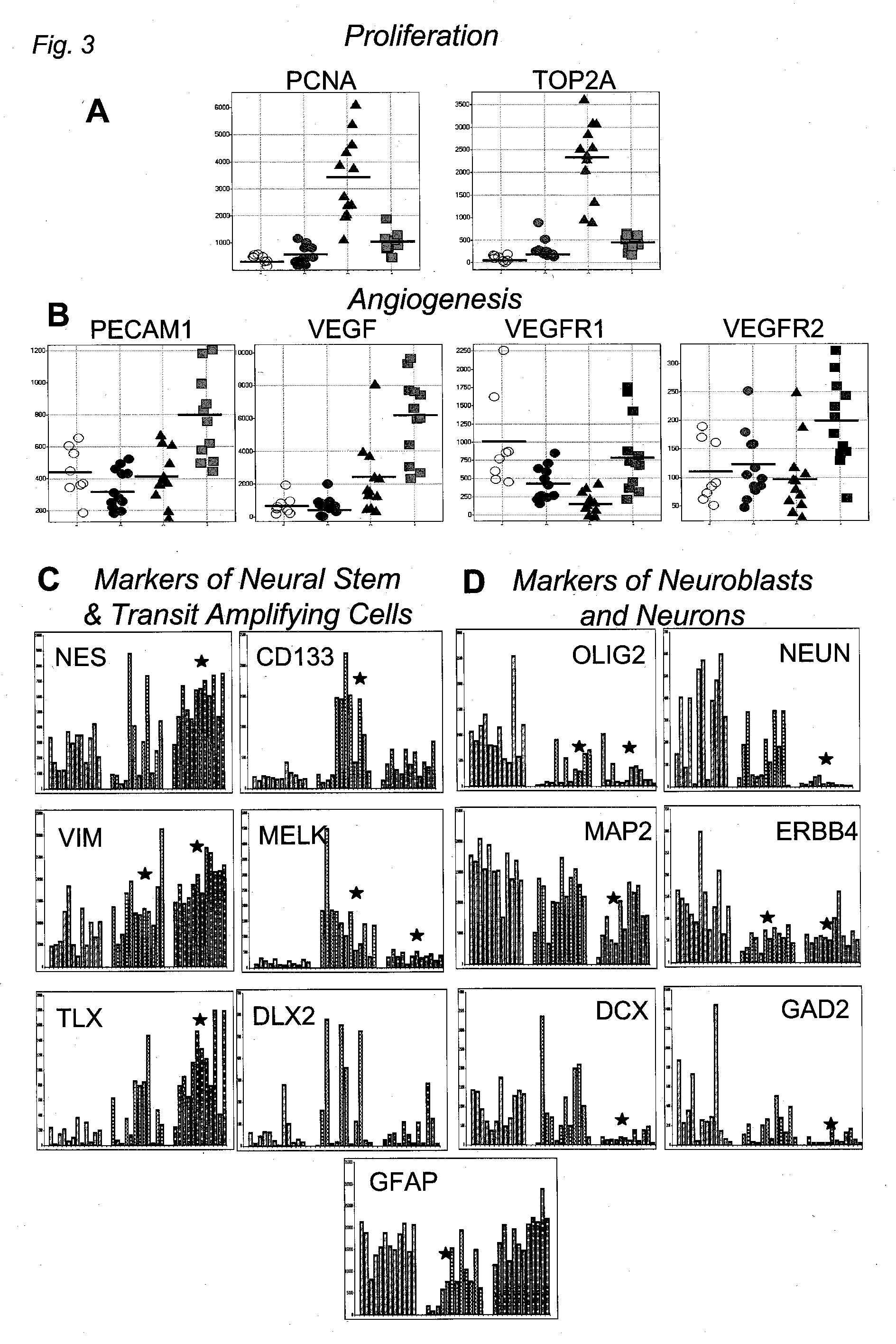
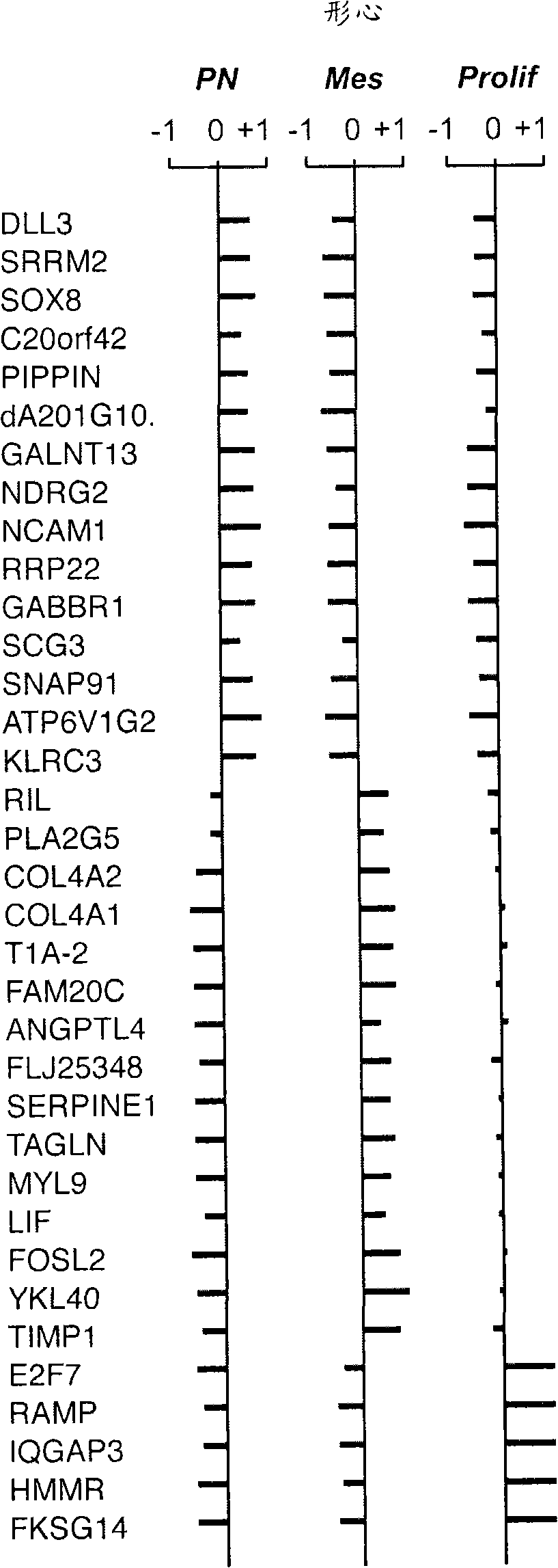
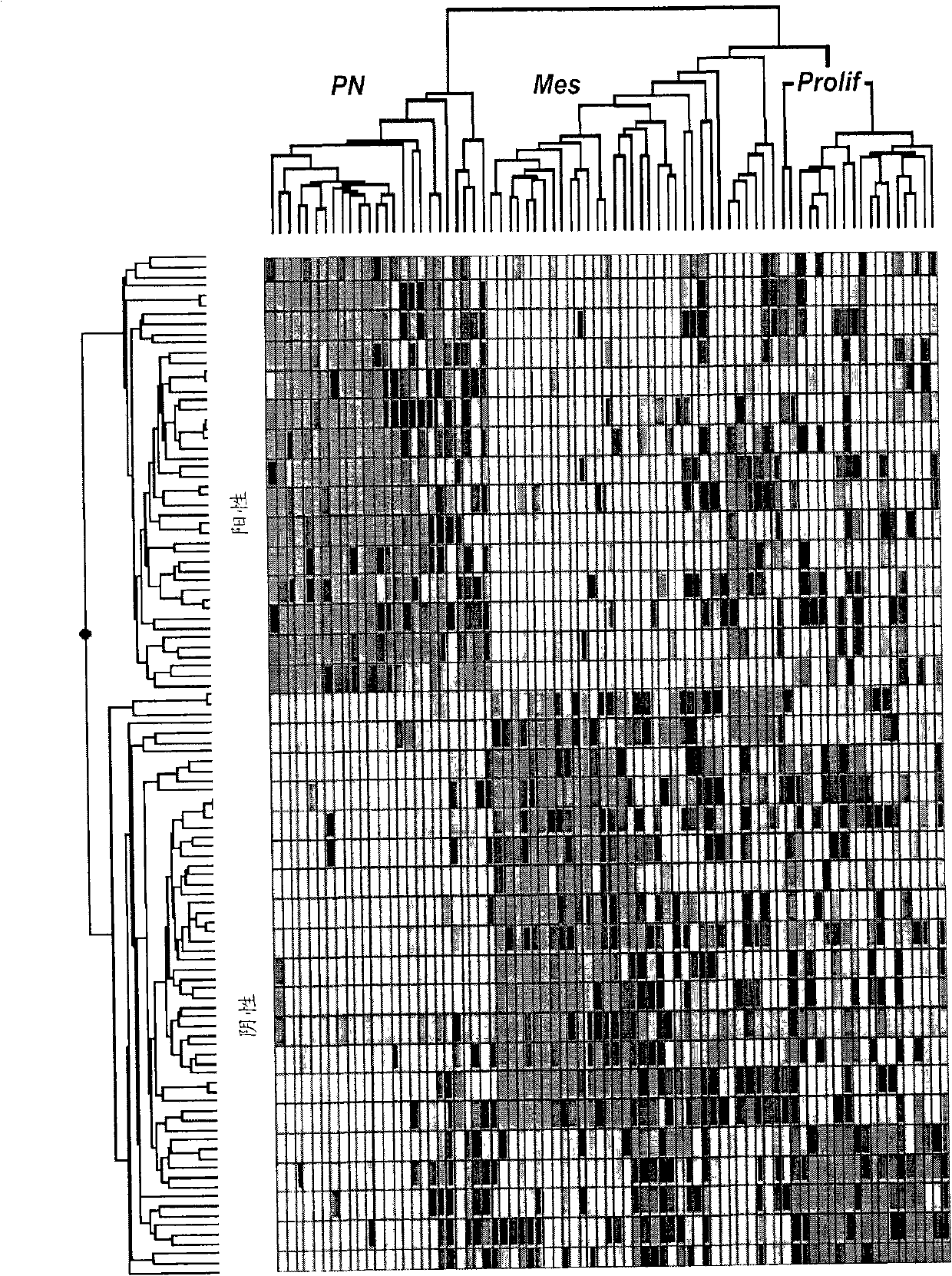
The invention provides generally a method of monitoring, diagnosing, prognosing and treating glioma. Specifically, the invention provides for three (3) prognostic subclasses of glioma, which are differentially associated with activation of the akt and notch signaling pathways. Tumor displaying neural or proneural PN lineage markers (including notch pathway elements) show longer median patient survival, while the two remaining tumor markers Prolif and Mes are associated with shortened survival. Tumors classified in this manner may also be treated with the appropriate PN- Prolif- or Mes-therapeutic corresponding to the subclassification in combination with anti-mitotic agents, anti-angiogenic agents, Akt antagonists, and neural differentiation agents. Alternatively, the invention also provides for method of prognosing and diagnosing glioma with a two-gene model based on the expression levels of PTEN and DLL3.
