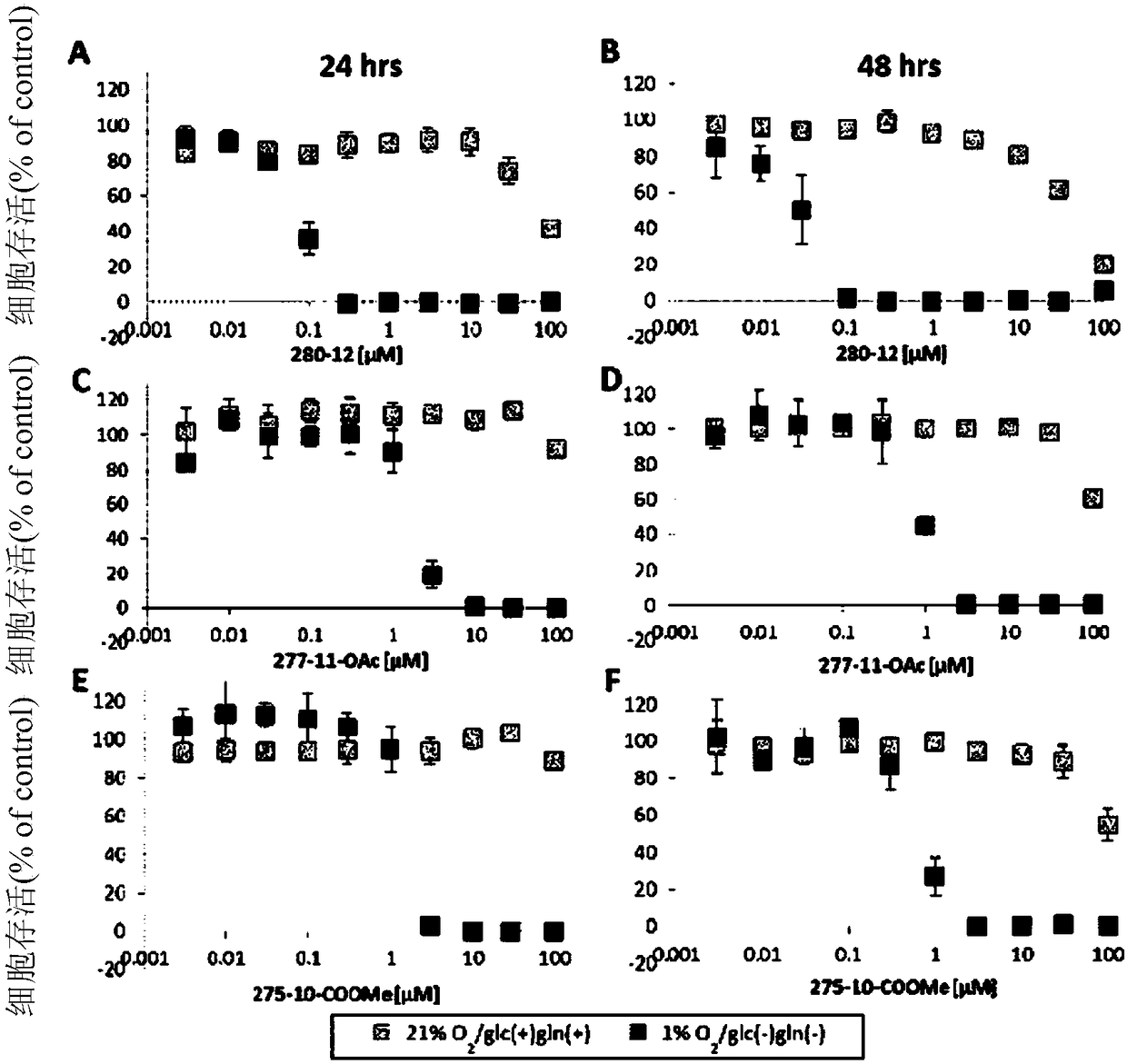
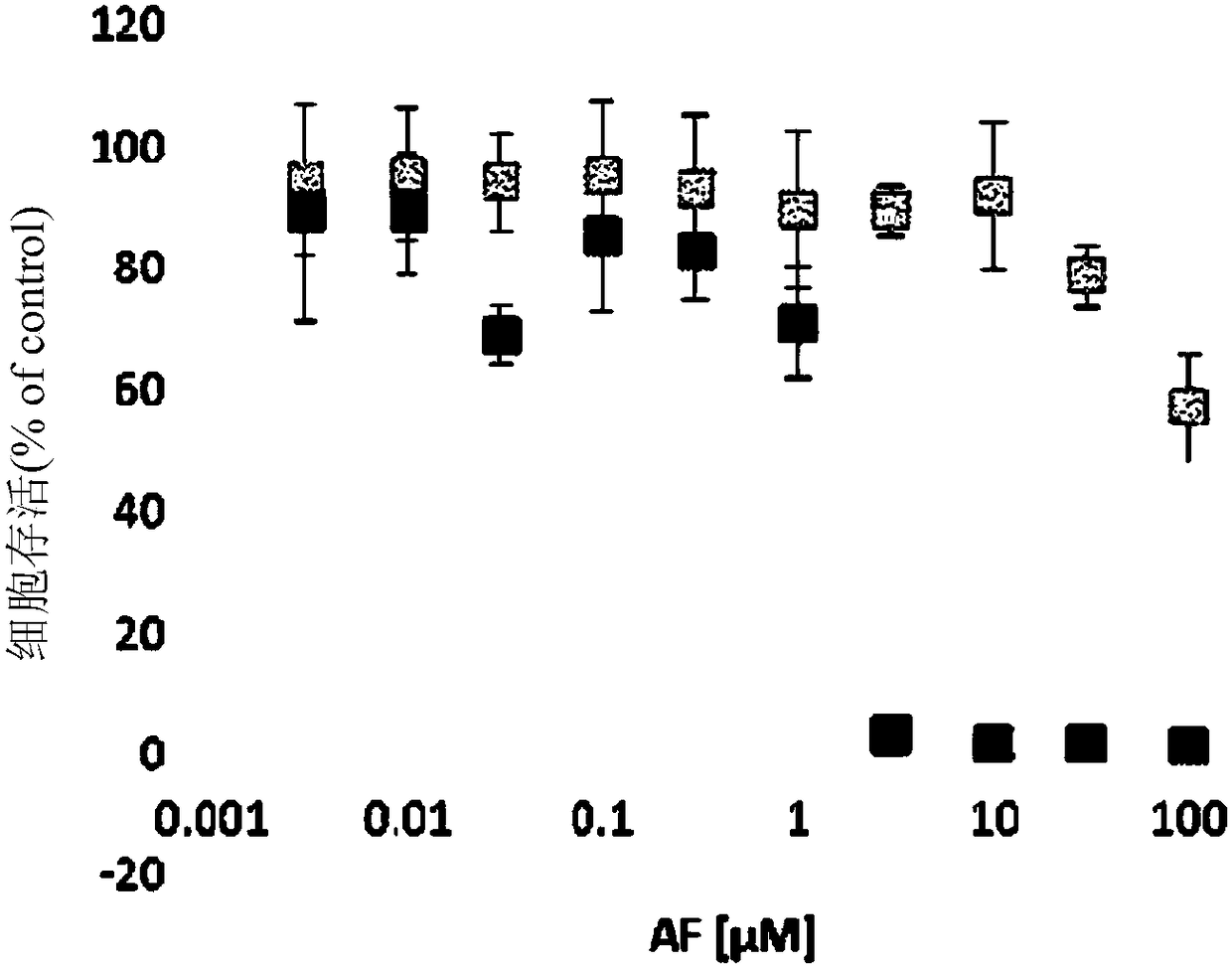
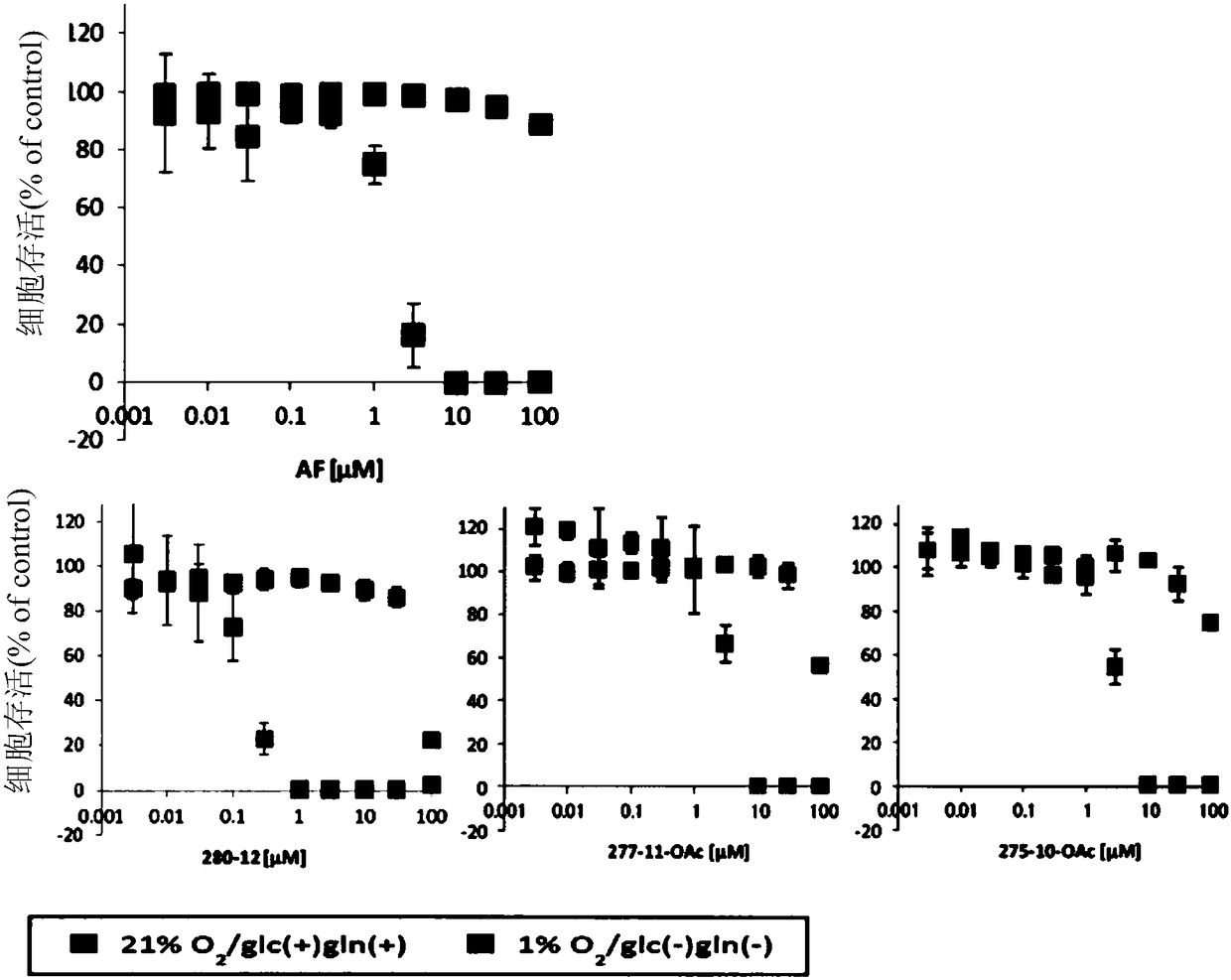
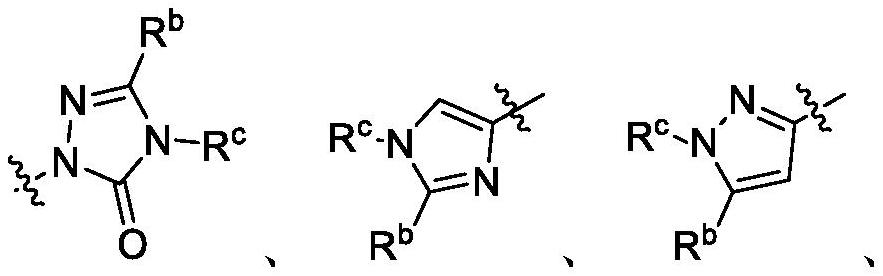
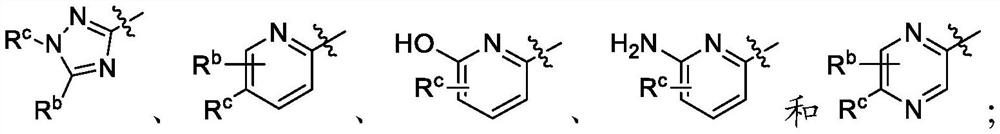
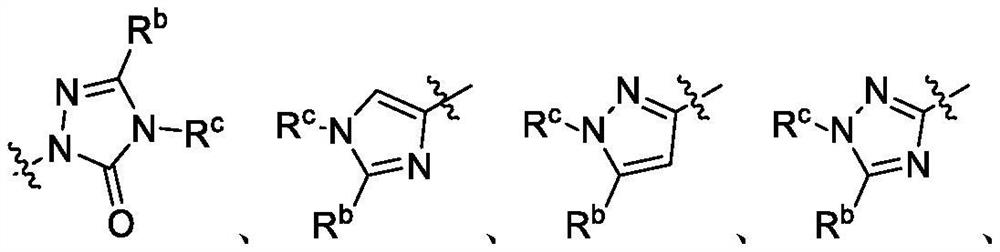
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Dihydroorotate Dehydrogenase Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
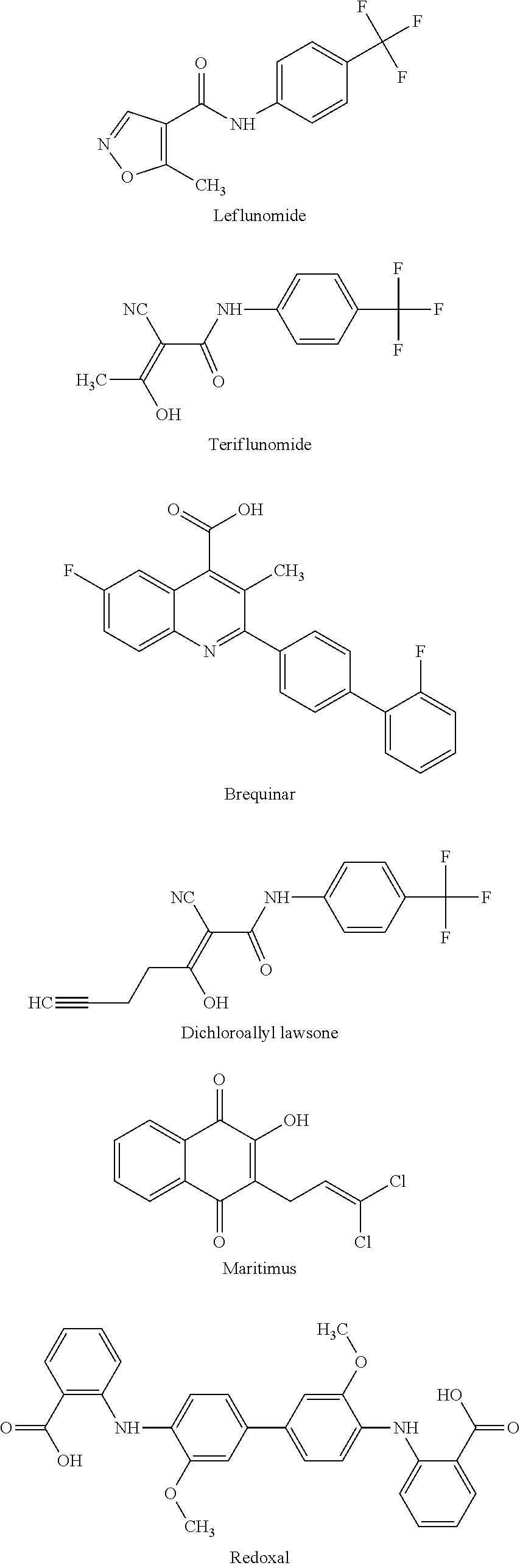
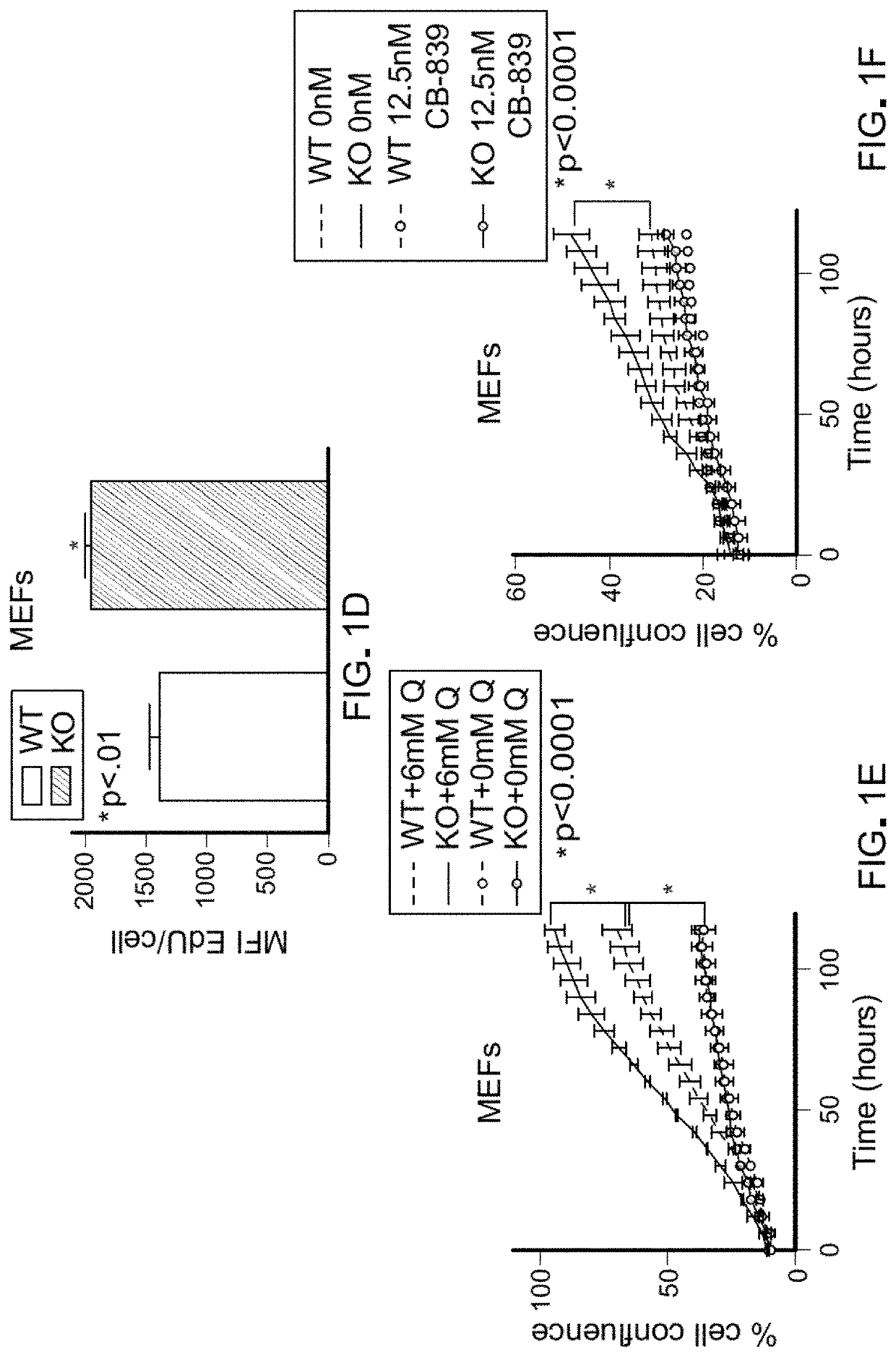
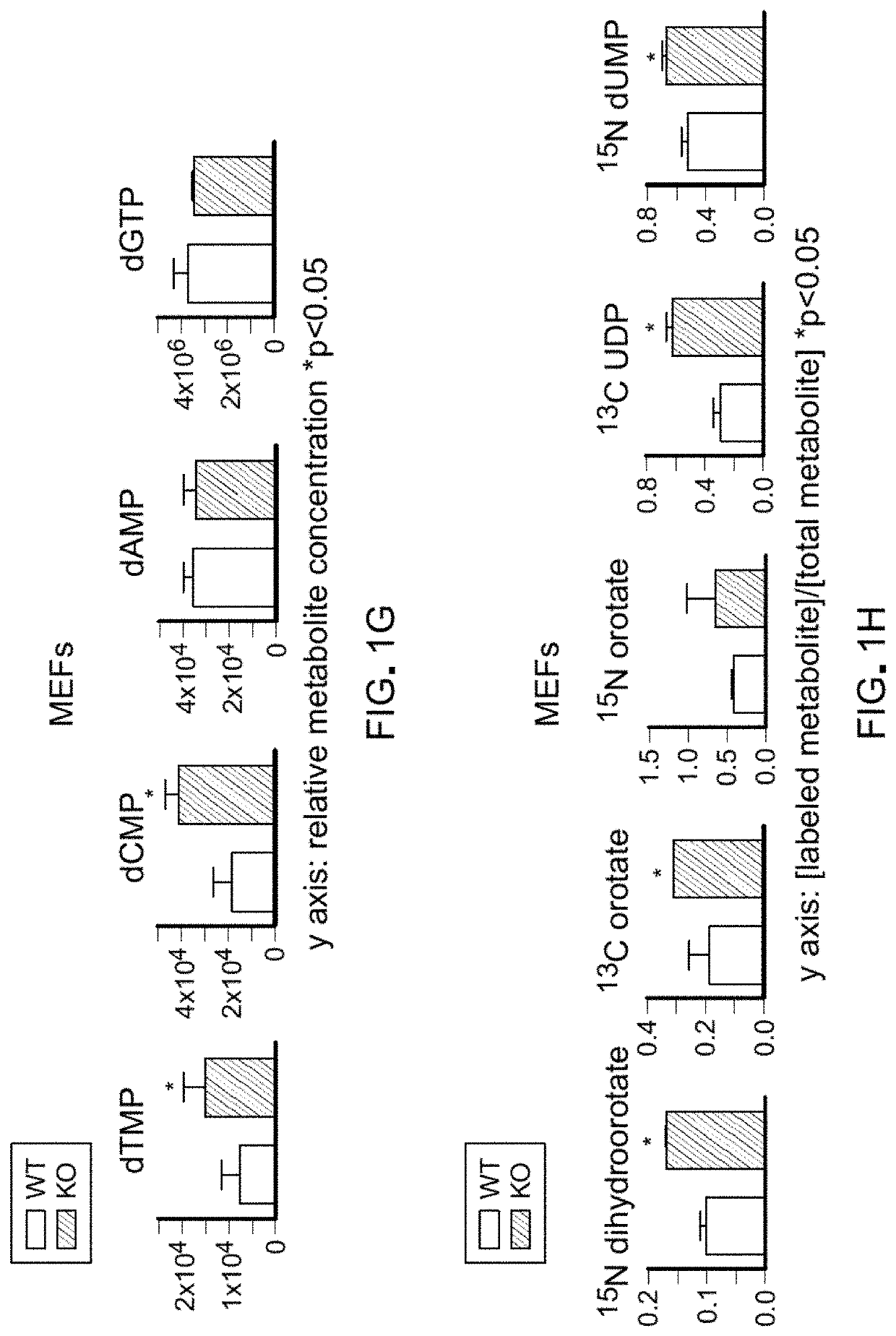
Any substance that inhibits dihydroorotate reductase, an enzyme required for de novo biosynthesis of pyrimidine. Inhibition of dihydroorotate reductase interferes with DNA and RNA synthesis.
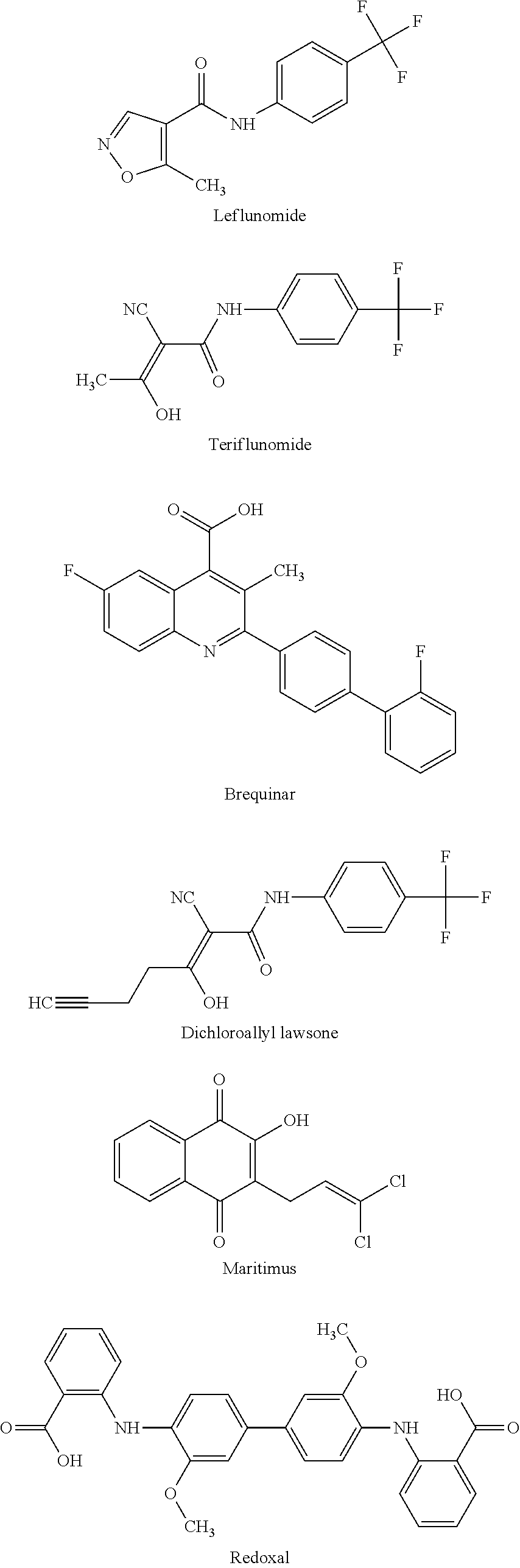
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Novel immunomodulator and Anti-inflammatory compounds
The present invention provides dihydroorotate dehydrogenase inhibitors, methods of preparing them, pharmaceutical compositions containing them and methods of treatment, prevention and / or amelioration of diseases or disorders wherein the inhibition of Dihydroorotate dehydrogenase is known to show beneficial effect.
