Compound used for manufacturing auxiliary therapeutic agent for chemotherapy cancer patient
A technology of adjuvant therapy and composition, applied in the direction of drug combination, medical raw materials derived from algae, medical raw materials derived from fungi, etc., can solve problems such as numbness of extremities, poor blood circulation, and low quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
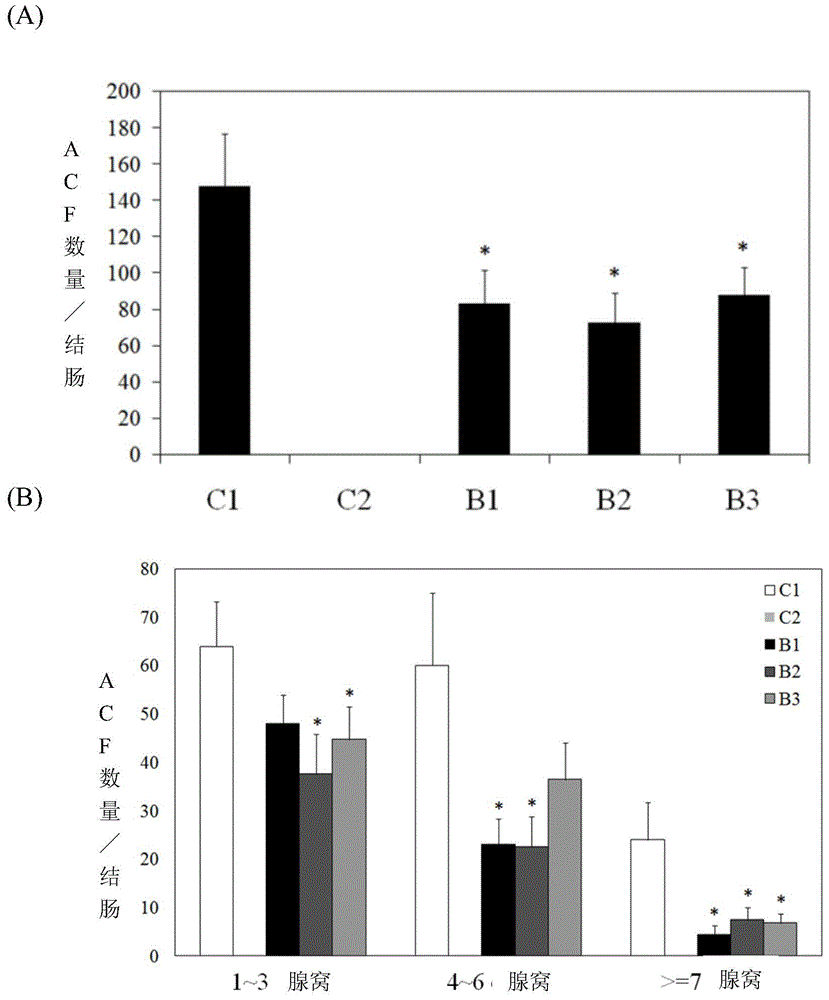
[0044] Example 1: Inhibition of abnormal fossa (ACF) induced by 1,2-dimethylhydrazine (DMH)
[0045] 1.1 Materials and methods
[0046] Animal research is approved by the Laboratory Animal Management and Use Committee of National Taiwan University, and all animals are handled in accordance with the standard guidelines for the care and use of laboratory animals. F344 male rats about 6 weeks old were purchased from the Laboratory Animal Center of National Taiwan University School of Medicine.
[0047] Male F344 rats were randomly divided into groups by weight, with 8 rats in each group. In the first week after adaptation, rats were fed Harlan AIN-76 purified rodent diet containing 17.7% by weight protein, 64.9% carbohydrate and 5.2% fat (Harlan AIN-76 purified rodent diet, Indiana, USA Harlan Laboratory, Indianapolis) (Harlan AIN-76 purified food rodent feed information page, http: / / www.harlan.com / download.axd / 1131a6e1617348b9a99c8d2af933f796.pdf?d=170481). The rats were kept in sta...