Owner:GENENTECH INC
Methods for the identification, assessment, and treatment of patients with cancer therapy
ActiveUS20080064055A1Prolong survival timeEliminate inefficienciesMicrobiological testing/measurementBiological testingShort term survivalPatient survival
The present invention is directed to the identification of predictive markers that can be used to determine whether patients with cancer are expected to demonstrate long term or short term survival times. In particular, the present invention is directed to the use of certain individual and / or combinations of predictive markers, wherein the expression of the predictive markers correlates with expected short term or long term survival. Thus, by examining the expression levels of individual predictive markers and / or predictive markers comprising a marker set, it is possible to determine predicted patient survival.
Owner:MILLENNIUM PHARMA INC
Method of predicting acute cardiopulmonary events and survivability of a patient
Owner:SINGAPORE HEALTH SERVICES PTE LTD +1
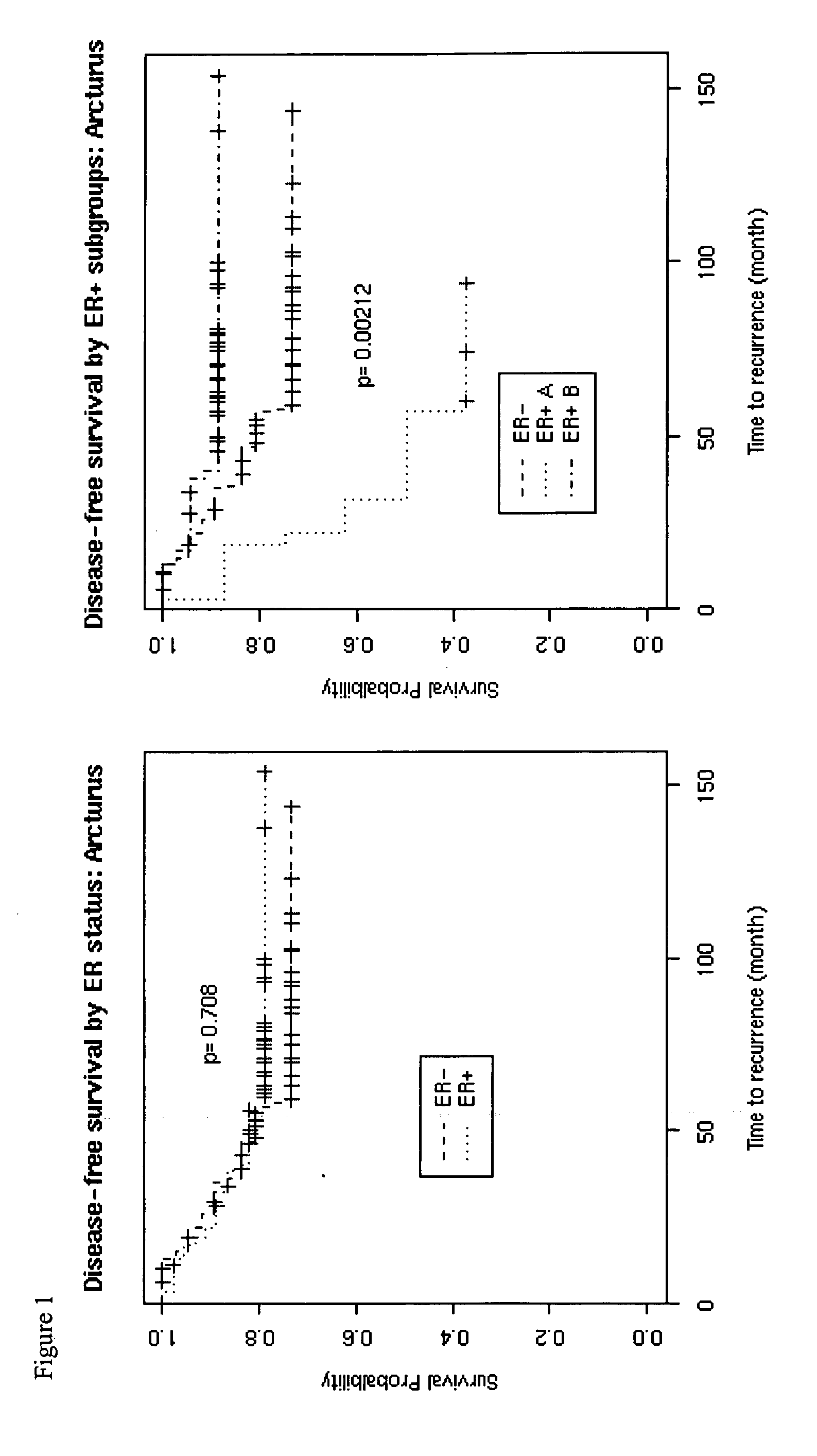
Breast cancer survival and recurrence
InactiveUS20050100933A1Accurate assessmentDetermining prognosisMicrobiological testing/measurementFermentationPatient survivalInvasive breast carcinoma
The invention provides for the identification and use of gene expression profiles, or patterns, with clinical relevance to breast cancer. In particular, the invention provides the identities of genes that are correlated with patient survival and breast cancer recurrence. The gene expression profiles may be embodied in nucleic acid expression, protein expression, or other expression formats and used to predict the survival of subjects afflicted with breast cancer and to predict breast cancer recurrence and. The profiles may also be used in the study and / or diagnosis of breast cancer cells and tissue, including the grading of invasive breast cancer, as well as for the study and / or determination of prognosis of a patient. When used for diagnosis or prognosis, the profiles may be used to determine the treatment of breast cancer based upon the likelihood of life expectancy and recurrence.
Owner:UNIV OF LOUISVILLE RES FOUND INC +1
Method for diagnosing, prognosing and treating glioma
The invention provides generally a method of monitoring, diagnosing, prognosing and treating glioma. Specifically, the invention provides for three (3) prognostic subclasses of glioma, which are differentially associated with activation of the akt and notch signaling pathways. Tumor displaying neural or proneural PN lineage markers (including notch pathway elements) show longer median patient survival, while the two remaining tumor markers Prolif and Mes are associated with shortened survival. Tumors classified in this manner may also be treated with the appropriate PN- Prolif- or Mes- therapeutic corresponding to the subclassification in combination with anti-mitotic agents, anti-angiogenic agents, Akt antagonists, and neural differentiation agents. Alternatively, the invention also provides for method of prognosing and diagnosing glioma with a two- gene model based on the expression levels of PTEN and DLL3.
Owner:GENENTECH INC
Carcinoma diagnosis and treatment, based on ODC1 genotype
ActiveUS8329636B2Reduce contentInhibition formationBiocideOrganic active ingredientsTreatment choicesCancers diagnosis
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Preparation and use of human body immunocompetent cell DCCIK anti-tumor cell preparation
InactiveCN1990044AAnti-rotation transferAnti-relapseMammal material medical ingredientsBlood/immune system cellsHuman bodyAbnormal tissue growth
The invention discloses the human body immunocompetent cell (DCCIK) anti-tumor cell agent and preparation methods. The method induces patients peripheral blood to DC and CIK using relevant cytokine, mixes the DC and CIK cells according proportion and cultures together after using alpha-Galcer or CI repeatedly impact DC, then gets DCCIK cells. Compared with homologous CIK, its proliferative activity and cytotoxicity are improved. The DCCIK has specific targeting and highly effective wide spectrum kill tumor effect without relevant tumor antigen impact. It can prevent tumour transition and recurrence after tumour surgery, radiotheraphy and chemotherapy. It can extend patient survival time and improve life quality with the assistance of other therapies.
Owner:SHANGHAI ZHONGKE YINGDA BIO TECH
Combination antibody kit for risk assessment of postoperative recurrence of primary hepatocellular carcinoma
InactiveCN102298053AThe experimental method is matureEasy to detectBiological testingPatient survivalCvd risk
The invention discloses a multi-antibody detection kit used in postoperative recurrence risk assessment after a resection operation is performed on a primary hepatocellular carcinoma patient. The kit comprises 8 immunohistochemical antibodies used for paraffin sections. The invention also discloses a method for determining postoperative recurrence risk of a liver cancer patient by using the kit. The prediction effect provided by the invention is superior to an existing clinical index. The kit and the method provided by the invention have characteristics of objective, convenient, direct, and repeatable. The kit can be used in the predictions of recurrence and death after clinical liver cancer resection operations. The kit assists in screening liver cancer patients with high postoperative recurrence risk. Therefore, appropriate auxiliary treatments can be directed, recurrence can be effectively reduced, and patient survival time can be prolonged.
Owner:SUN YAT SEN UNIV CANCER CENT
Maca superfine powder as well as preparation method and application thereof
ActiveCN104352543AStable conditionImprove the quality of lifePowder deliveryAntinoxious agentsSuperfine grindingMicrometer
The invention discloses maca superfine powder as well as a preparation method and application thereof. The grain size of the maca superfine powder is measured by a dry method laser granulometry meter and is 0.5-3 micrometers. The preparation method of the powder comprises the following steps: 1 removing impurities and cleansing; 2 freeze-drying, and carrying out sterilization treatment after drying; 3 grinding dry maca powder into coarse powder, then carrying out superfine-grinding in a jet mill, and thus obtaining the superfine powder with the grain size being 0.5-3 micrometers. The conditions of superfine-grinding are as follows: the grinding temperature is 18-25 DEG C, the pressure of a superfine-grinding machine when the powder enters the superfine-grinding machine is 1.5-3Mpa, the grinding pressure is 3-7Mpa, the airflow velocity is 190-300m / s, and the grinding time is 1-3h. The maca superfine powder is used for treating low immunity, physical fatigue and sexual dysfunction and has the advantages of being significant in curative effect and stable in condition of patients, improving the survival quality of patients, increasing the weight of patients, improving the immunologic function of patients, and being safe in medication.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
Method and system for calorimetry probe
InactiveUS9586060B2Calorimetric dosimetersX-ray/gamma-ray/particle-irradiation therapyDosimetry radiationEquipment Operator
Radiotherapy is one of the most effective treatments for cancer and its success depends critically on accurate targeting and delivery of the correct radiation dose. Accurate dosimetry is therefore essential to maintain and improve patient survival rates. However, size and long wait times currently limit water and graphite based calorimeters to standards laboratories leaving field-based dosimetry to ionization chamber measurements which depend upon a reference field-specified calibration factor. It would therefore be beneficial to provide radiotherapy equipment operators a direct approach of clinical reference dosimetry wherein the dosimeter provides increased independence on dose, dose rate, radiation energy, and energy type, etc. It would be further beneficial for such novel clinical dosimeters to be compact, function as secondary standards used routinely for measurements and allow radiotherapy doses to be measured directly and in an absolute manner. According to embodiments of the invention novel compact graphite probe calorimeters are provided.