Owner:RHIZEN PHARMACEUTICALS AG +1
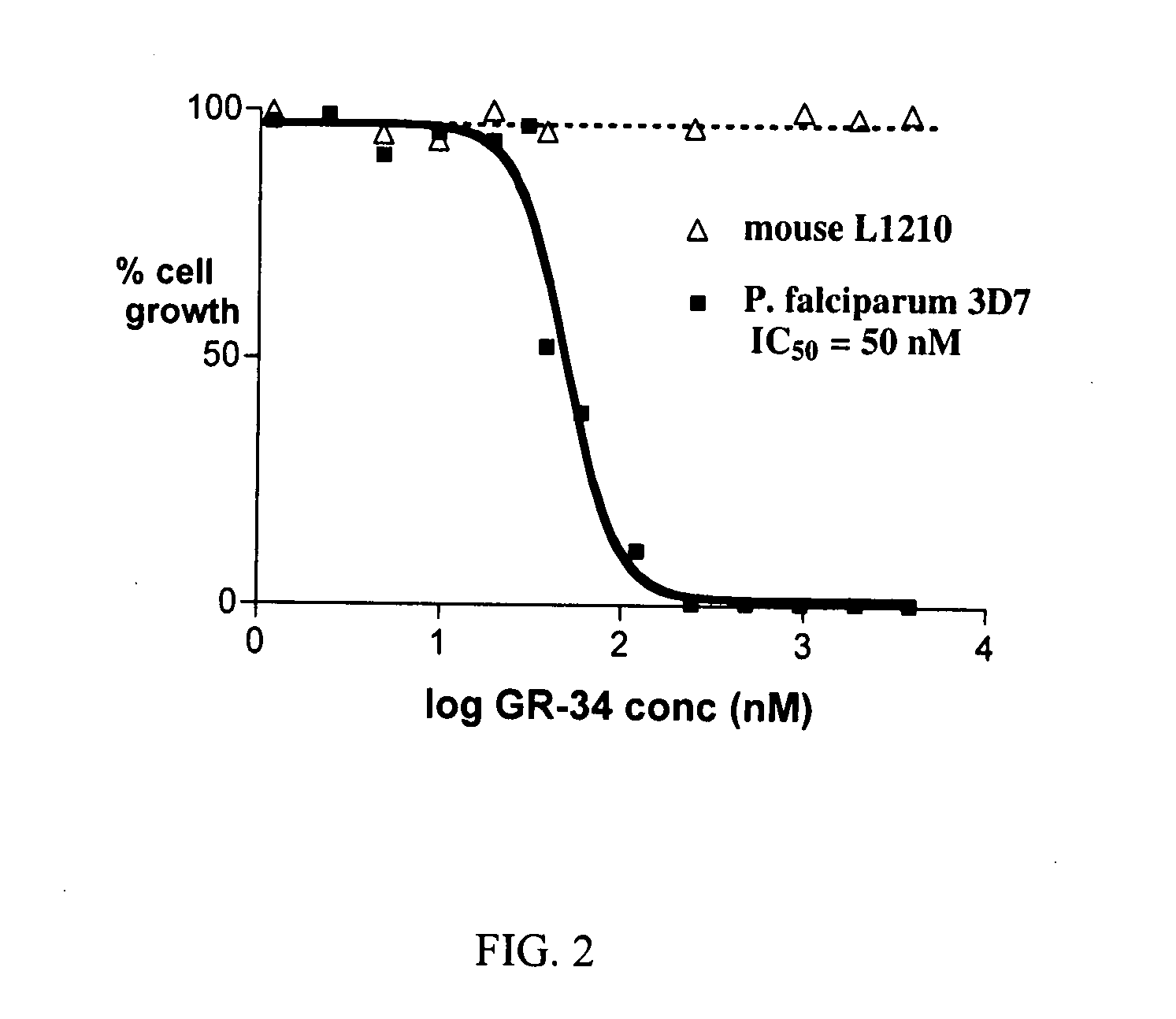
Dihydroorotate dehydrogenase inhibitors with selective Anti-malarial activity
InactiveUS20080027079A1Not be inhibitBiocideOrganic active ingredientsMedicineDihydroorotate Dehydrogenase Inhibitor
Pharmaceutical compositions comprising compounds of the formula where R1, R2, and R3 are described here, have therapeutic utility in selectively inhibiting P. falciparum dihydroorotate dehydrogenase. Accordingly, such compositions have use in the treatment and prevention of malaria.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
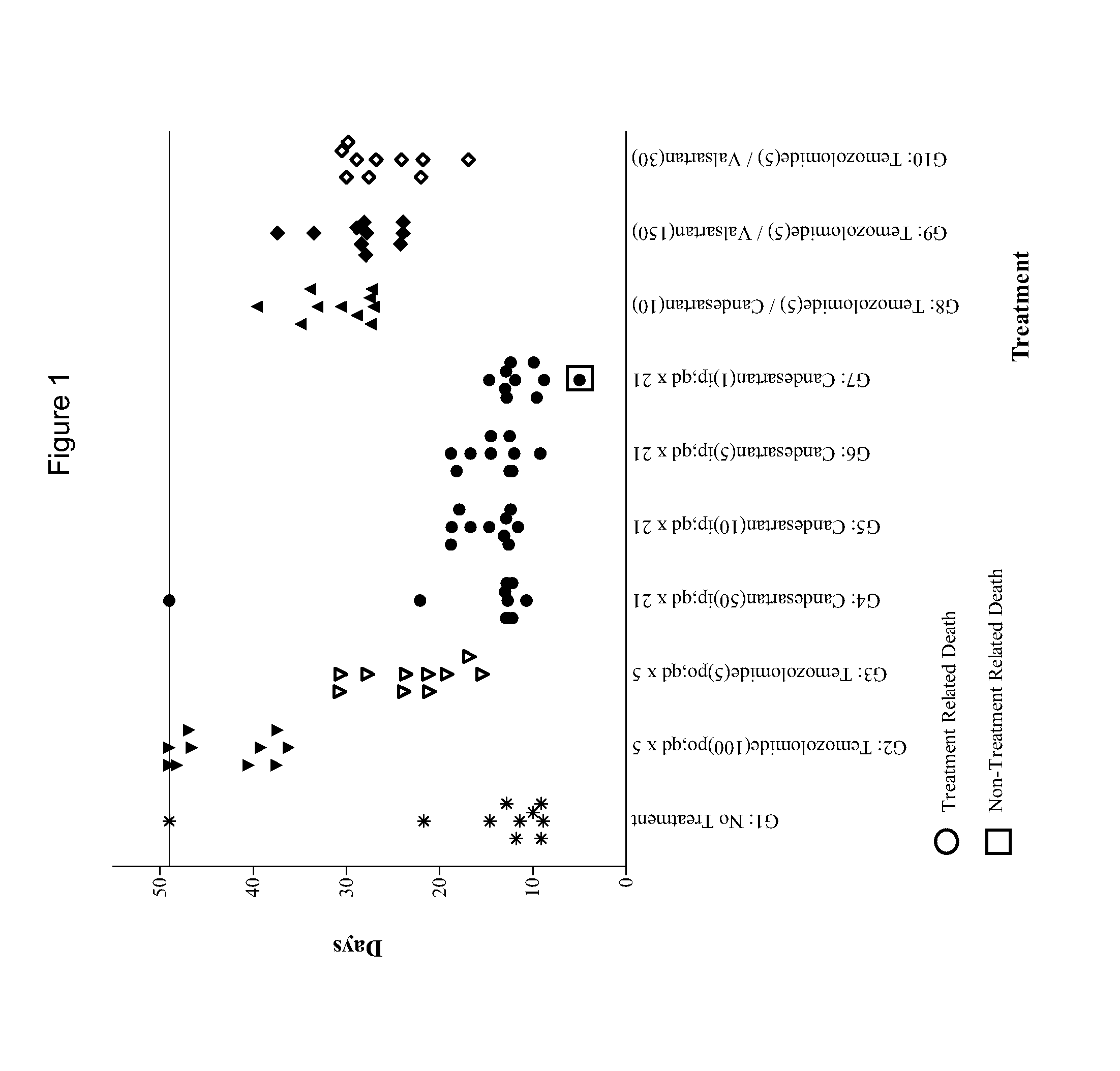
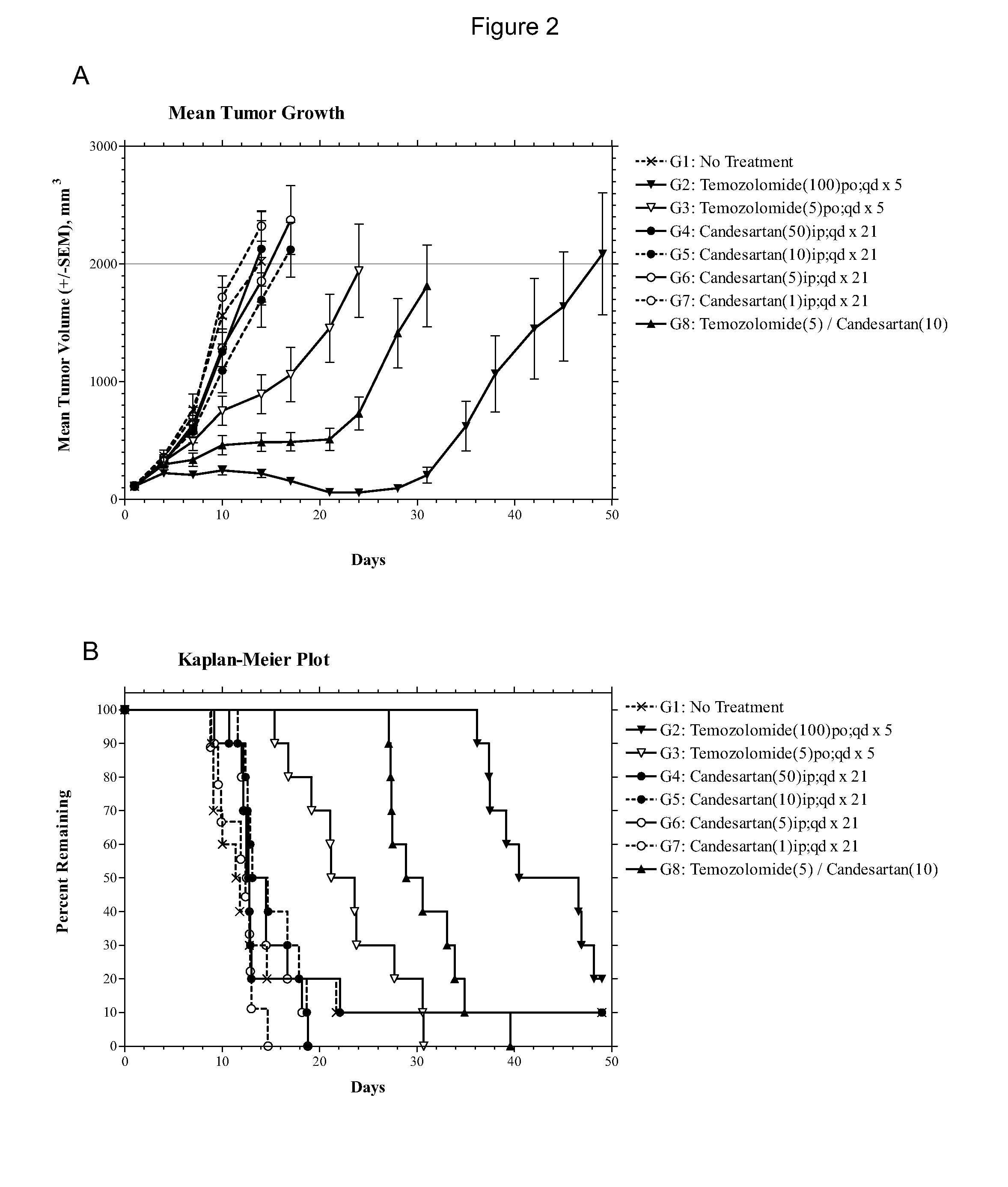
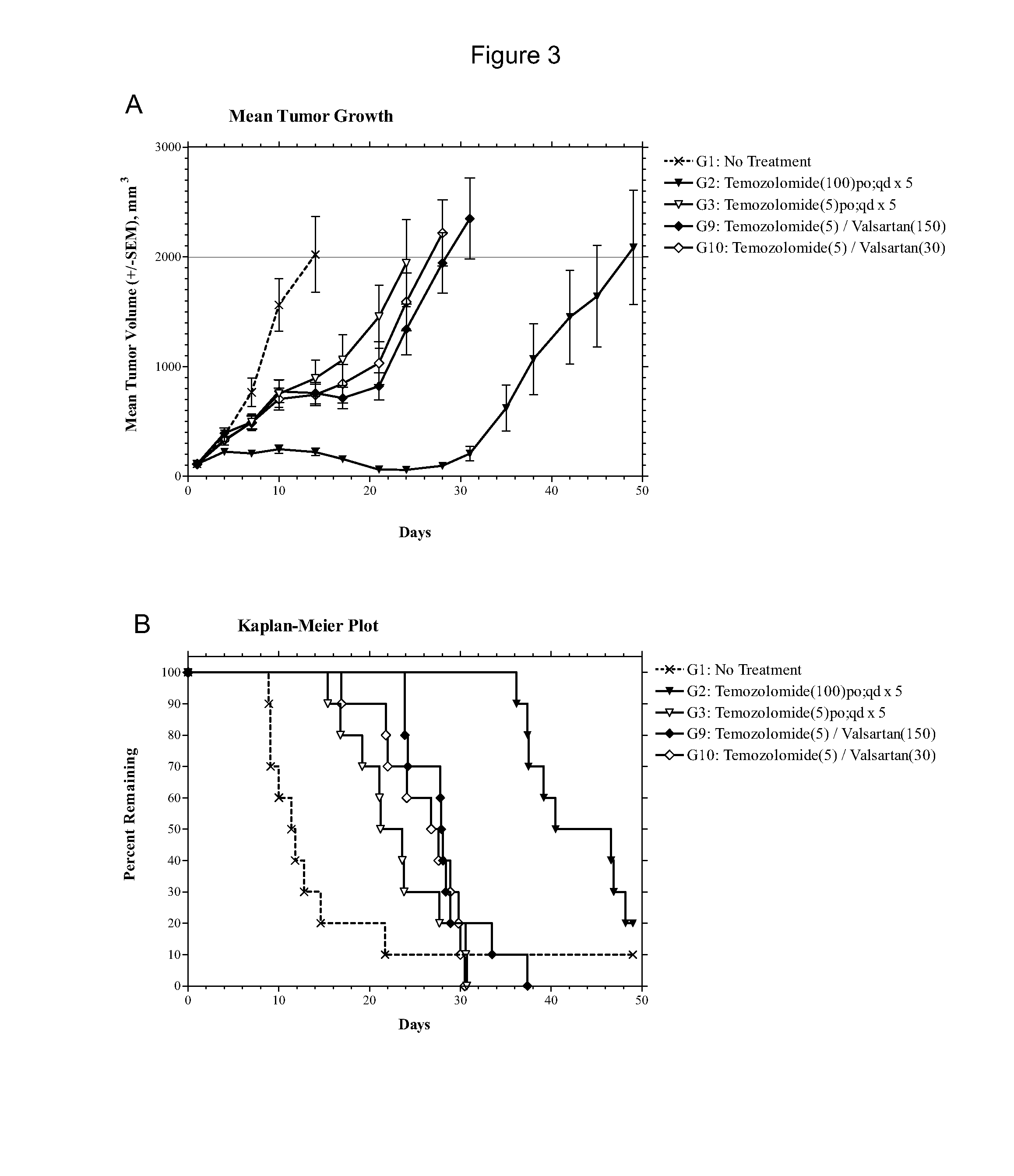
Compositions and methods of treating gliomas