Embodiment 2
[0073] Example 2: Inhibition of colorectal cancer caused by the formation of 1,2-dimethylhydrazine (DMH)
[0074] 2.1 Materials and methods
[0075] The purchased F344 male rats aged about 6 weeks were randomly divided into groups after one week of adaptation. There are 30 animals in each group. The animals were divided into the following groups: C1 group: injection 1,2-dimethylhydrazine (DMH) control group; C2 group: normal saline injection control group; B2 group: taking a middle dose of the six-component formula of the present invention (34.6 mg / rat / Days) Experimental group. DMH was dissolved in brine (0.9% NaCl solution) to form a 2% solution. The pH was adjusted to 6.7, then aliquoted and stored at -20°C until needed. DMH was injected intraperitoneally (IP) at a dose of 30 mg / kg every week for 8 weeks (equivalent to an injection of 1.5 ml of 2% solution per kg body weight). The control group was injected with normal saline (1.5 ml saline per kg body weight). See Table 6 ...
Embodiment 3
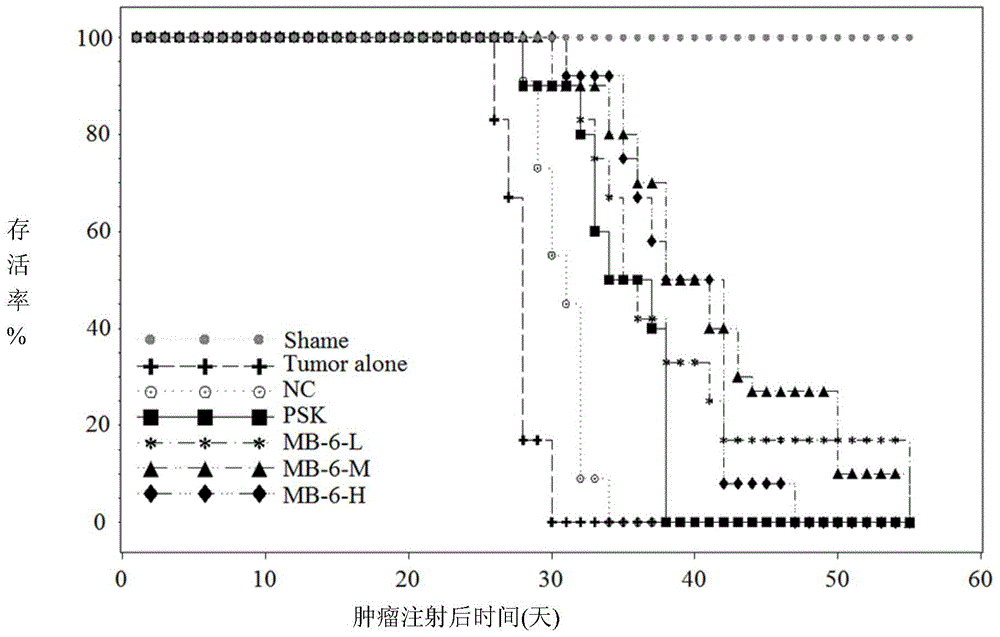
[0095] Example 3: Reduce the volume of mouse colorectal cancer and prolong animal survival
[0096] 3.1 Materials and methods
[0097] Male BALB / c mice were purchased from the National Laboratory Animal Center in Taiwan, and maintained at 21±2°C in a 12-hour light / dark cycle. AIN-76 purified feed and distilled water are freely consumed by mice.
[0098] The mice were randomly divided into 7 groups, with 12 mice in each group and 3 mice in each cage. The mouse colon cancer cell line CT-26-VD is prepared in complete Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (Dulbecco's Modified Eagle Medium (DMEM); GIBCO, catalog number 11995) at 37°C Incubate in an incubator containing 5% carbon dioxide. The establishment of a mouse colon cancer model has been described previously (Wittke M et al., Int J Radiat Oncol Biol Phys 2014; 88:1188-95). Briefly, mice were weighed and anesthetized with an appropriate dose of sodium pentobarbital (Somnotol, Canada, 10 μl / g, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com