Owner:SUN NUCLEAR
Primer pair and probe used for detecting AIDS treatment medicine 3TC and FTC drug-resistance mutation sites and application thereof
ActiveCN104911252AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationPatient survivalPharmaceutical drug
The invention discloses a primer pair and a probe used for detecting AIDS treatment medicine 3TC and FTC drug-resistance mutation sites, which comprise an ARMS primer and a Taqman probe of mutation sites M184V, M184I, Q151M and K65R at 184th site, 151st site and 65th site of pol gene for detecting HIV-1 virus RNA. The invention also provides the application of the primer pair and the probe on detection of 3TC and FTC main drug-resistance mutation sites M184V, M184I, Q151M and K65R. The kit has the advantages of high detection sensitivity, good specificity, and low detection cost, and provides medicine usage guidance for treating clinic AIDS patient, individuation treatment for AIDS patient can be realized, medicine effectiveness can be increased, AIDS patient living time can be prolonged, and the primer pair and the probe have wide application prospect and social benefit.
Owner:江苏亦文生物科技有限公司
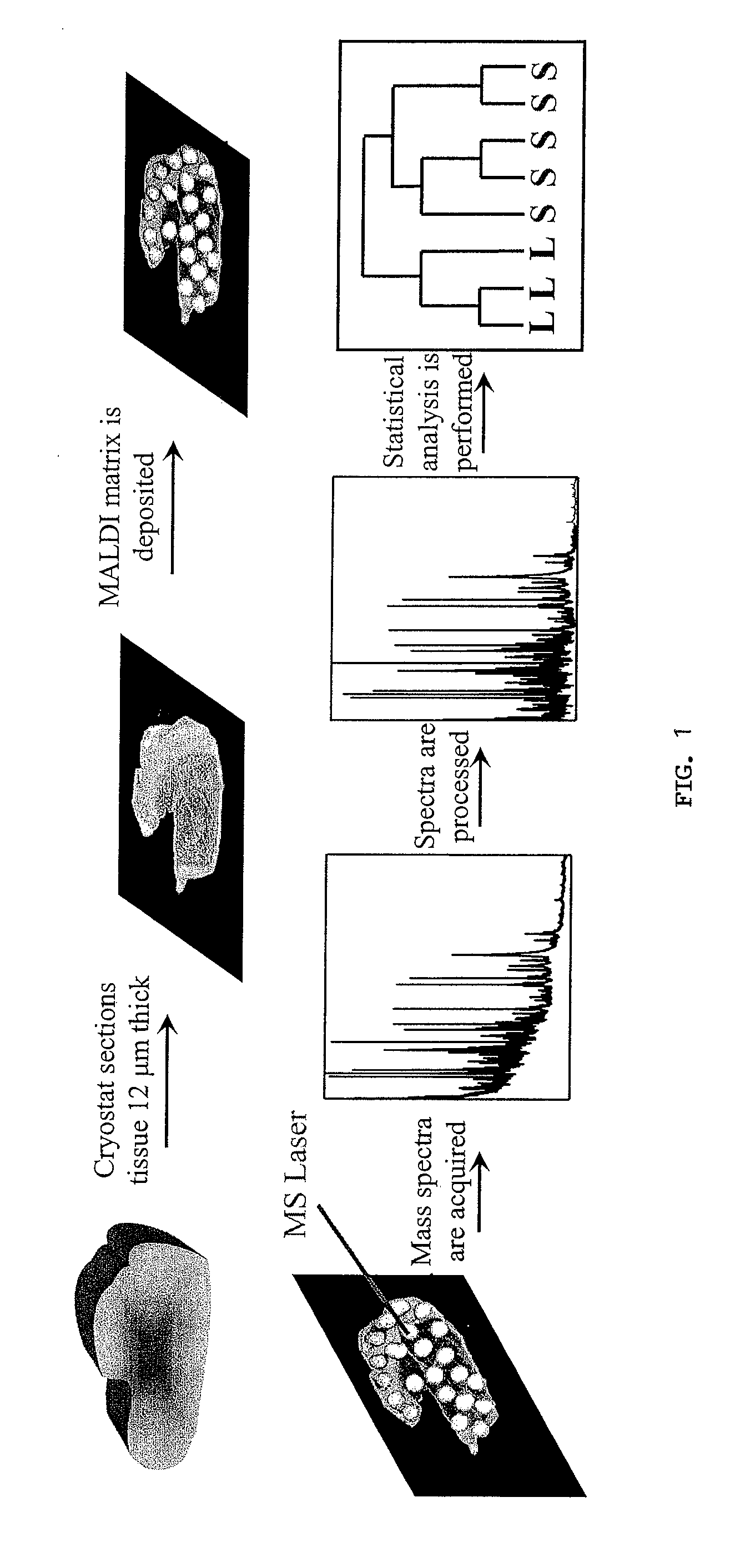
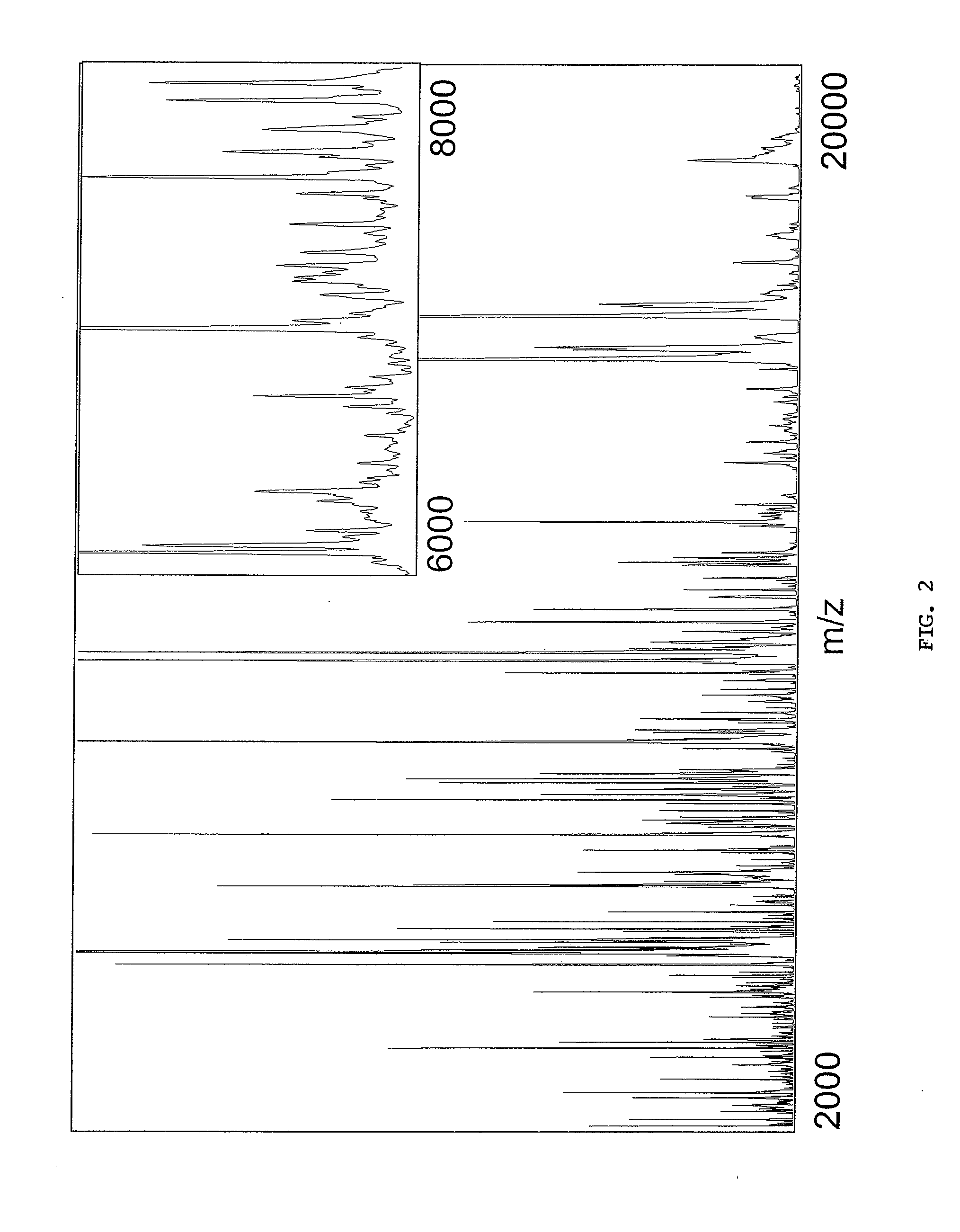
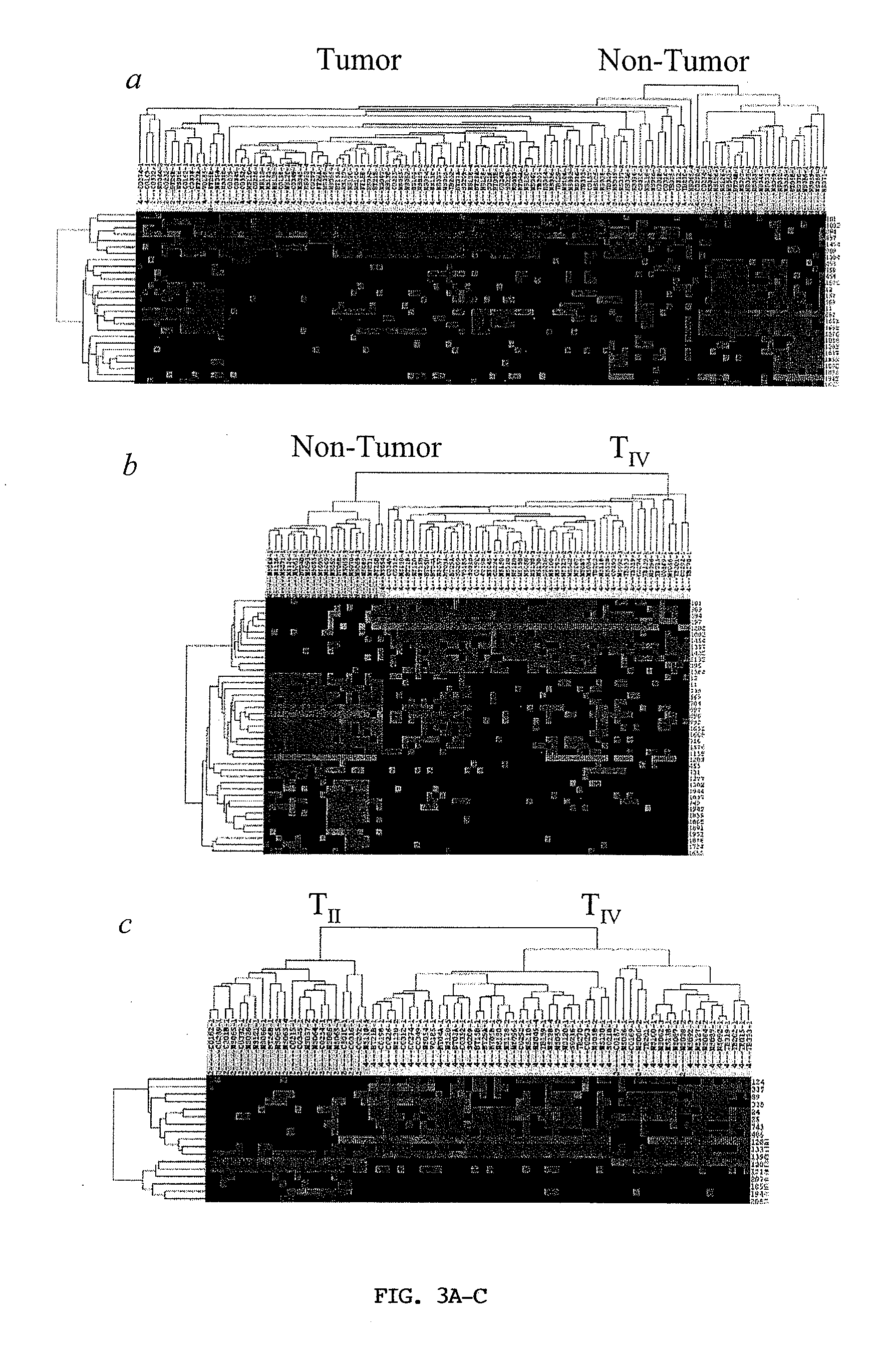
Diagnosing and Grading Gliomas Using a Proteomics Approach
InactiveUS20070031900A1Microbiological testing/measurementBiological testingProteomics methodsPatient survival