The present invention provides, inter alia, methods for treating or ameliorating the effects of a glioma. Methods of this invention include administering to a subject in need thereof an effective amount of a first active agent, such as e.g., an angiotensin receptor blocker, an antifungal agent, a bisphosphonate, an oxytocin inhibitor, an interleukin-1 (IL-1) inhibitor, a cyclooxygenase inhibitor, an α2δ voltage-dependent calcium channel (VDCC) inhibitor, a dihydroorotate dehydrogenase inhibitor, a calcium channel blocker, a renal sodium-chloride symporter inhibitor, an a2 adrenergic agonist, a phenothiazine antipsychotic, a calcineurin inhibitor, a 5-HT agonist, an angiotensin-converting enzyme (ACE) inhibitor, a direct rennin inhibitor, or combinations thereof, and a second active agent, which is a chemotherapeutic agent. Compositions for treating or ameliorating the effects of a glioma are also provided.
Owner:BIOMED VALLEY DISCOVERIES INC
Small molecule inhibitors of plasmodium falciparum dihydroorotate dehydrogenase
ActiveUS20110130381A1Strong specificityEffective inhibitorBiocideOrganic chemistryANTU toxicityCytotoxicity
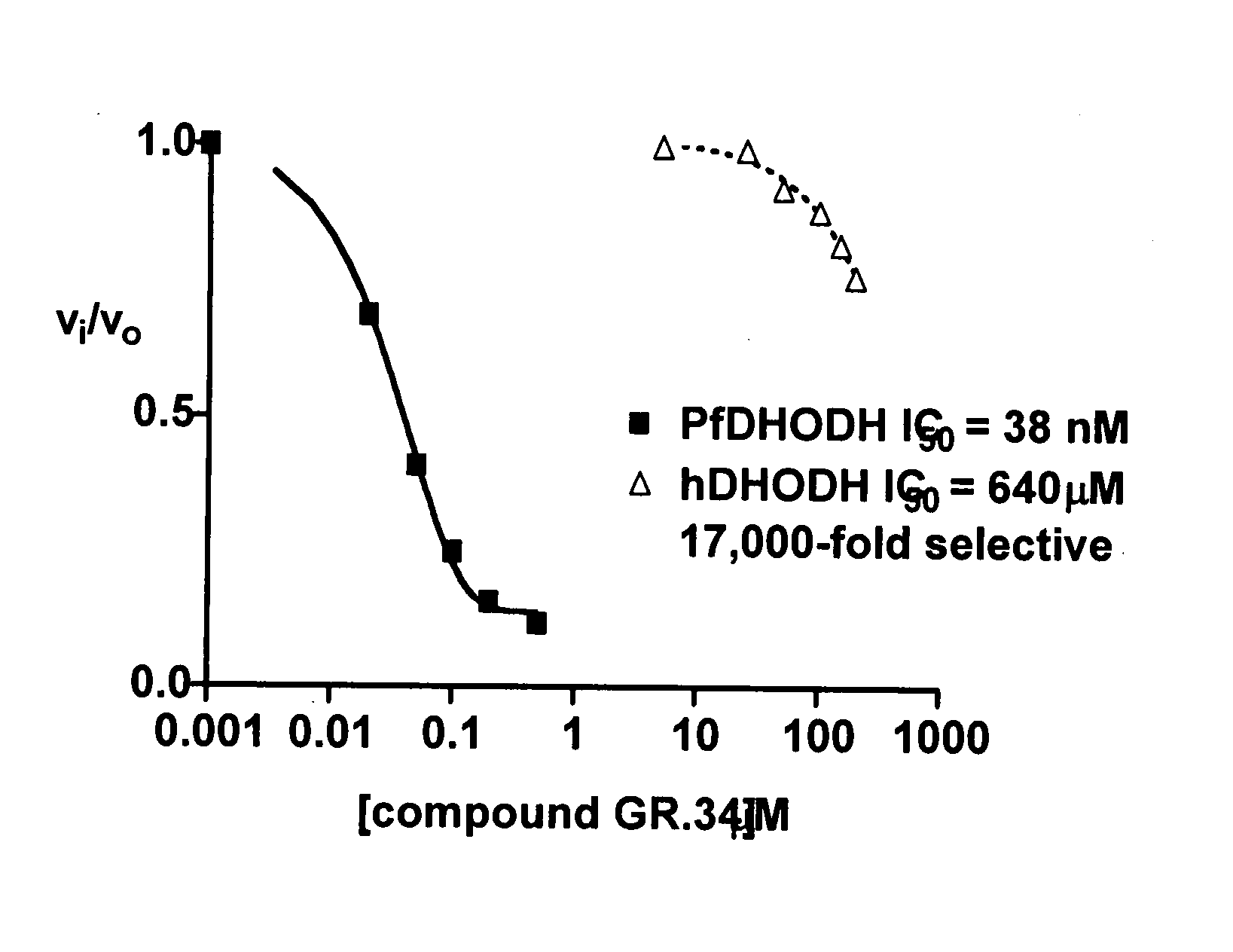
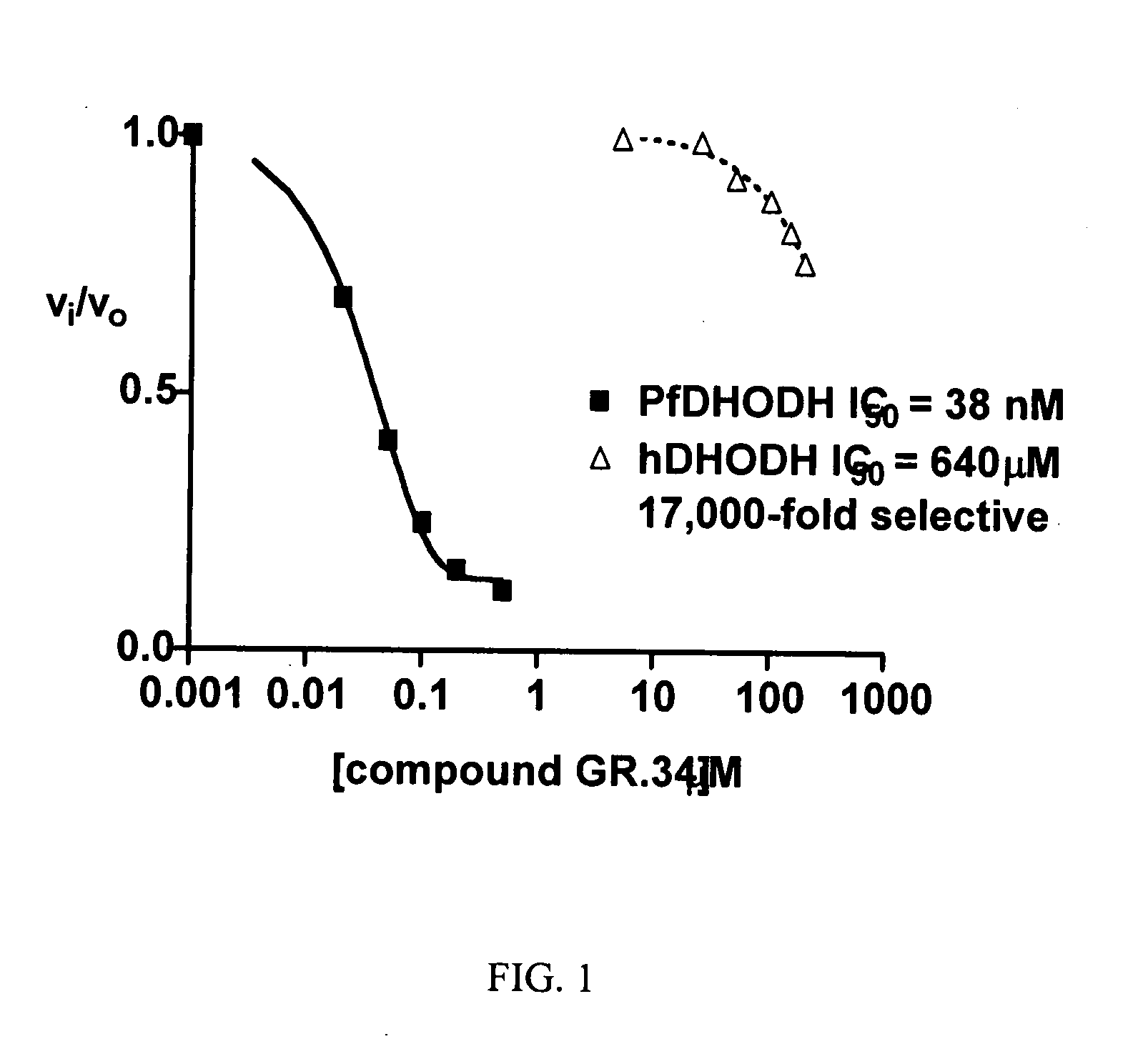
Inhibitors of dihydroorotate dehydrogenase (DHODH) for the Plasmodium enzyme have been identified and characterized. The inhibitors have high specificity, submicromolar efficacy against cultured parasite strains, exhibit drug-like properties, and are not overtly cytotoxic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
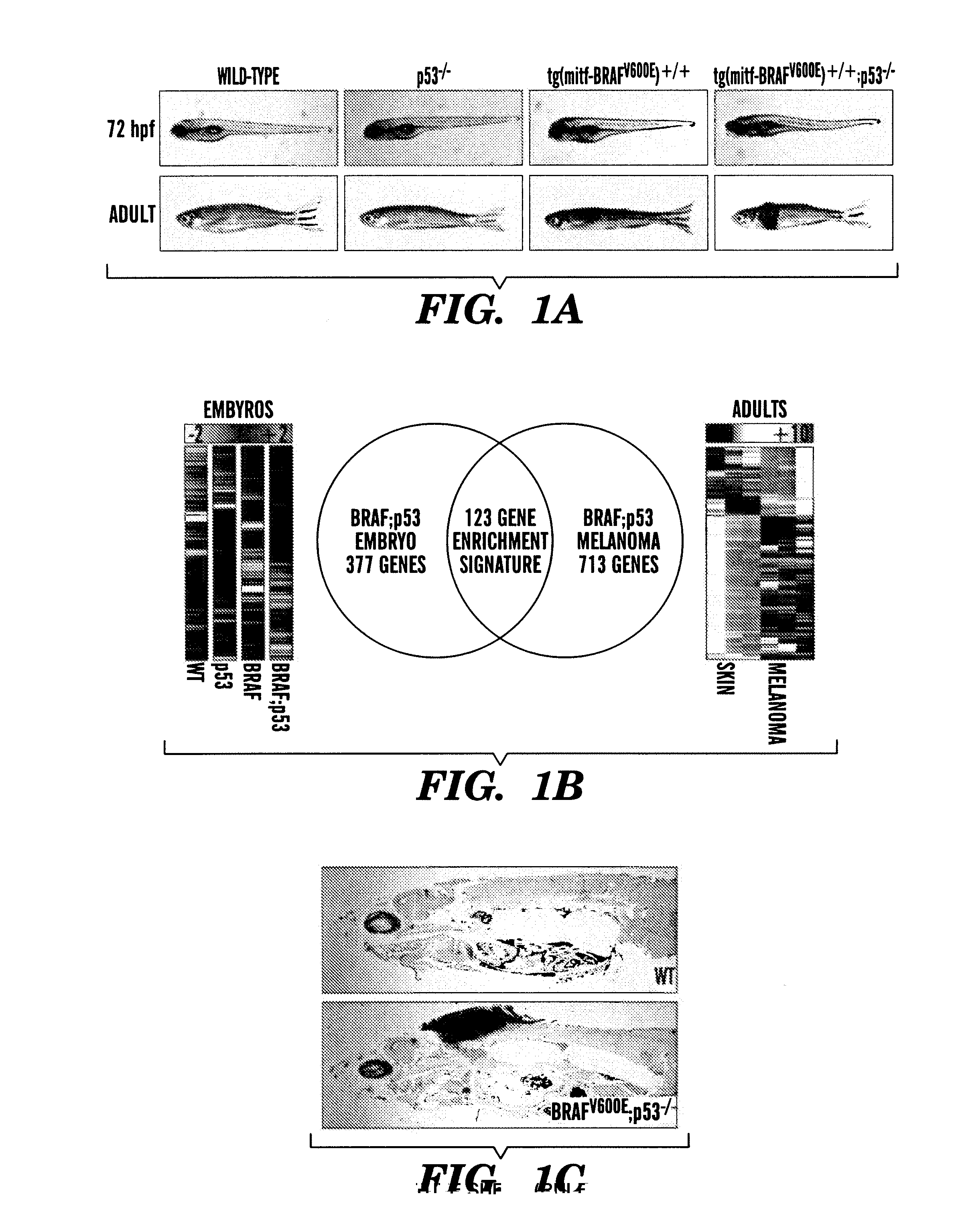
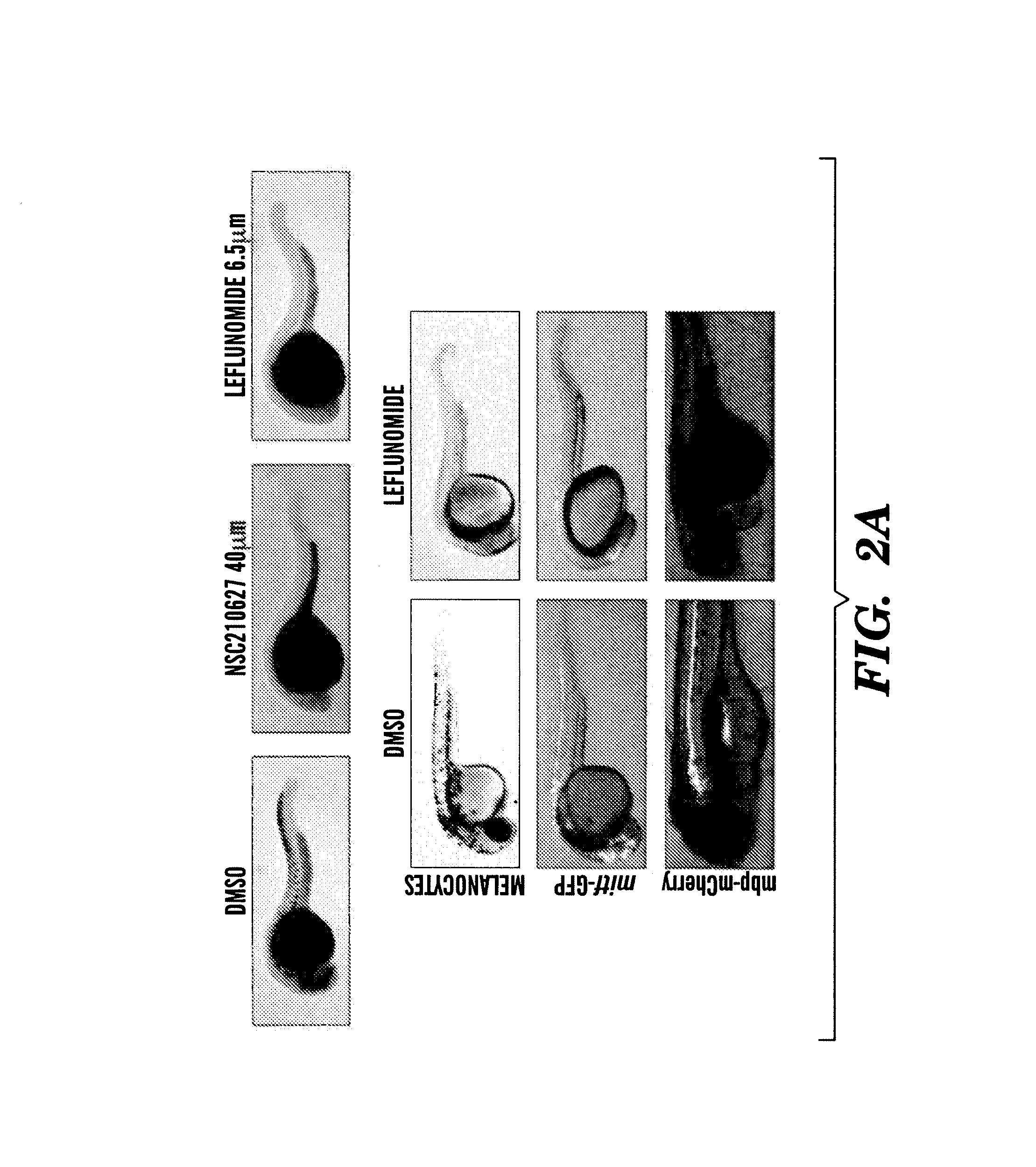
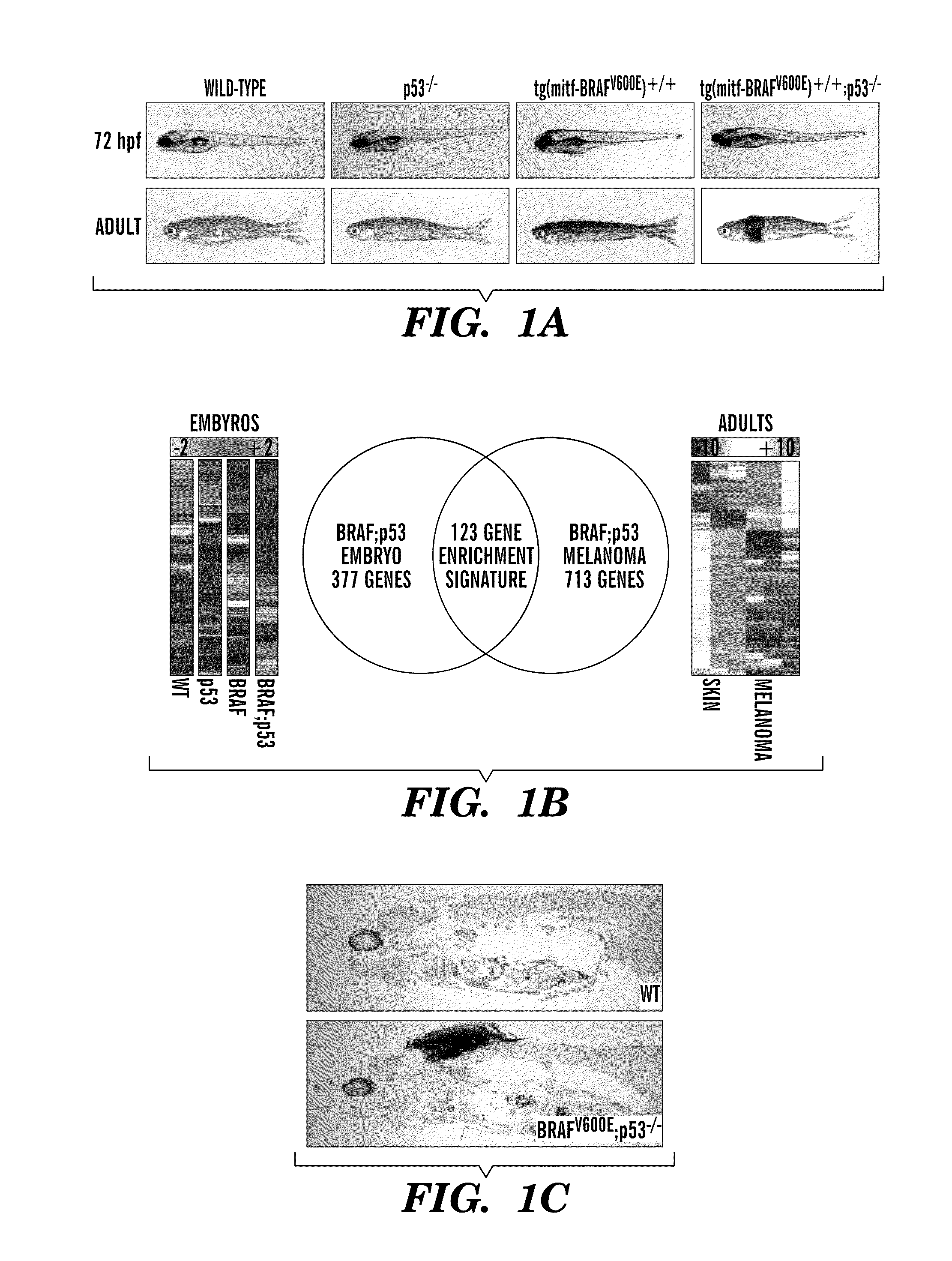
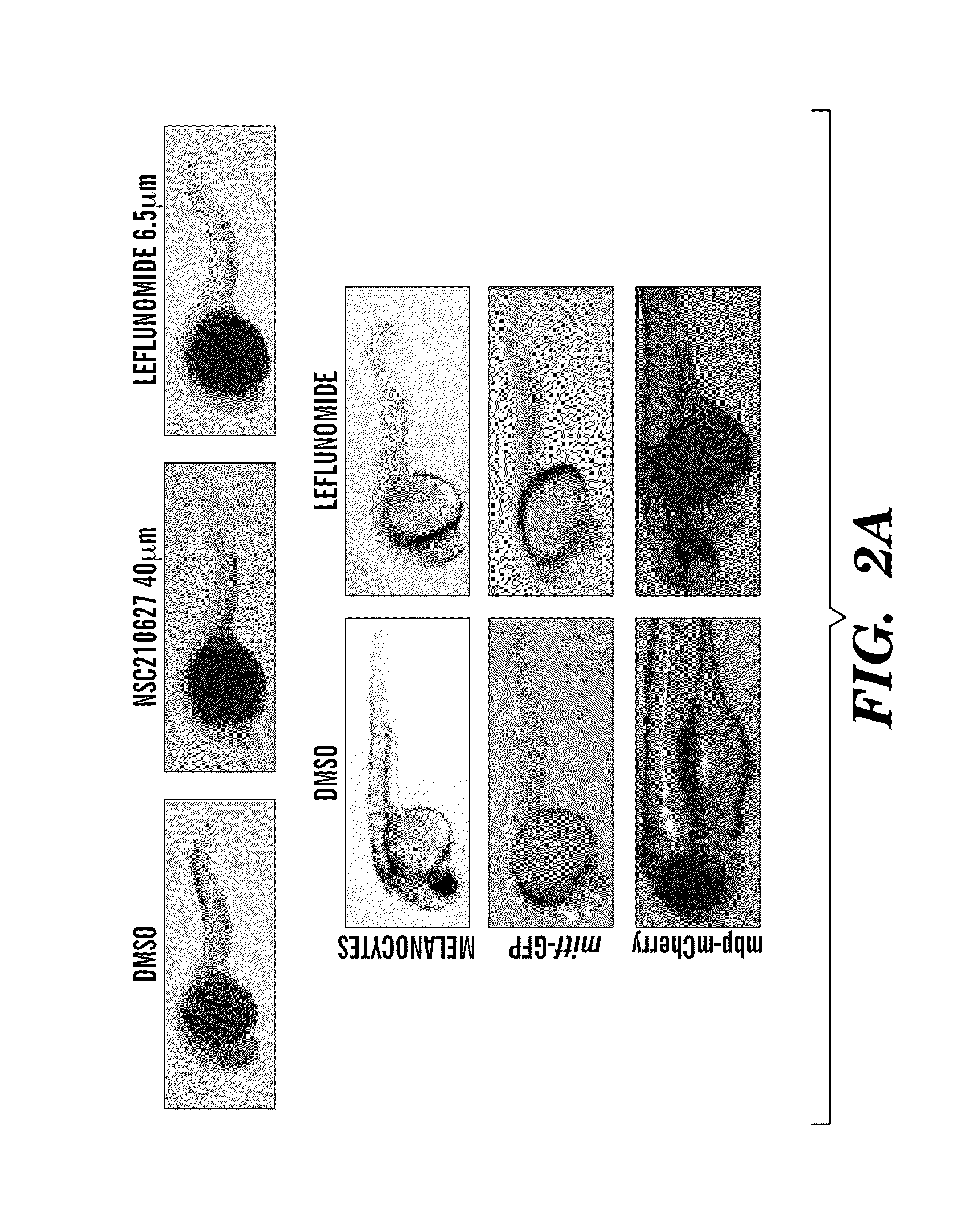
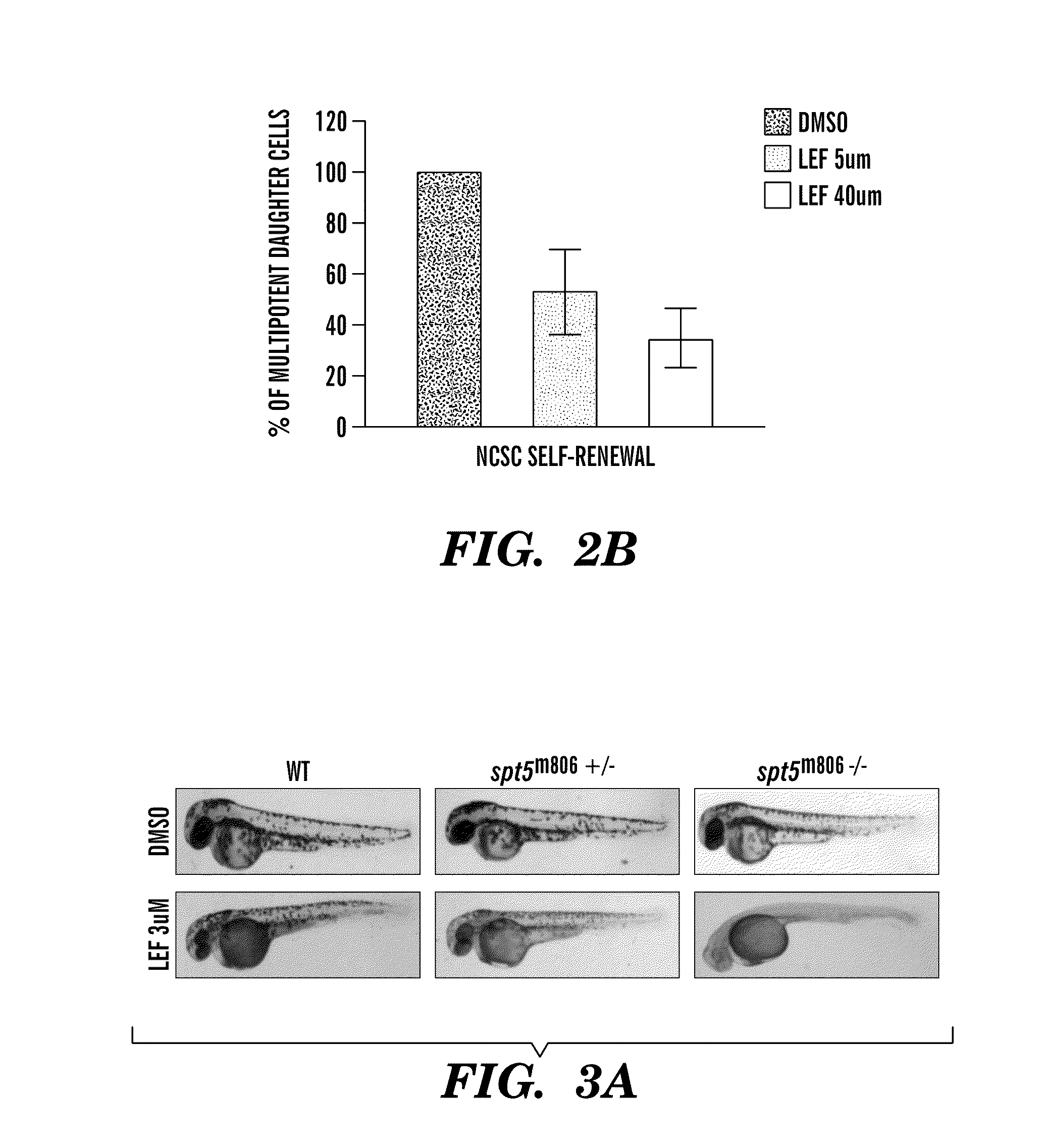
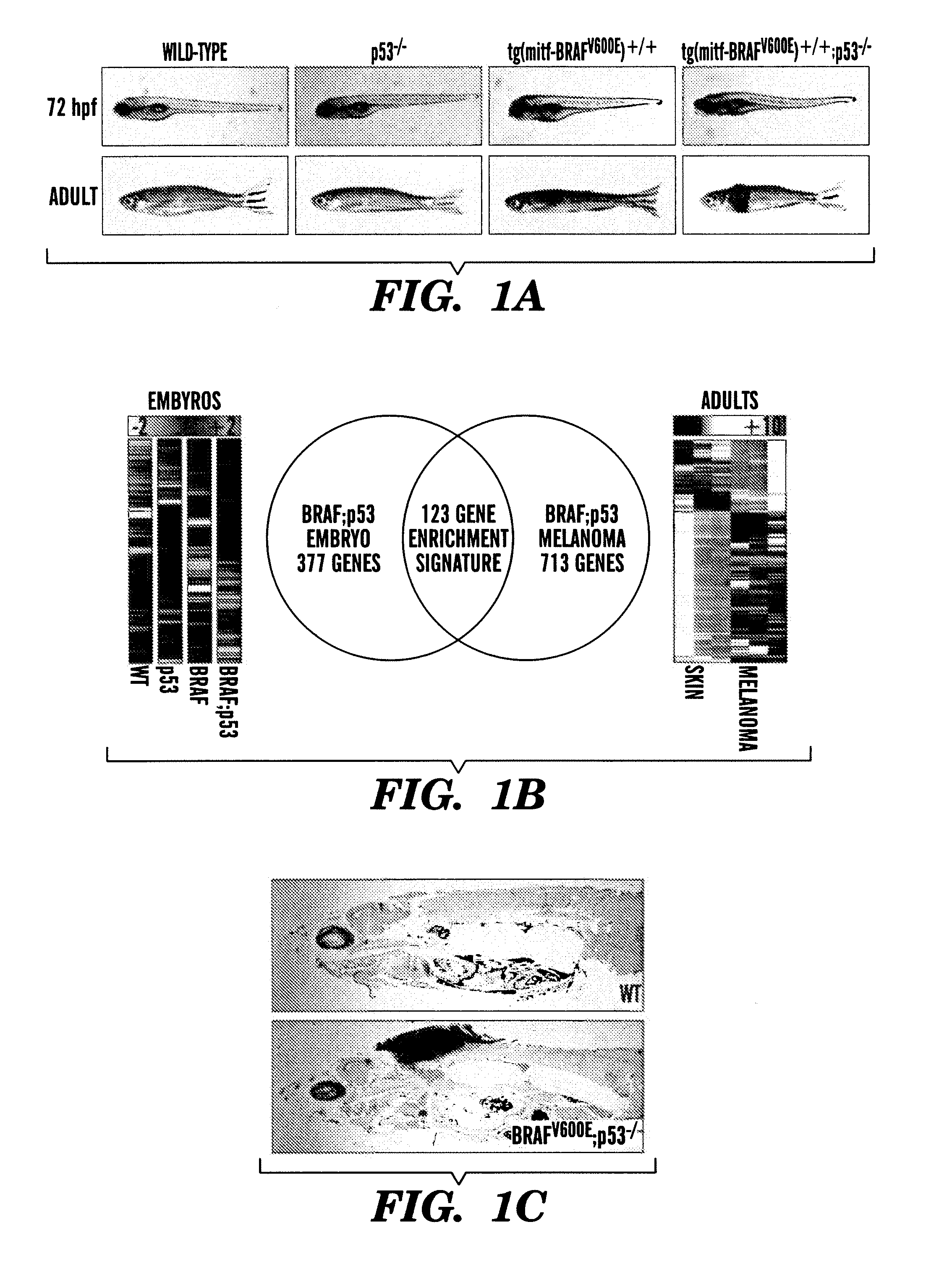
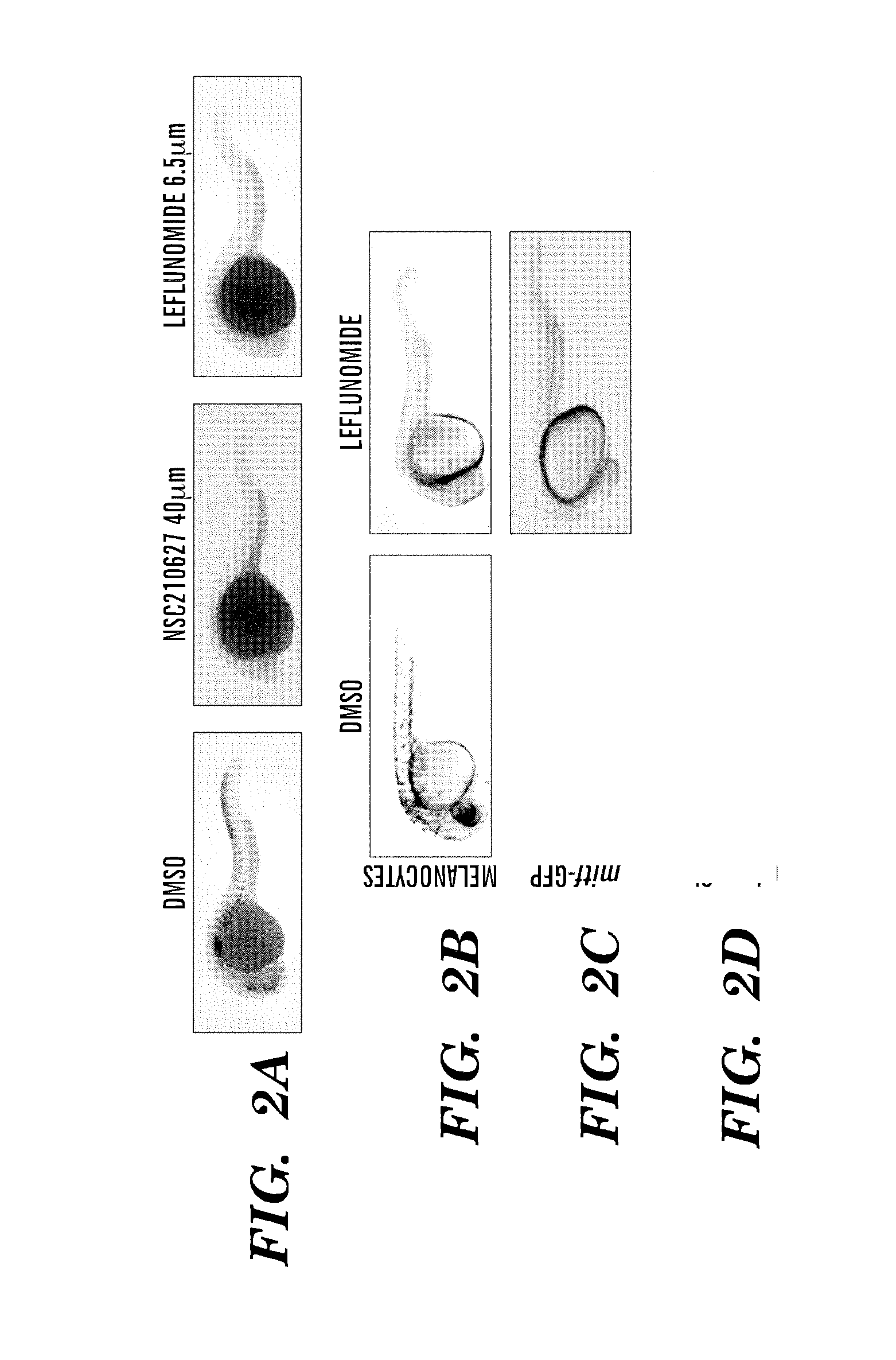
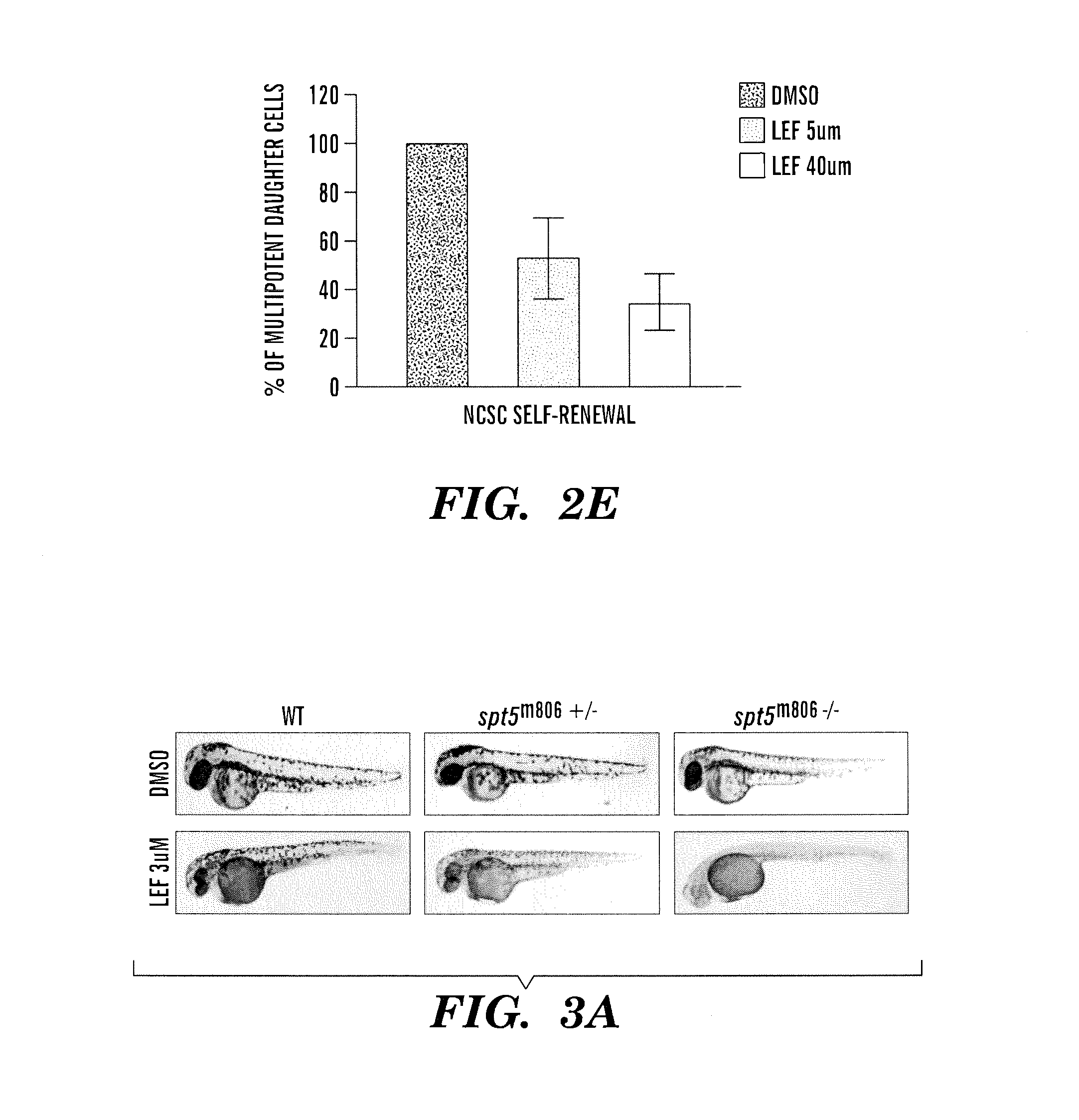
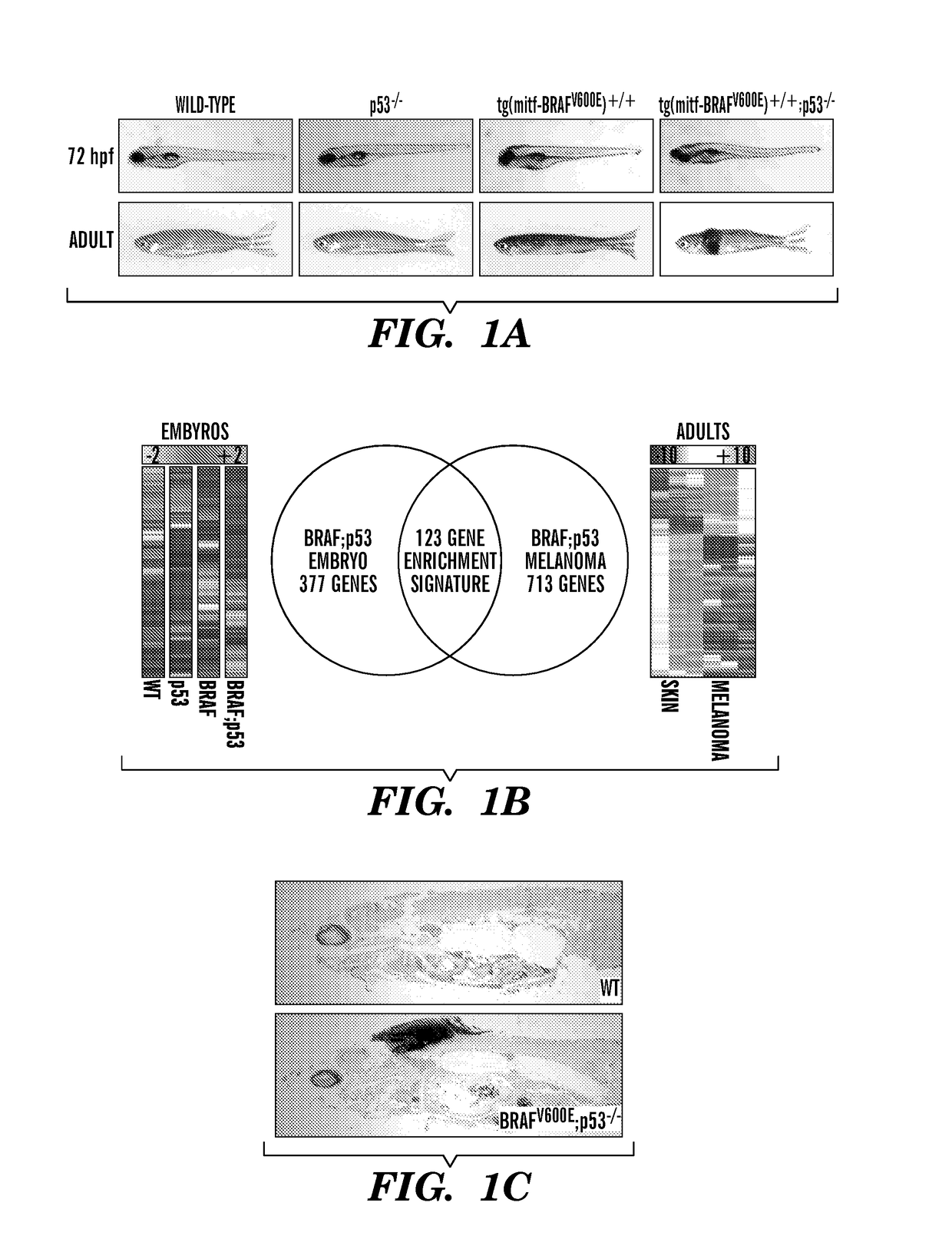
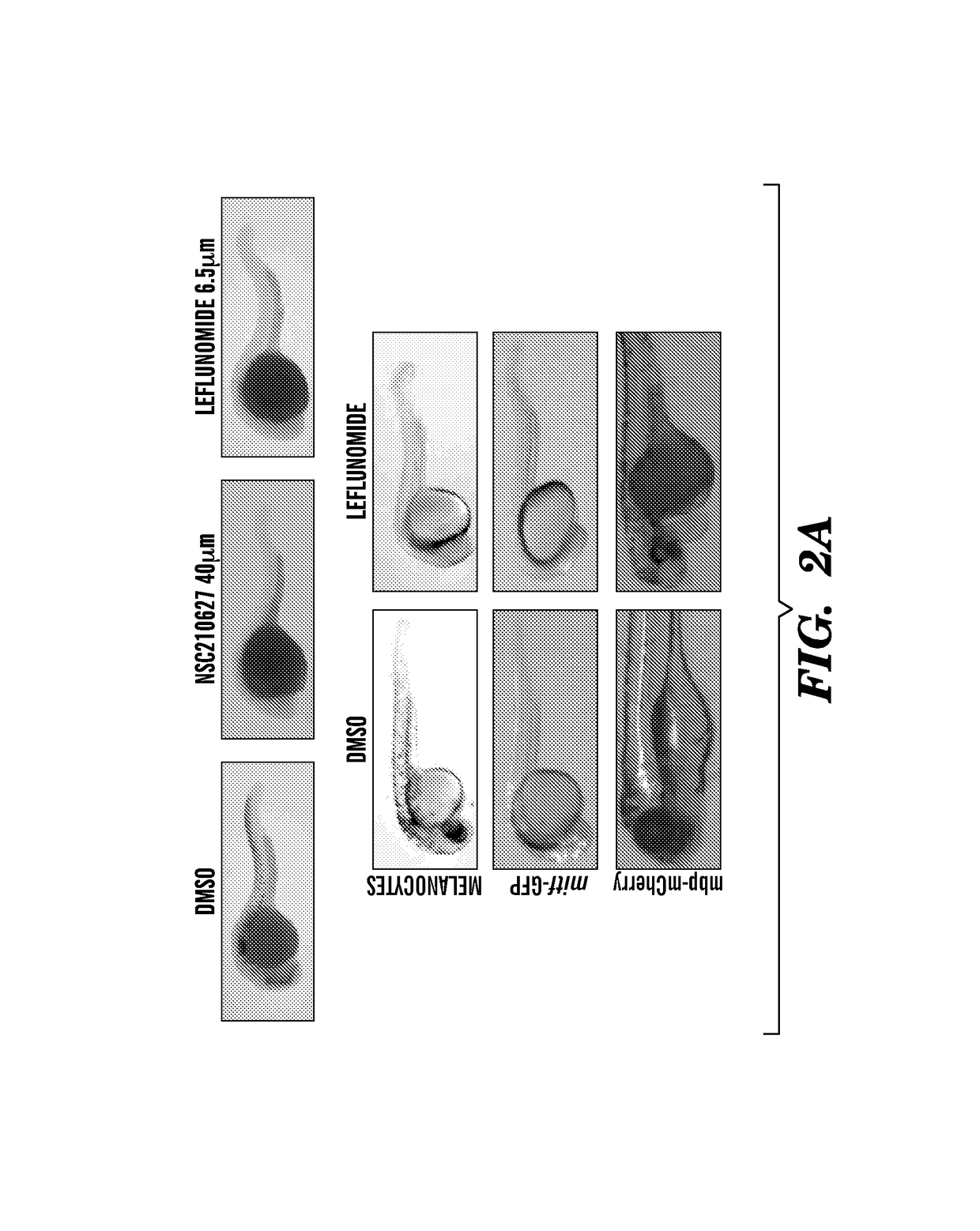
Methods for treatment of melanoma
InactiveUS20140031383A1Utility in treatmentUseful in treatmentBiocideMicrobiological testing/measurementProgenitorNeural crest
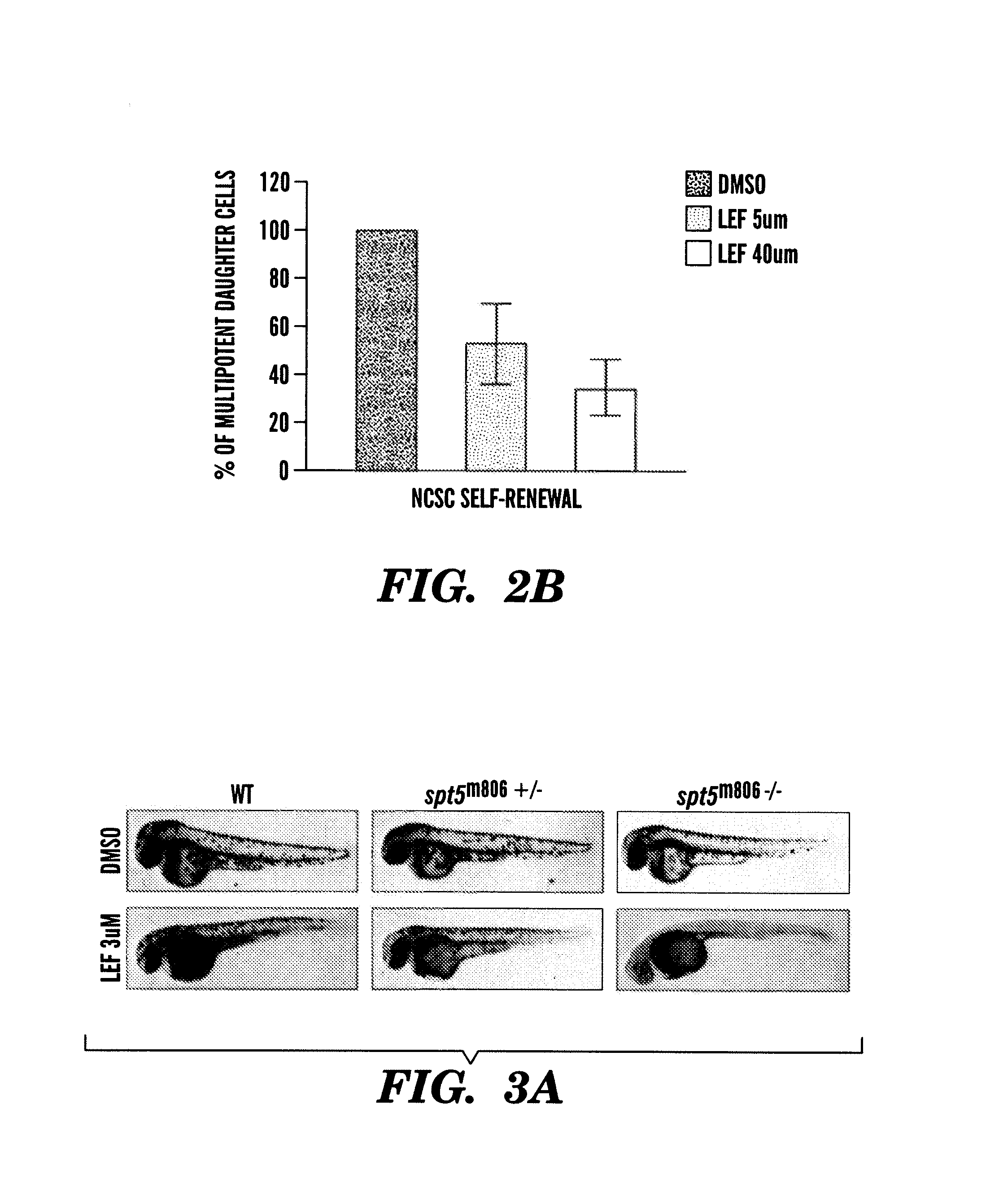
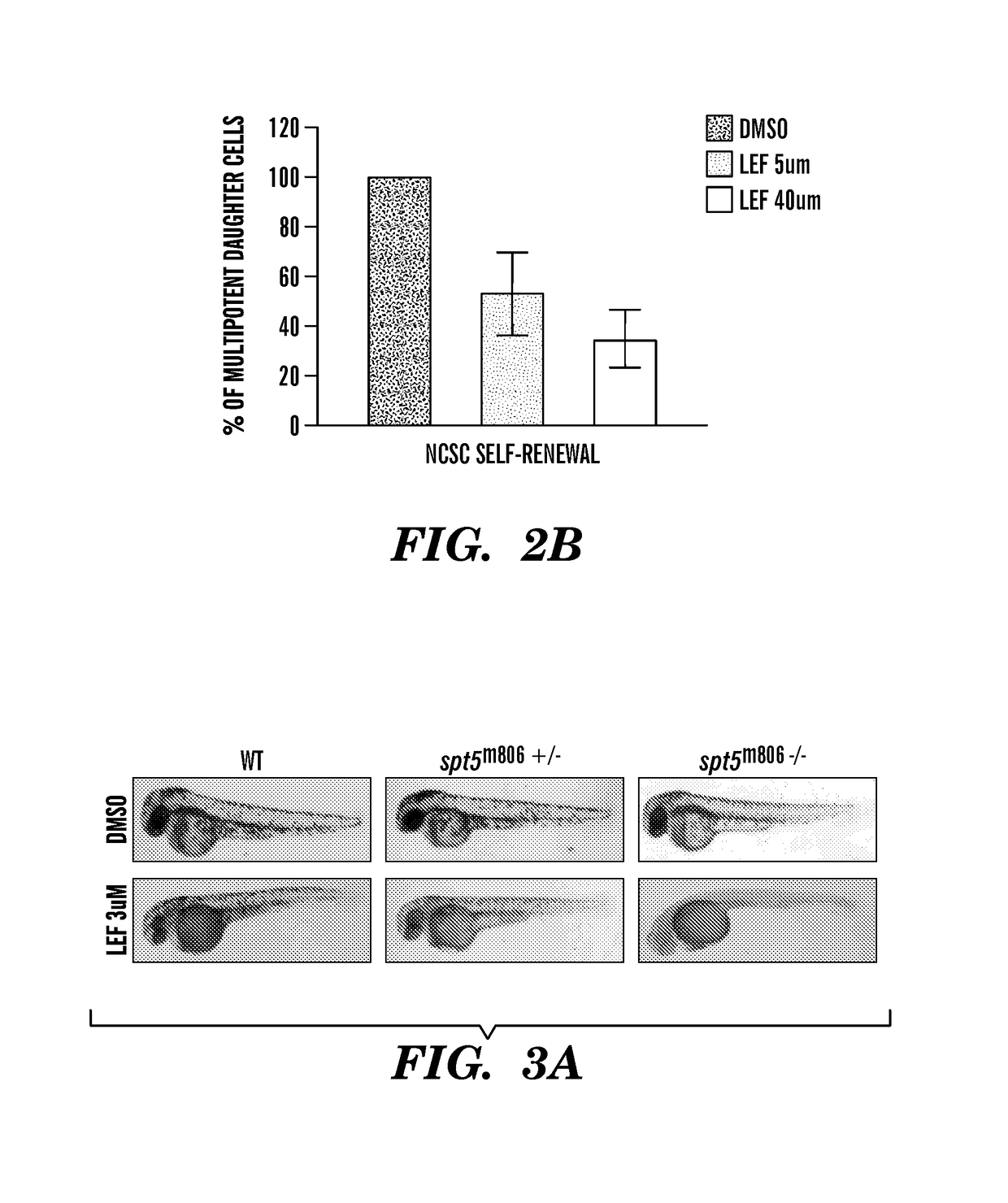
The present invention is directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods herein are directed to screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
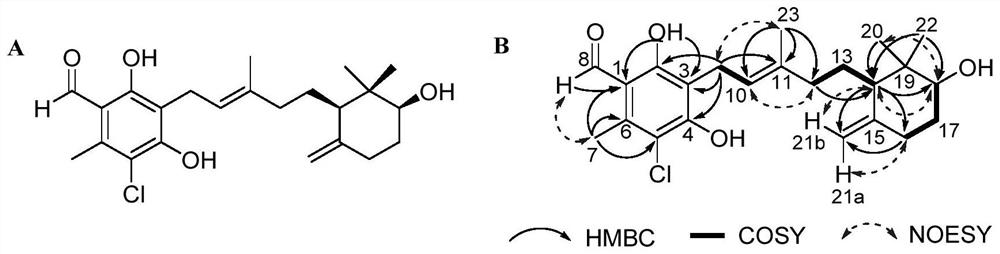
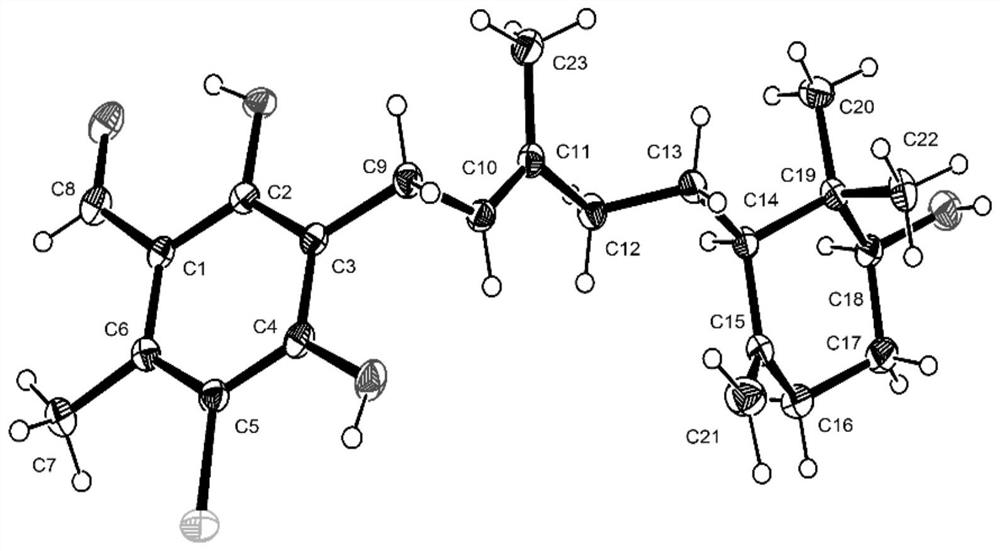
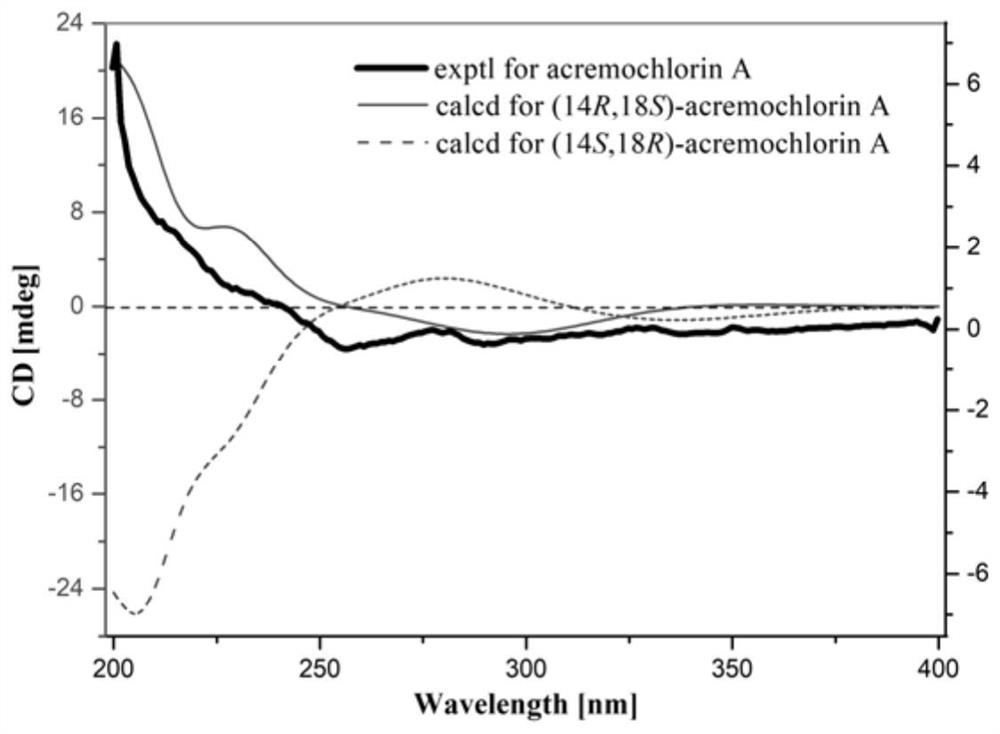
Ascochlorin compound and application thereof in preparation of antitumor drugs or dihydroorotate dehydrogenase inhibitor drugs
ActiveCN111943828AGrowth inhibitionHigh activityFungiOrganic chemistryAscochlorinDihydroorotate Dehydrogenase Inhibitor
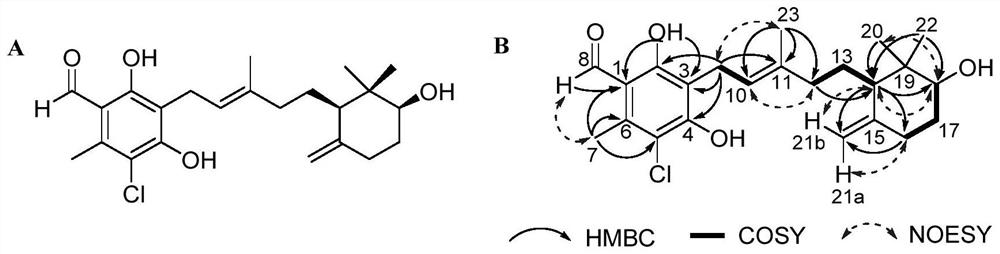
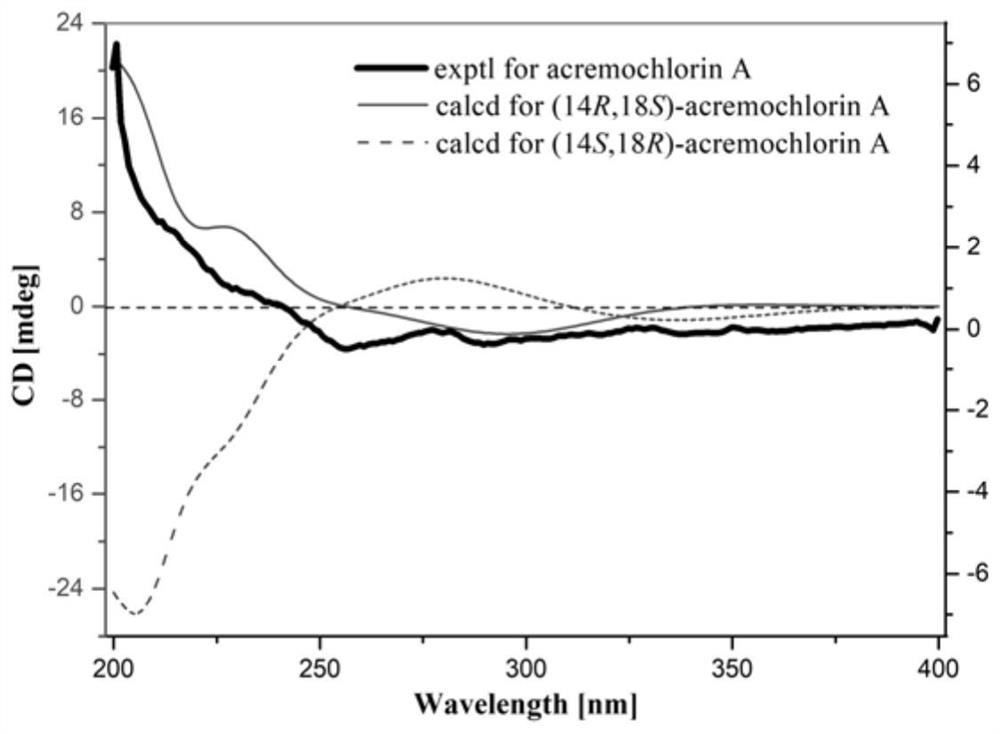
The invention provides an ascochlorin compound and application thereof in preparation of antitumor drugs or dihydroorotate dehydrogenase inhibitor drugs, and relates to the field of marine natural products. The structural formula of the disclosed ascochlorin compound is shown as a formula (I), a