The present invention provides for a proteomic approach to grading gliomas, and for predicting patient survival. In addition to employing global protein expression patterns, such as by mass spectrometry, particular target proteins whose expression is altered in various gliomas can be used to predict the stage / classificiation of a glioma, as well as to indicate whether a given patient will be a short- or long-term survivor.
Owner:VANDERBILT UNIV
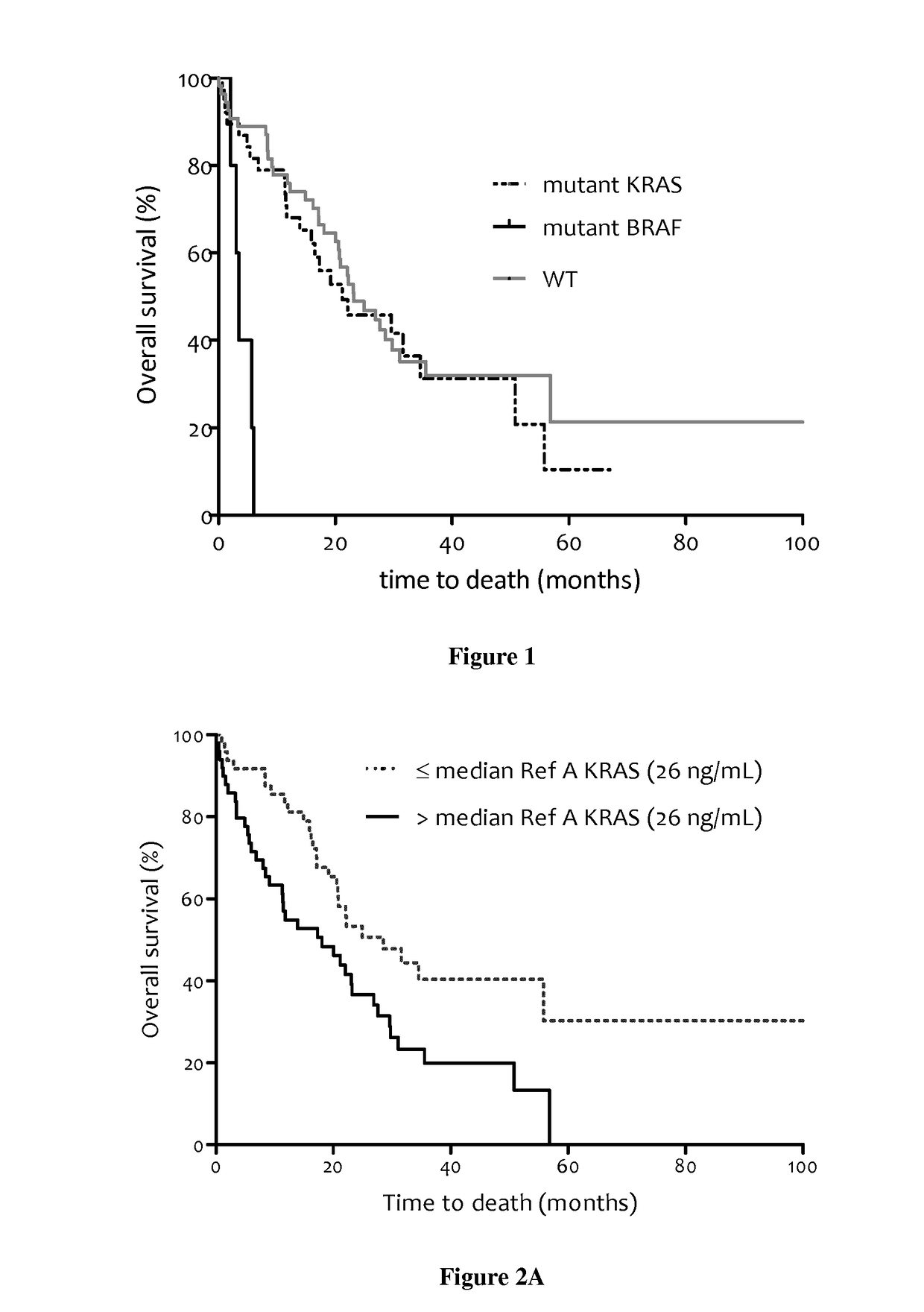
Methods for predicting the survival time of patients suffering from cancer
InactiveUS20170183742A1Short survival timeKeep for a long timeMicrobiological testing/measurementPatient survivalBiology
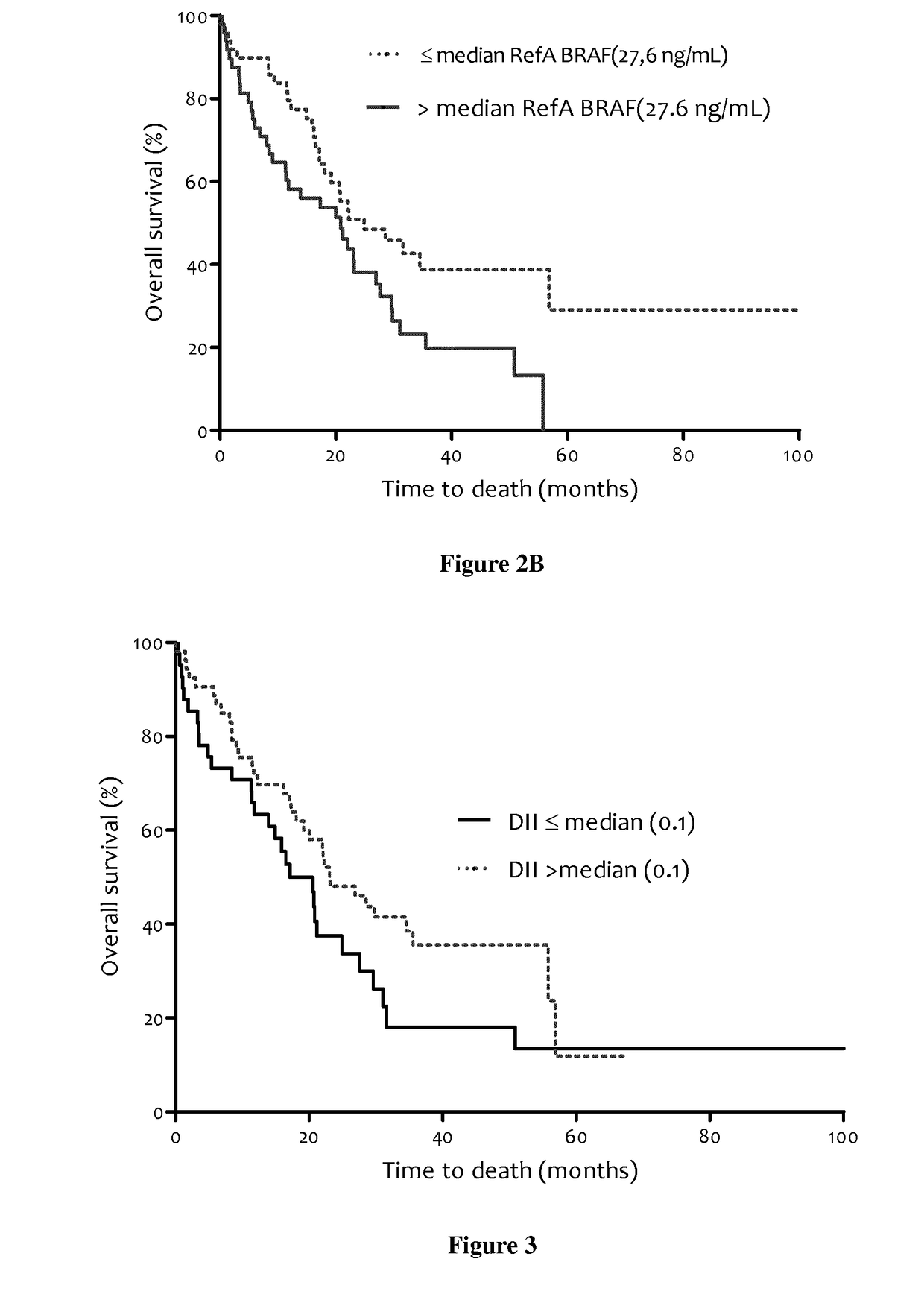
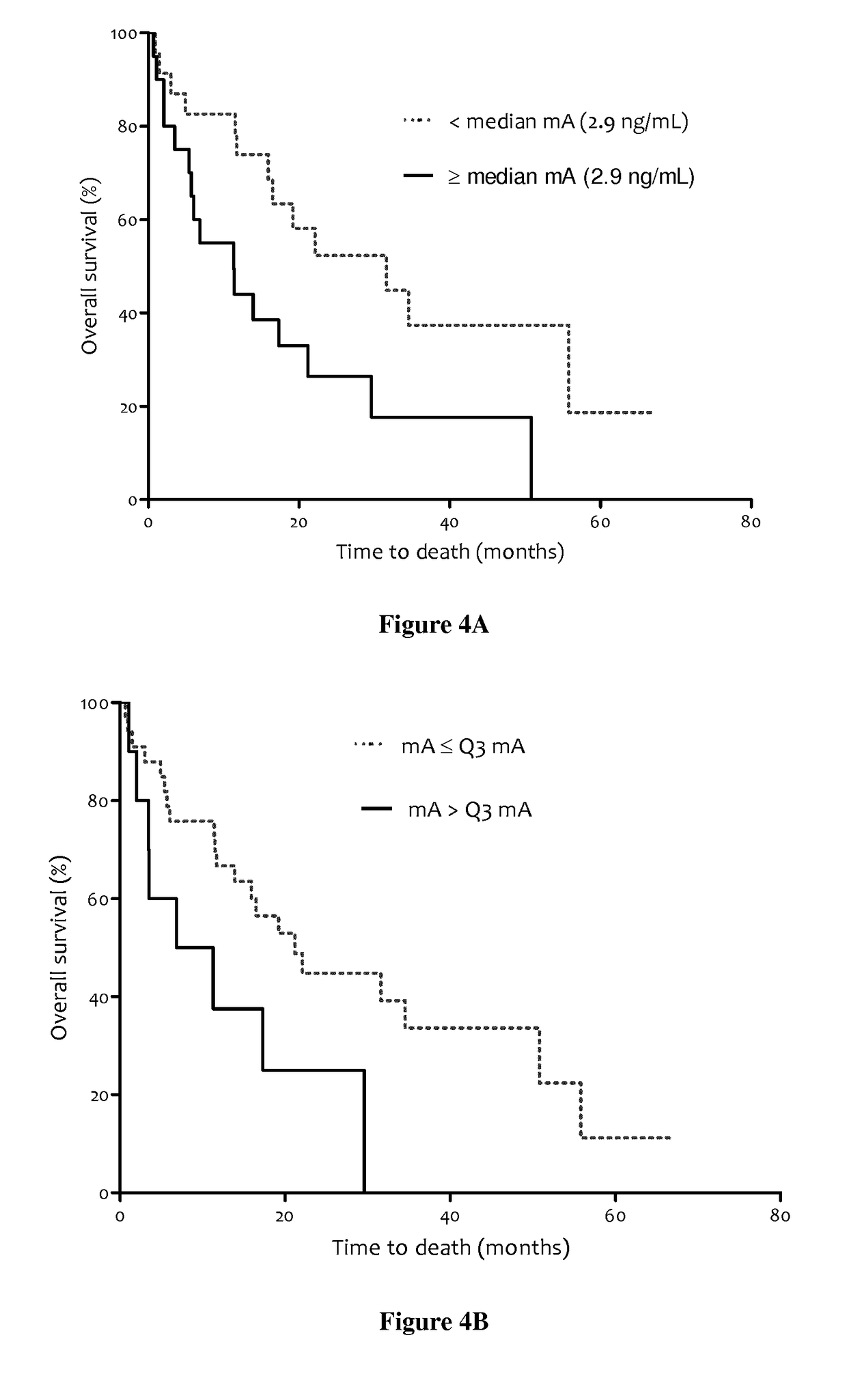
The present invention relates to methods for predicting the survival time of patients suffering from cancer. Said methods are based on the quantification and analysis of the cell free nucleic acids that are present in a sample from the patient and typically include the determination of the level of the mutant nucleic acid which contains a mutation of interest, the calculation of the mutation load for said mutation of interest, the calculation of the DNA integrity index or a combination thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Nlrc5 as a biomarker for cancer patients and a target for cancer therapy
InactiveUS20170321285A1Reduce capacityHigh expressionMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellBiomarker identification
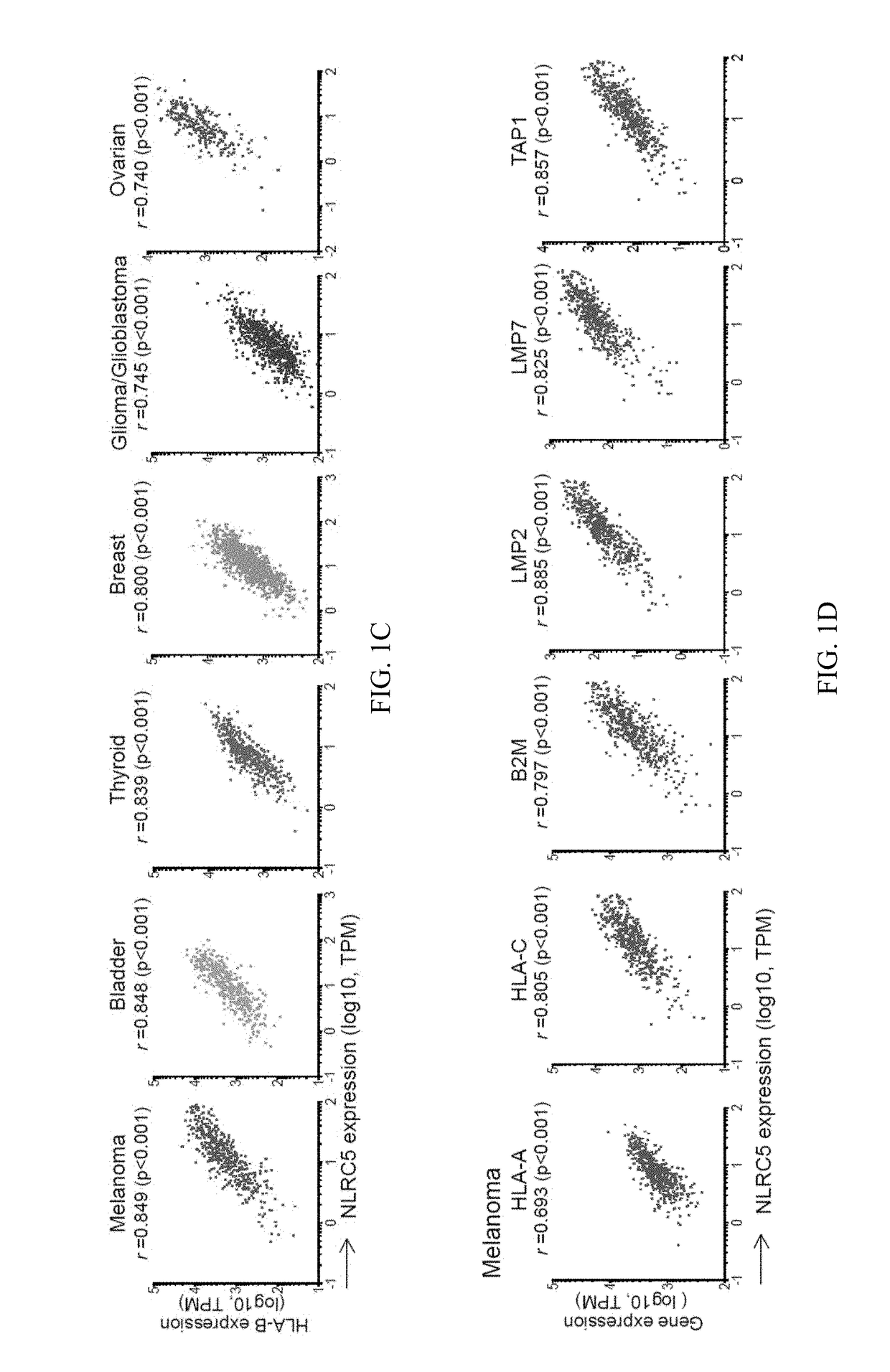
The invention pertains to biomarkers for identifying a cancer that is likely or not likely to evade the immune system of a subject, thus, is likely or not likely to show better prognosis (prognostic biomarker) and / or better responses to cancer therapies (predictive biomarker). The invention provides a method of identifying a subject as having a cancer that is likely to evade the immune system of the subject based on one or more of the following biomarkers in the cancer cells of the subject: a) reduced amount of NLRC5 mRNA or protein; b) reduced activity of NLRC5 protein; c) a mutation that reduces the activity of NLRC5 protein; d) increased methylation of nlrc5 or a portion thereof; and e) reduced copy number of nlrc5. These variables are useful to predict both patient survival (prognostic biomarker) and patient responses to immunotherapies (predictive biomarker). Furthermore, this invention provides a method of identifying a subject as having a cancer that is likely to evade the immune system of the subject with greater prediction power by utilizing multiple variables, in addition to above a)-e) variables, including neoantigen load, mutation number, or expression of genes involved in immune responses, including but not limited to CTLA4, PD1, PD-L1 and PD-L2. The invention also pertains to a method of treating a cancer likely to evade the immune system of the subject by administering an immunotherapy and a therapy designed to activate the MHC class I antigen presentation pathway by activating the expression and / or activity of NLRC5 protein.