cyclohexanol fragment of the ascochlorin compound has rare gem-dimethyl and exocyclic double bonds, andthe compound has broad-spectrum remarkable tumor cell proliferation inhibition activity and dihydroorotate dehydrogenase inhibition activity, and therefore, the compound is an ideal candidate compound developed into an antitumor drug or a dihydroorotate dehydrogenase inhibitor drug which is novel in structure and high in activity.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Methods for treatment of melanoma
ActiveUS20150306080A1Utility in treatmentUseful in treatmentBiocidePeptide/protein ingredientsProgenitorNeural crest
Embodiments of the present invention are directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods comprise screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
Dihydroorotate dehydrogenase inhibitors
Owner:JANSSEN BIOTECH INC
Dihydroorotate dehydrogenase inhibitors
Owner:JANSSEN BIOTECH INC
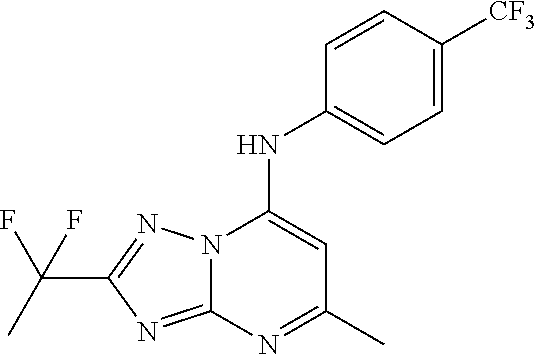
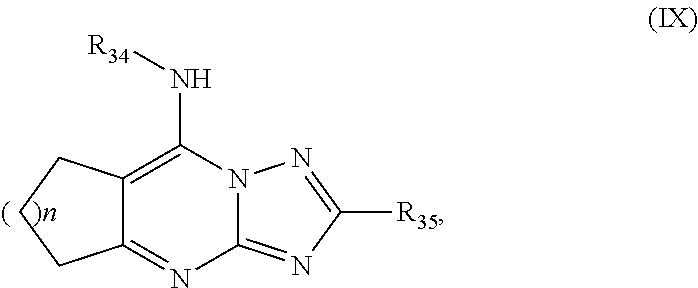
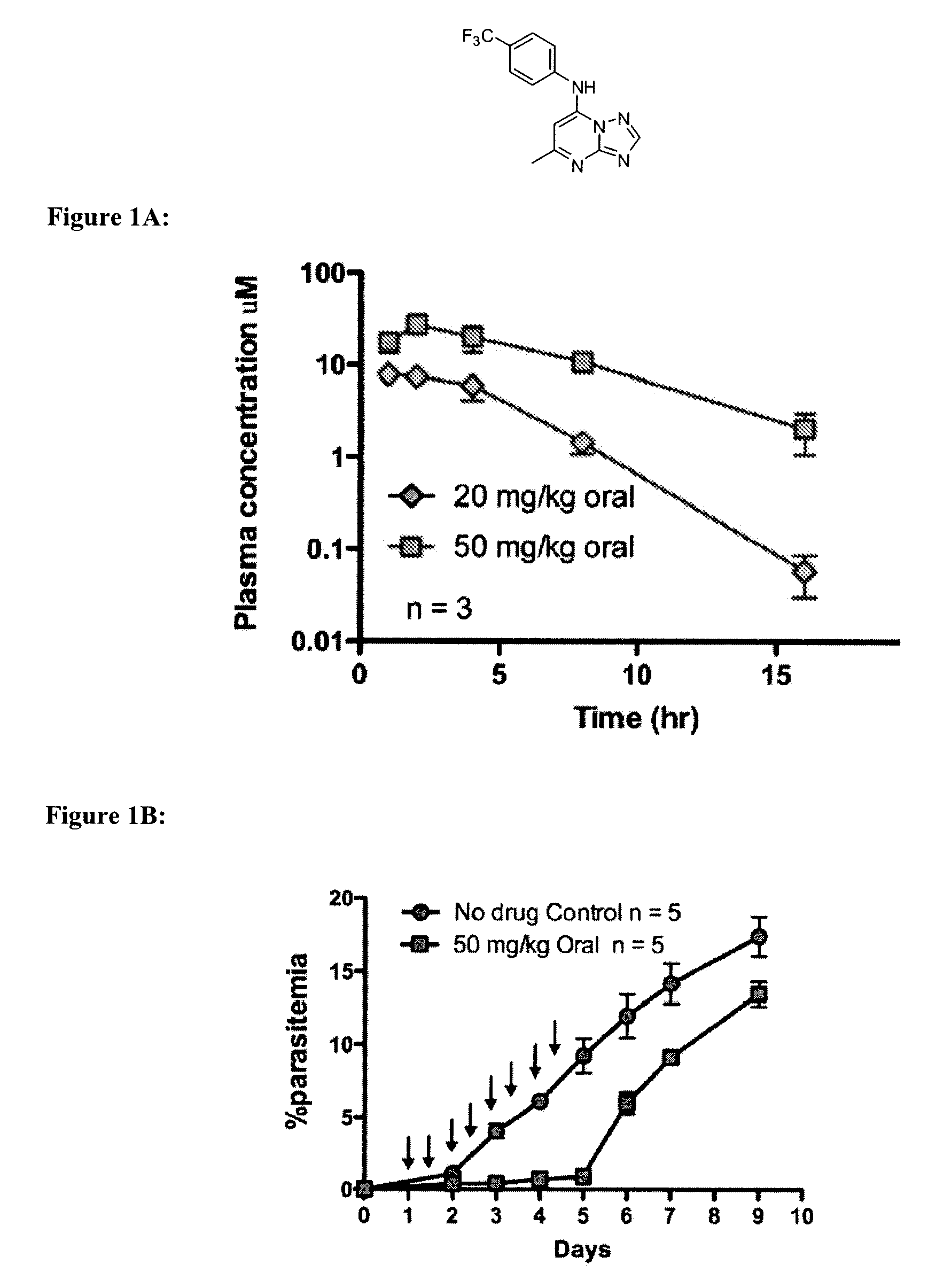
Antimalarial agents that are inhibitors of dihydroorotate dehydrogenase
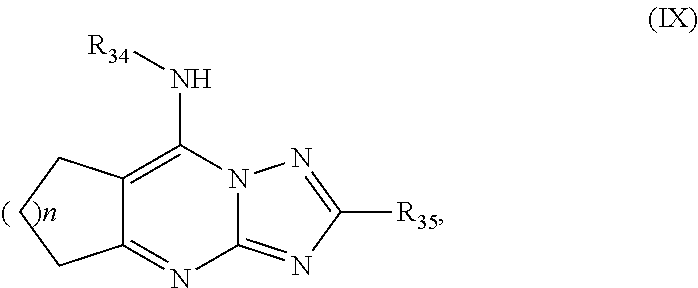
Inhibitors of parasitic dihydroorotate dehydrogenase enzyme (DHOD) are candidate therapeutics for treating malaria. Illustrative of such therapeutic agents include the compound:and a triazolopyrimidine class of compounds that conform to Formula IX:and their solvates, stereoisomers, tautomers and pharmaceutically acceptable salts.
Owner:MMV MEDICINES FOR MALARIA VENTURE +2
Immunomodulator and anti-inflammatory compounds
ActiveUS8686048B2Enhanced inhibitory effectBiocideSenses disorderMedicineDihydroorotate Dehydrogenase Inhibitor
The present invention provides dihydroorotate dehydrogenase inhibitors, methods of preparing them, pharmaceutical compositions containing them and methods of treatment, prevention and / or amelioration of diseases or disorders wherein the inhibition of Dihydroorotate dehydrogenase is known to show beneficial effect.