Owner:TEXAS A&M UNIVERSITY
Application of miR-425 in tumor diagnosis, treatment and prognosis
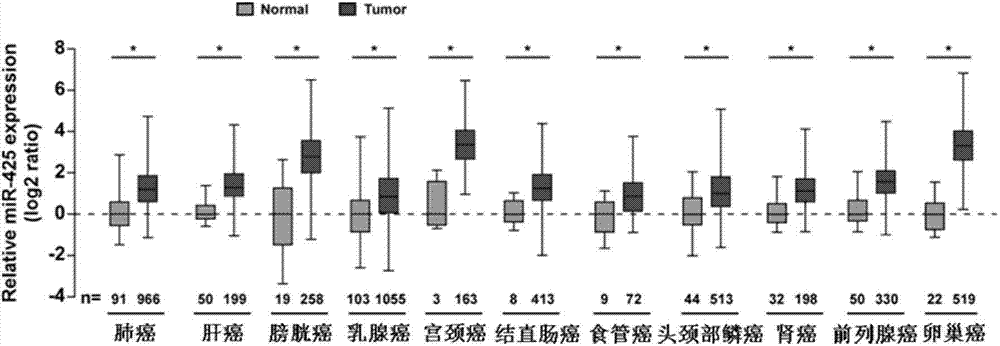
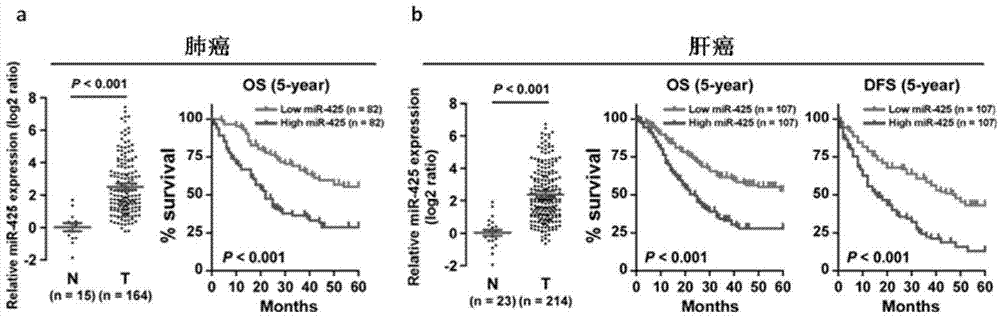
ActiveCN104726584APrevent proliferationInhibit transferGenetic material ingredientsMicrobiological testing/measurementTumor therapyApoptosis
The invention discloses application of miR-425 in tumor diagnosis, treatment and prognosis. Multiple biological characteristics of tumor including apoptosis, proliferation, drug susceptibility and the like disclosed by the invention are closely related to the expression quantity of miR-425. MiR-425 antagonist antimiR-425 can be used for significantly inhibiting proliferation of multiple types of tumor cells and the tumorigenic ability of the tumor cells in a nude mouse, and enhancing the tumor treatment capacity of chemotherapy drug cisplatin and the like in the nude mouse. The invention discloses a kit for tumor assisted diagnosis and patient survival prognosis, and the kit contains a primer sequence for the quantitative determination of miR-425; the invention also discloses a pharmaceutical composition for treating tumor, wherein the composition contains the miR-425 antagonist antimiR-425. The invention provides a novel method for assisted diagnosis and prognosis diagnosis of cancer. The invention discovers that the antimiR-425 has high clinical application value in the preparation of a tumor-treating medicine, and particularly provides a novel medicine and a treatment method for the effective treatment of lung cancer and liver cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
Carcinoma diagnosis and treatment, based on odc1 genotype
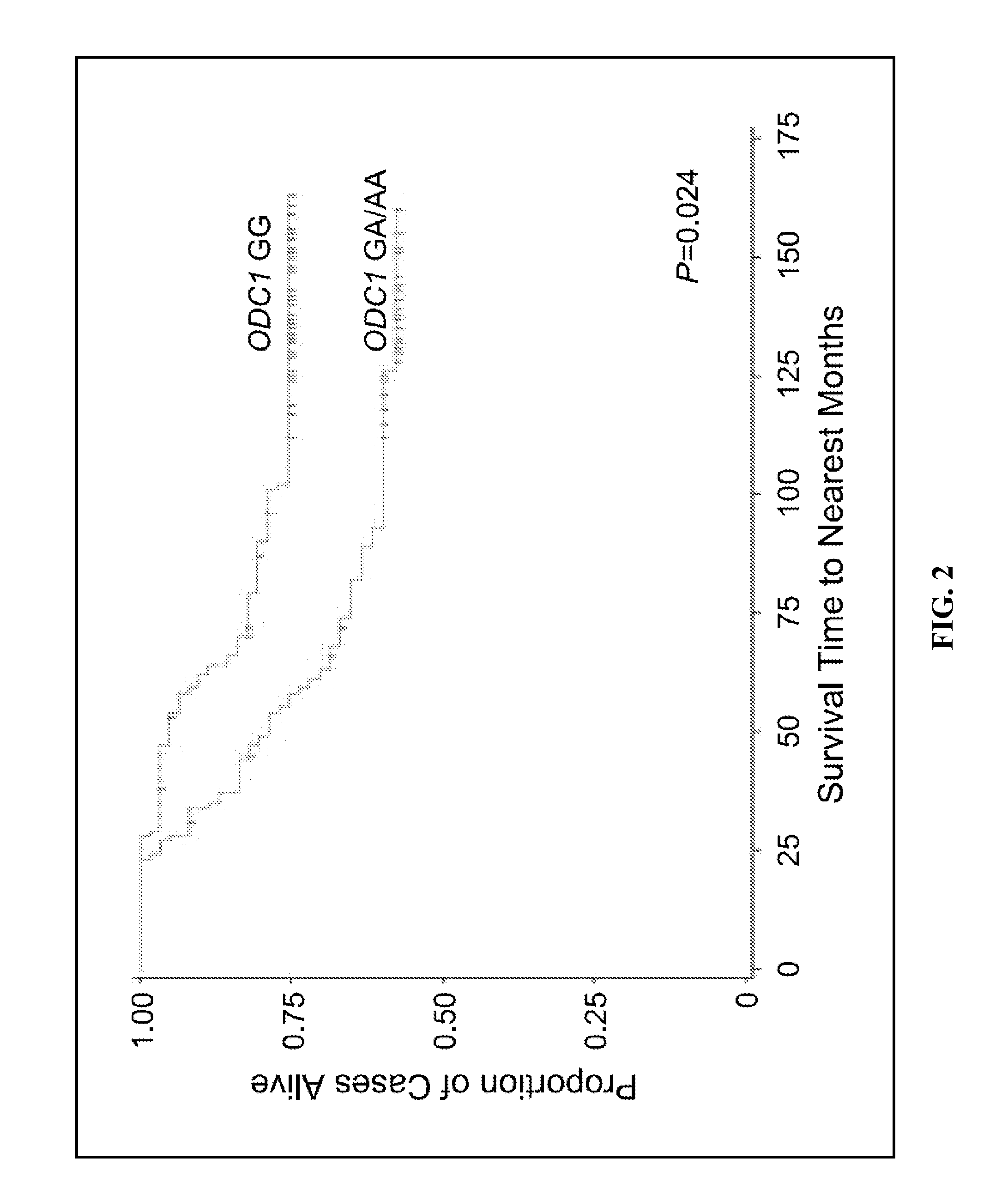
ActiveUS20100317708A1Reduce overall polyamine contentHigh expressionBiocideOrganic active ingredientsTreatment choicesTreatment options
The present invention provides methods and kits a) for predicting colorectal cancer patient survival, as well as the survival of patients harboring other invasive cancers where cellular proliferation and carcinogenesis is linked, in part, to high levels of ODC activity and increased cellular polyamine contents, and b) for selecting the corresponding treatment options for such patients based on the allelic nucleotide sequence or SNP at position +316 of the ODC1 promoter gene as well as cancer treatment methods, in each case, which include the determination of the ODC1 promoter +316 position genotype, as a means to guide treatment selection.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Application of POU5F1B in diagnosis, treatment, prognosis and relapse prediction of tumor
ActiveCN104762375ALower survival rateImprove survival rateOrganic active ingredientsMicrobiological testing/measurementParanasal Sinus CarcinomaOncology
The invention discloses an application of POU5F1B in diagnosis, treatment, prognosis and relapse prediction of tumor. In the invention, it is found that the copy number and the expression level of the gene POU5F1B in various tumors are closely related to transfer, relapse, patient prognosis and dryness of the tumor cells. A research result proves that by means of high expression of the POU5F1B, formation of TIC is promoted and further the in-vivo tumorigenic capability of esophagus cancer cells is enhanced, thereby reducing the survival rate, and oppositely, by means of inhibition of the expression of the POU5F1B, the formation of the TIC is promoted and further the in-vivo tumorigenic capability of the esophagus cancer cells is reduced, thereby increasing the survival rate, so that it is obviously that by means of inhibition of the endogenous POU5F1B, the in-vivo tumorigenic capability of the esophagus cancer cells can be significantly reduced, thereby increasing the survival rate of patients, which proves that the POU5F1B can be used as a marker for diagnosis, treatment, prognosis and relapse prediction. An inhibitor of the POU5F1B has a great clinical application value in preparation of a drug composition for tumor, so that the invention provides a new drug and a new method for effective treatment of tumor.
Owner:SUN YAT SEN UNIV CANCER CENT
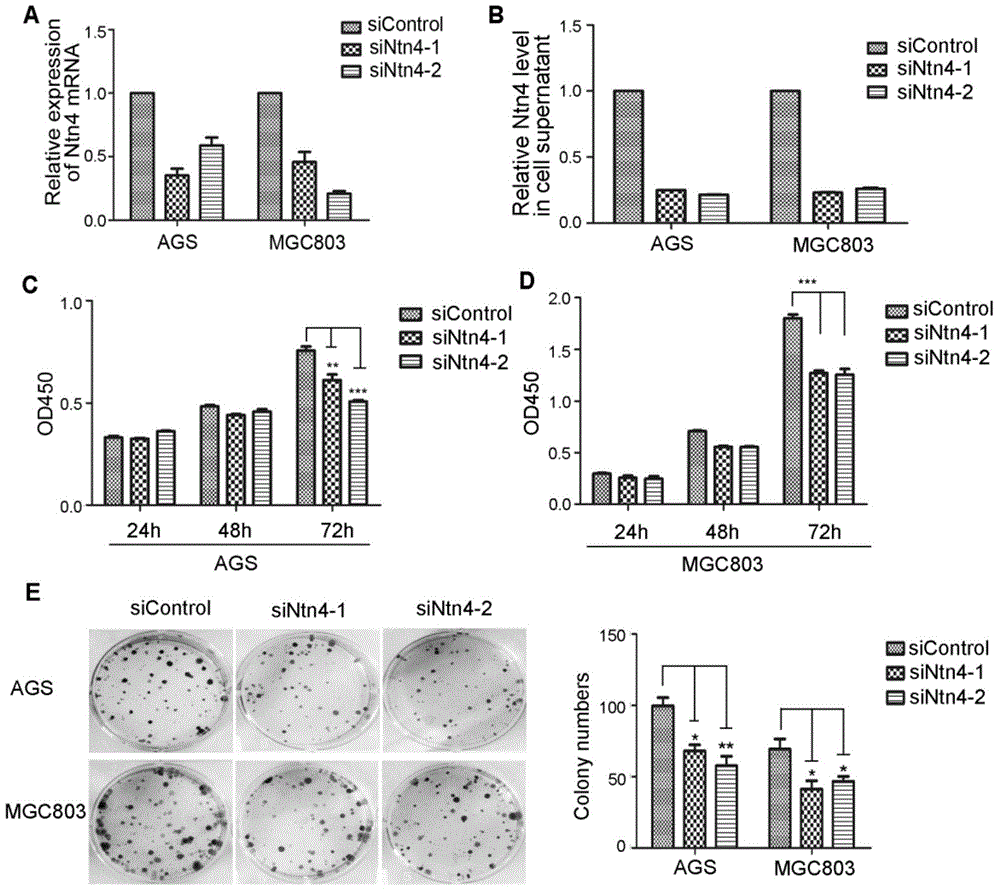
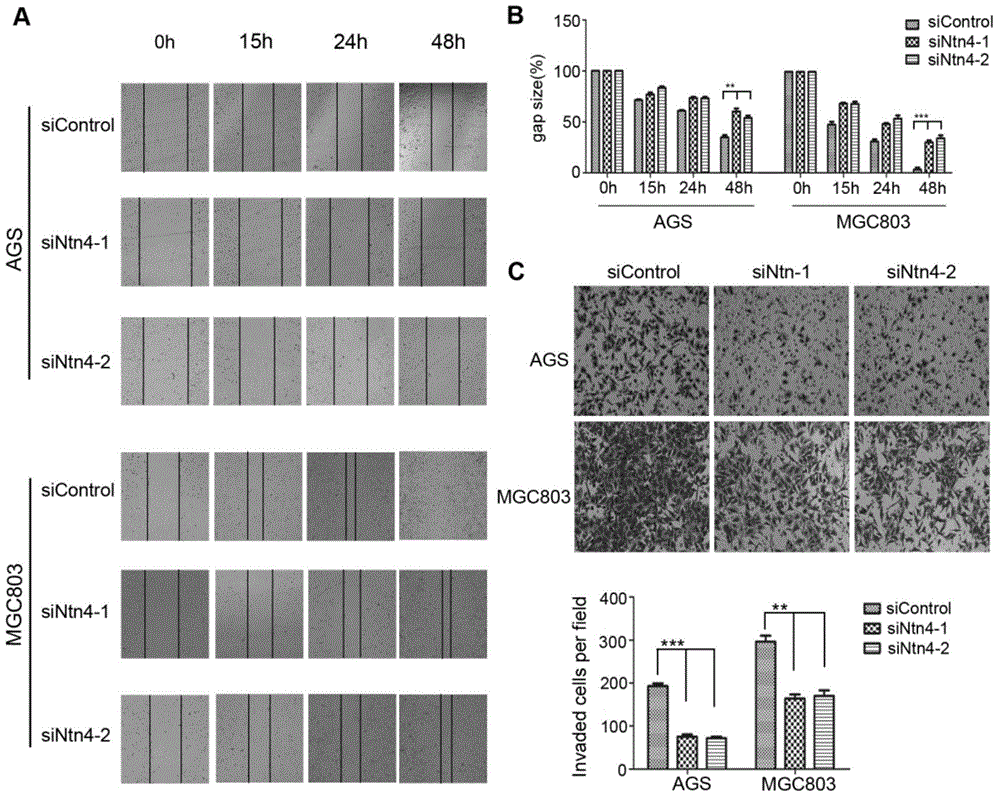
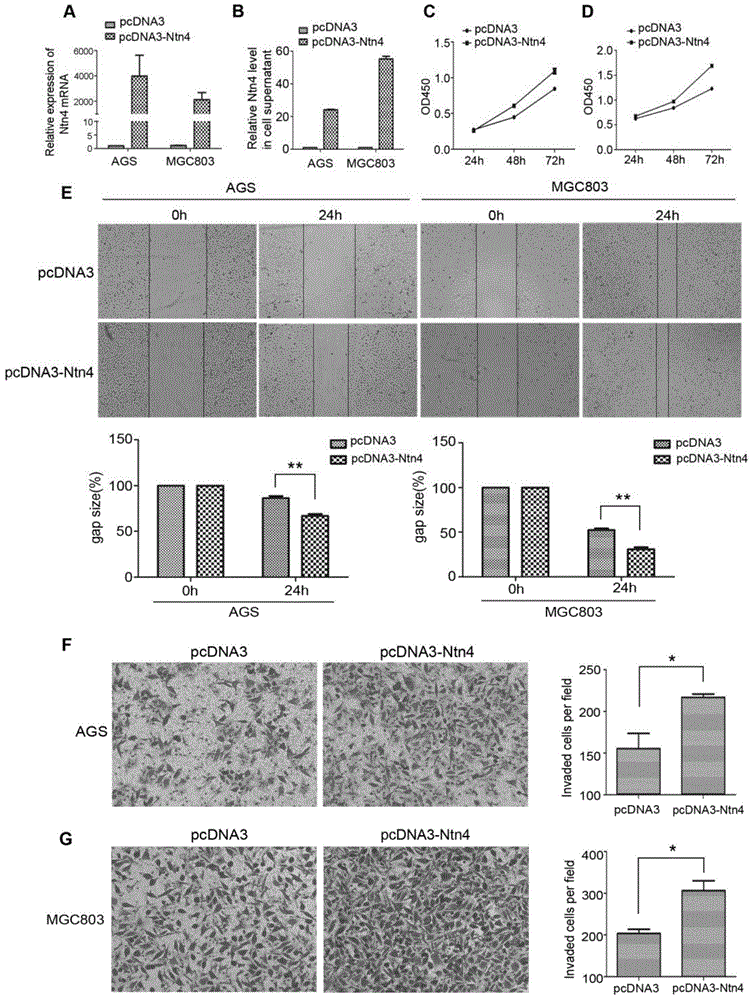
Application of Netrin-4 in preparing preparation for detecting stomach cancer and prognosis marker
The invention belongs to the technical field of cell biology, and relates to application of Netrin-4 in preparing a preparation for detecting stomach cancer and a prognosis marker. Experiments display that Ntn4 is combined with a ligand neogenin thereof to activate cancer promoting paths such as Jak / Stat, PI3K / Akt and ERK / MAPK, thereby achieving the effects of promoting gastric cancer cell proliferation and invasion. Experiment results show that Ntn4 has remarkable high expression in both a serum sample and a tumor tissue sample of a patient with gastric cancer, high expression is in negative correlation with the lifetime of the patient and in positive correlation with TNM staging, it shows that Ntn4 can be used as potential serological indicator for gastric cancer and prognosis and used for preparing the preparation for detecting stomach cancer and the prognosis marker. The preparation comprises a reagent and a kit which are used for detecting stomach cancer and the prognosis marker.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Compound used for manufacturing auxiliary therapeutic agent for chemotherapy cancer patient
The invention discloses a compound used for manufacturing an auxiliary therapeutic agent for chemotherapy cancer patients; the compound mainly comprises fermentation soybean abstract, green tea abstract, spirulina, turmeric abstract, antrodia mycelium, and grape seed abstract; the compound can prevent colorectal polyps from canceration, and improves chemotherapy effect, prolongs cancer patient survival period, and reduces non-deteriorate survival period / and chemotherapy side effects; the compound also provides anti-inflammation activity, and effectively reduces generation of inflammation mediums like PGE2, NO, -alpha (TNF-alpha) and -6(IL-6).
Owner:MICROBIO CO LTD
Bad phosphorylation determines ovarian cancer chemo-sensitivity and patient survival
Despite initial sensitivity BAD-protein phosphorylation were evaluated in patient samples and cell lines as determinants of chemo-sensitivity and / or clinical outcome, and as therapeutic targets. Induced in-vitro OVCA cisplatin-resistance was associated with BAD-pathway expression. Expression of the pathway was also associated with resistance of 7 different cancers cell-types to 8 chemotherapeutic agents. Phosphorylation of the BAD-protein was associated with platinum-resistance in OVCA cells and primary OVCA specimens, and also overall patient survival. Targeted modulation of BAD-phosphorylation levels influenced cisplatin-sensitivity. A 47-gene BAD-pathway signature was associated in-vitro phospho-BAD levels and with survival of 838 patients with ovarian, breast, colon, and brain cancer. The survival advantage associated with both BAD-phosphorylation and also the BAD-pathway signature was independent of surgical cytoreductive status. The BAD apoptosis pathway influences human cancer chemo-sensitivity and overall survival. The pathway is useful as a biomarker of therapeutic response, patient survival, and therapeutic target.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Training method of early warning model of primary liver cancer
ActiveCN110991536AImprove bindingEasy way to getCharacter and pattern recognitionPatient survivalData set
The invention provides a training method of the early warning model of primary liver cancer. According to the training method, a primary liver cancer early screening model is established by utilizinga machine learning algorithm on the basis of clinical examination data. The method comprises the following steps that: (a) carrying out data preprocessing on the obtained clinical examination data; (b) carrying out data set division on a preprocessed data set; (c) training a model according to divided data sets; and (d) evaluating and verifying the performance of the classification model. According to the screening model, the clinical examination data are utilized; on the basis of actual clinical requirements, a threshold value is selected through the performance index curve of the optimizedmodel so as to perform test verification, so that primary liver cancer clinical early warning can be carried out; conditions are provided for the early successful diagnosis of liver cancer; and the life quality of a patient is improved.
Owner:SHANGHAI INST OF TECH
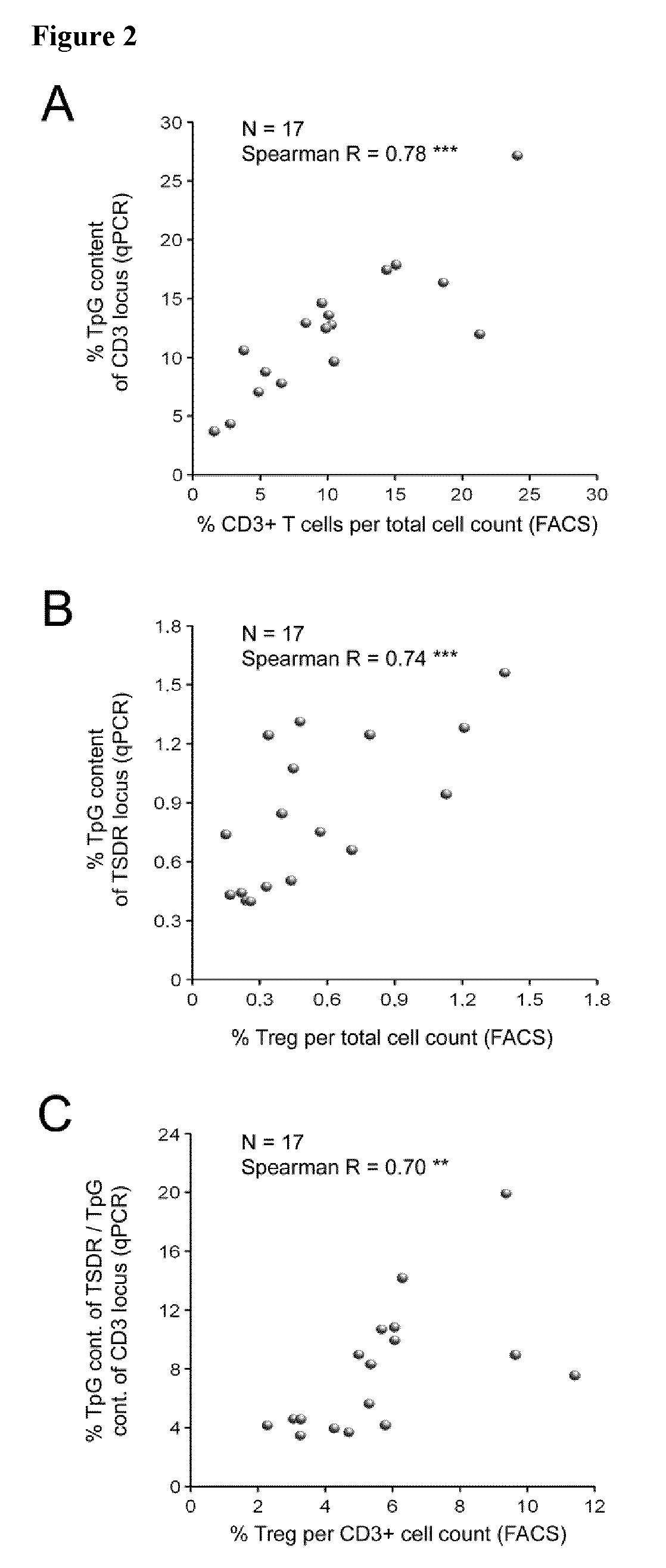
Method for determining cancer patient survival based on analyzing tumorinfiltrating overall t-lymphocytes
The present invention relates to a method, in vitro or in vivo, for determining cancer patient survival, comprising analyzing the number and / or amount of tumor-infiltrating overall T-lymphocytes (oTLs) based on the methylation status of at least one CpG position in one or more of the genes for CD3 γ, -δ, and -ε in a tumor sample derived from said cancer patient, wherein a high number and / or amount of oTLs is indicative for a better survival of said cancer patient in a non-breast cancer, wherein in breast cancer a high number and / or amount of oTLs is indicative for an inferior survival of said patient. The present invention also relates to a respective kit for use in the methods of the invention.
Owner:EPIONTIS GMBH
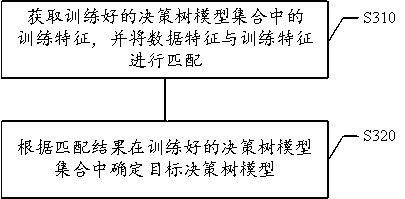
Prediction method for survival probability of infectious disease, and training method and device of prediction model
ActiveCN111564223ASolve problems that cannot be accurately predictedAvoid wastingEpidemiological alert systemsCharacter and pattern recognitionPatient survivalPredictive methods
The invention belongs to the technical field of data processing, and relates to a prediction method for the survival probability of an infectious disease, and a training method and device for a prediction model. The prediction method comprises the steps of acquiring diagnosis and treatment data of an infectious disease patient to be diagnosed and treated, and extracting a plurality of data features of the diagnosis and treatment data; encoding the plurality of data features to obtain feature vectors, and determining a target decision tree model in a trained decision tree model set according tothe data features; and inputting the feature vectors into the target decision tree model to enable the target decision tree model to output the survival probability of the infectious disease patientto be diagnosed and treated. According to the invention, the problem that accurate estimation cannot be carried out due to lack of data characteristics in clinical practice is solved; the applicationscenarios for predicting the survival probability of infectious disease patients are enriched, inaccurate manual estimation is abandoned in the automatic and intelligent processing process, targeted treatment measures can be conveniently, efficiently and accurately taken for different infectious disease patients, and the situation of medical resource waste caused by missed examination and wrong examination is avoided.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
Gene expression profile for predicting ovarian cancer patient survival
InactiveUS20100292303A1Reduce biological activityAltered expressionOrganic active ingredientsOrganic compound preparationPatient survivalFhit gene
A gene profiling signature for predicting ovarian cancer patient survival is disclosed herein. The gene signature can be used to diagnosis or prognosis ovarian cancer, identify agents to treat an ovarian tumor, to predict the metastatic potential of an ovarian tumor and to determine the effectiveness of ovarian tumor treatments. Thus, methods are provided for diagnosing and prognosing an ovarian tumor, such as ovarian cancer, in a subject. Methods are also provided for identifying agents that can be used to treat an ovarian tumor, for determining the effectiveness of an ovarian tumor treatment, or to predict the metastatic potential of an ovarian tumor. Methods of treatment are also disclosed which include administering a composition that includes a specific binding agent that specifically binds to one of the disclosed ovarian survival factor-associated molecules and ovarian tumor in the subject.
Owner:BIRRER MICHAEL J +3
Method and System for Calorimetry Probe
InactiveUS20150108356A1Address limitationsCalorimetric dosimetersMaterial analysis by optical meansDose rateDosimeter
Radiotherapy is one of the most effective treatments for cancer and its success depends critically on accurate targeting and delivery of the correct radiation dose. Accurate dosimetry is therefore essential to maintain and improve patient survival rates. However, size and long wait times currently limit water and graphite based calorimeters to standards laboratories leaving field-based dosimetry to ionization chamber measurements which depend upon a reference field-specified calibration factor. It would therefore be beneficial to provide radiotherapy equipment operators a direct approach of clinical reference dosimetry wherein the dosimeter provides increased independence on dose, dose rate, radiation energy, and energy type, etc. It would be further beneficial for such novel clinical dosimeters to be compact, function as secondary standards used routinely for measurements and allow radiotherapy doses to be measured directly and in an absolute manner. According to embodiments of the invention novel compact graphite probe calorimeters are provided.
Owner:SUN NUCLEAR
Method and system for evaluating colorectal cancer metastasis and recurrence risk and dynamically monitoring based on methyl marker combination
PendingCN112992354AImprove survival rateImprove the quality of lifeHealth-index calculationMicrobiological testing/measurementPatient survivalObserved Survival
The invention relates to a method and a system for evaluating colorectal cancer metastasis and recurrence risk and dynamically monitoring based on a methyl marker combination, and particularly discloses a colorectal cancer metastasis and recurrence risk monitoring method. The method comprises the following steps: S1) forming a training set database; S2) training data in the training set database by adopting a random forest model to obtain a mapping relationship between a methylation signal value of a ctDNA methylation block of a plasma sample of a colorectal cancer patient and non-recurrence lifetime information of a corresponding sample and a correlation model; S3) taking a methylation signal value of a ctDNA methylation block of a patient to be predicted as an input value, and predicting the recurrence progress risk of the patient through the correlation model. The prediction model can noninvasively, sensitively and quickly predict the progress of CRC, the progress risk monitoring after the radical treatment of CRC patients is realized, the clinical optimization treatment scheme is guided, and the survival rate and life quality of the patients are improved.
Owner:SOUTHERN MEDICAL UNIVERSITY
Esophageal squamous carcinoma patient survival risk prediction method based on convolutional neural network
InactiveCN113096810AImproved prognosisHealth-index calculationEpidemiological alert systemsSCC - Squamous cell carcinomaPatient survival
The invention provides an esophageal squamous carcinoma patient survival risk prediction method based on a convolutional neural network. The method comprises the steps of: collecting M clinical phenotype indexes and survival information of esophageal squamous carcinoma patients as original data; performing research by using a Kaplan-Meier method and a log-rank method to obtain a relationship between clinical phenotype indexes and lifetime information of the esophageal cancer patients; analyzing clinical phenotype indexes influencing survival prognosis of the patients by using Univariate Cox hazard analysis; extracting clinical phenotype indexes with higher correlation with the survival risk of the patients through a Relief feature selection algorithm and Pearson correlation analysis; and finally, constructing a survival risk prediction model of the esophageal squamous carcinoma patients by using a convolutional neural network and using clinical phenotype indexes with higher correlation, and further judging the prognosis survival risk of the patients. According to the method, the postoperative survival condition of the esophageal squamous carcinoma patients is accurately predicted, the prognosis risk prediction capability is improved, and the prognosis risk prediction cost is reduced.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Esophageal squamous cell carcinoma risk prediction method based on clinical phenotype and logistic regression analysis
ActiveCN112185549AEffective identification of characteristic variablesImprove the performance of risk predictionMedical data miningCharacter and pattern recognitionPatient survivalClinical phenotype
The invention provides an esophageal squamous cell carcinoma risk prediction method based on clinical phenotype and logistic regression analysis. The method is used for realizing prognosis survival risk assessment of esophageal squamous cell carcinoma patients. The method comprises the following steps: firstly, screening out characteristic indexes according to clinical detection data of the esophageal squamous cell carcinoma patients, and constructing a decision tree classifier according to the characteristic indexes; secondly, dividing the esophageal squamous cell carcinoma patients into early-stage esophageal squamous cell carcinoma patients and middle-late-stage esophageal squamous cell carcinoma patients by utilizing the decision tree classifier; then, obtaining blood index informationof the esophageal squamous cell carcinoma patient one week before the operation, and screening out blood indexes with high correlation with the survival risk of the esophageal squamous cell carcinomapatient, and constructiung a logistic regression model; inputting the classified blood indexes of the esophageal squamous cell carcinoma patient into the logistic regression model to obtain a prognosis survival risk probability value of the esophageal squamous cell carcinoma patient; and judging the prognosis survival risk. According to the method, the postoperative survival state of the esophageal squamous cell carcinoma patient can be accurately judged, the risk prediction performance is improved, and the risk prediction cost is reduced.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com