Owner:RHIZEN PHARMACEUTICALS AG +1
Methods for treatment of melanoma
InactiveUS20150328204A1Reduce the overall heightBiocidePeptide/protein ingredientsProgenitorMedicine
Embodiments of the present invention are directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods comprise screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
Methods for treatment of melanoma
ActiveUS10016402B2Reduce the overall heightHeterocyclic compound active ingredientsAssayNeural crest
Owner:DANA FARBER CANCER INST INC +1
Methods for treating pten-mutant tumors
InactiveUS20190192501A1Microbiological testing/measurementDisease diagnosisDihydroorotate Dehydrogenase InhibitorCancer therapy
Owner:MT SINAI SCHOOL OF MEDICINE
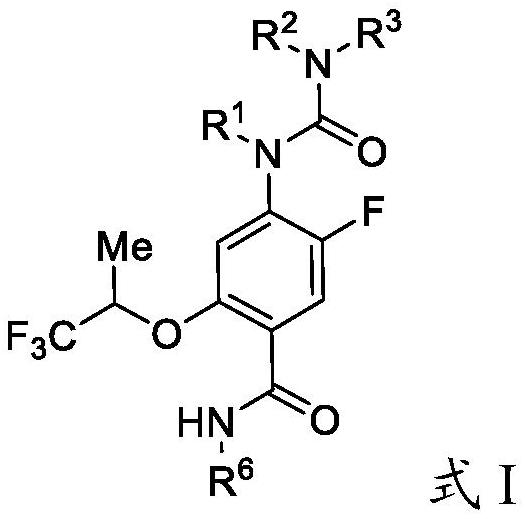
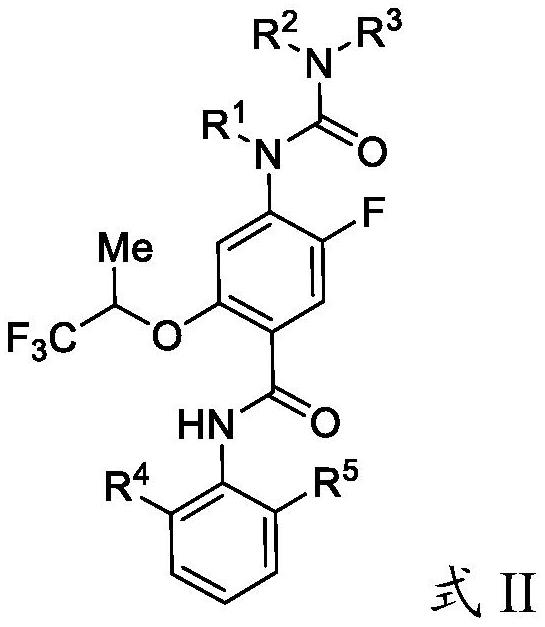
Substituted urea dihydroorotate dehydrogenase inhibitors
Owner:JANSSEN BIOTECH INC
Methods for treating pten-mutant tumors
InactiveUS20220211690A1Microbiological testing/measurementDisease diagnosisEfficacyDihydroorotate Dehydrogenase Inhibitor
Owner:MT SINAI SCHOOL OF MEDICINE
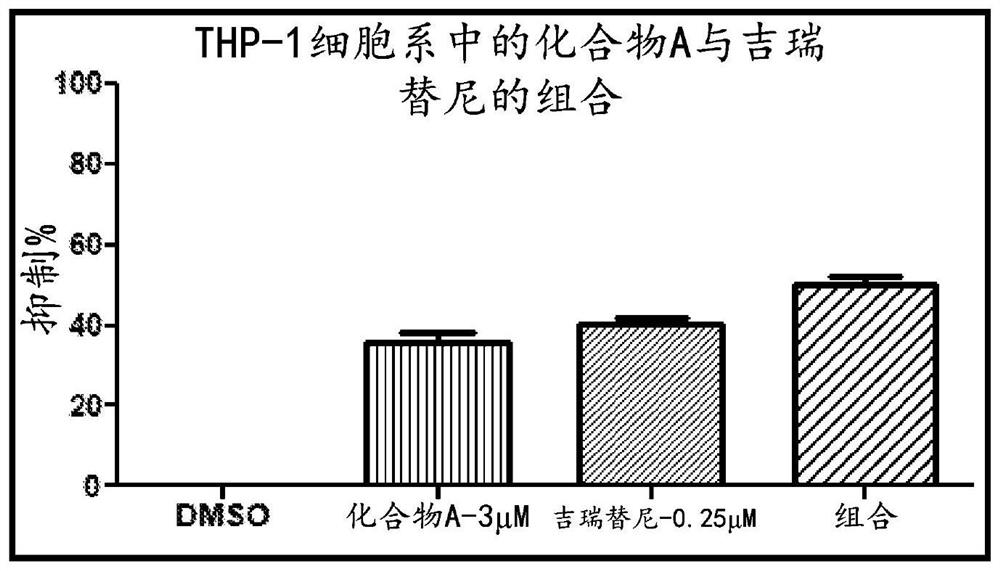
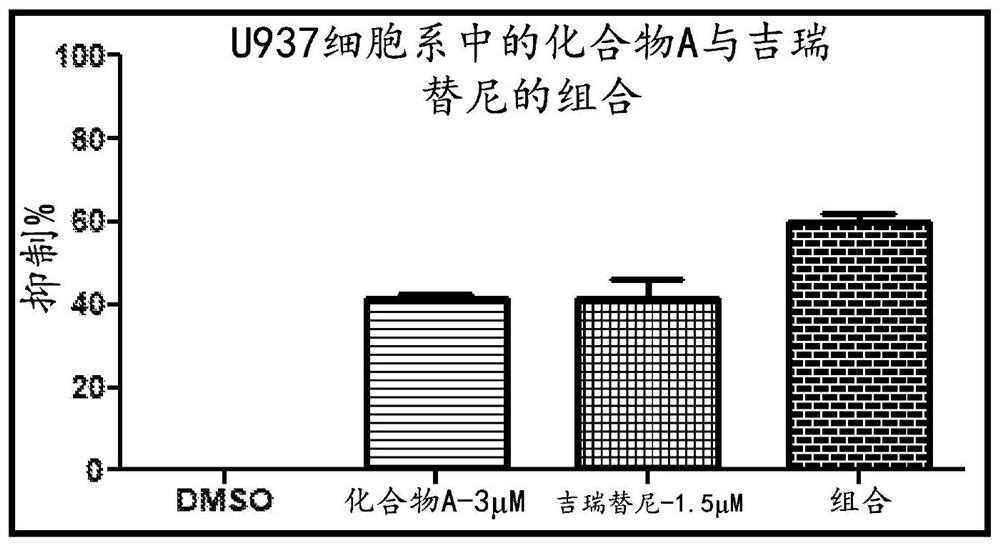
Compositions comprising DHODH inhibitors for treatment of acute myelogenous leukemia
PendingCN114828842AHigh activityEffective treatmentOrganic active ingredientsAntineoplastic agentsAcute myeloid leukemiasTyrosine-kinase inhibitor
The present invention relates to a method of treating acute myelogenous leukemia (AML). In one embodiment, the present invention relates to a method of treating acute myelogenous leukemia (AML), the method comprising administering a dihydroorotate dehydrogenase (DHODH) inhibitor, alone or in combination with at least one fms-like tyrosine kinase 3 (FLT-3) inhibitor and / or a DNA polymerase inhibitor, to a subject in need thereof.
Owner:RHIZEN PHARM SA
Composition for removing pluripotent stem cells and method of removing pluripotent stem cells
PendingUS20220364065A1EliminateEliminate undifferentiated pluripotent stem cellsMicrobiological testing/measurementCulture processDihydroorotate Dehydrogenase InhibitorBiochemistry
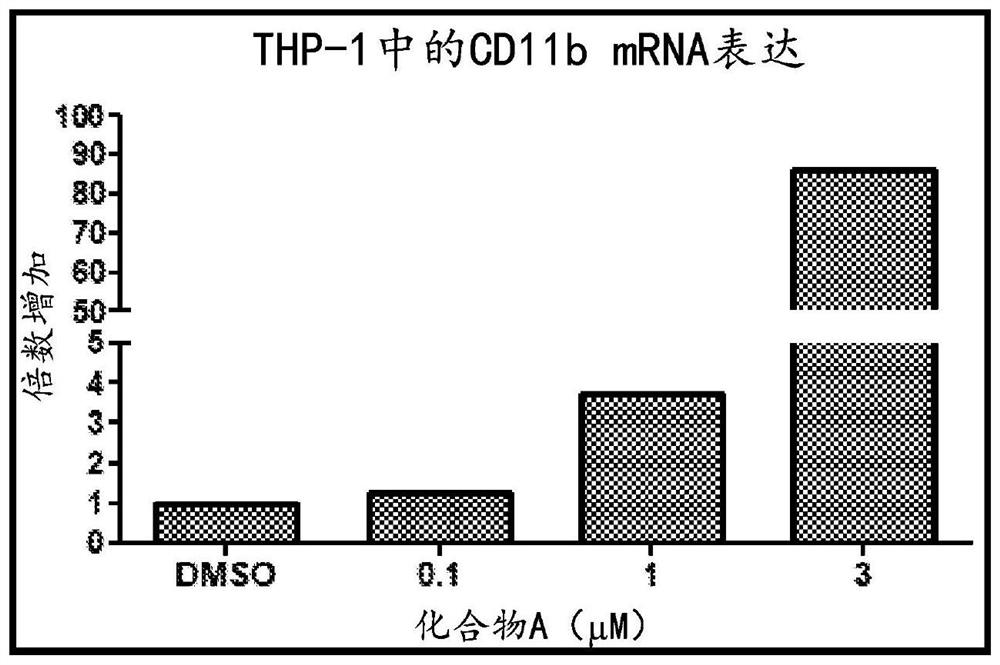
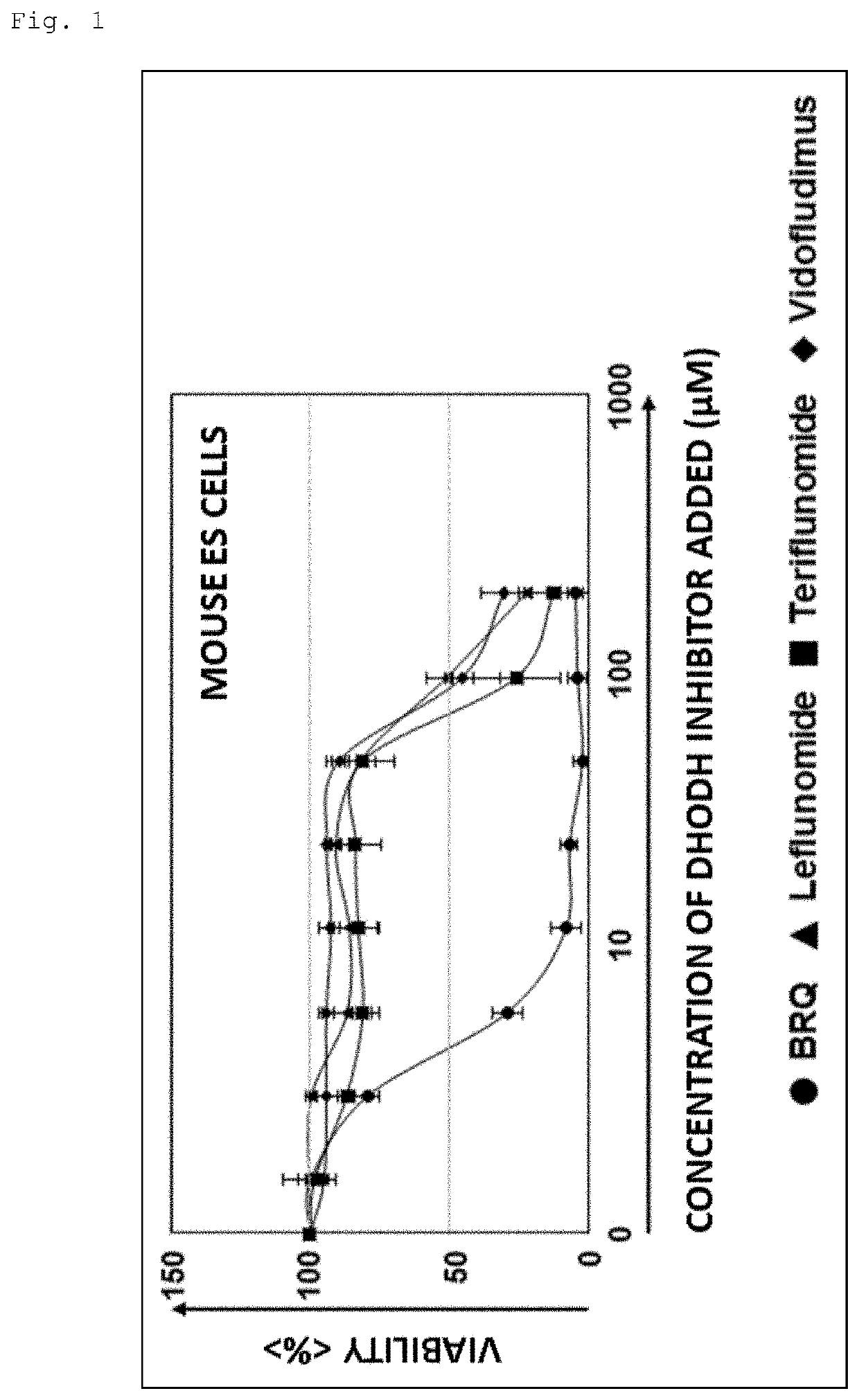
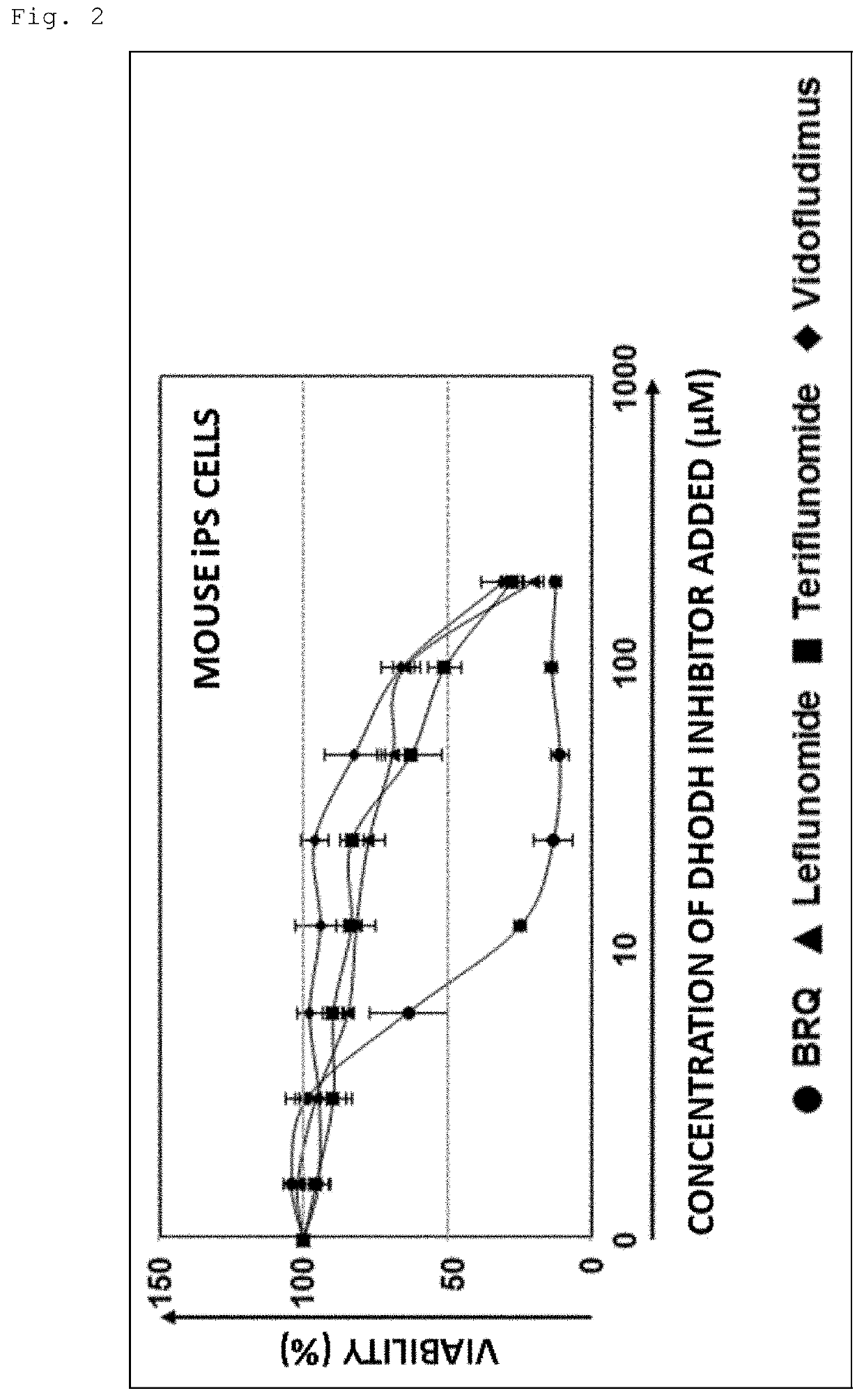
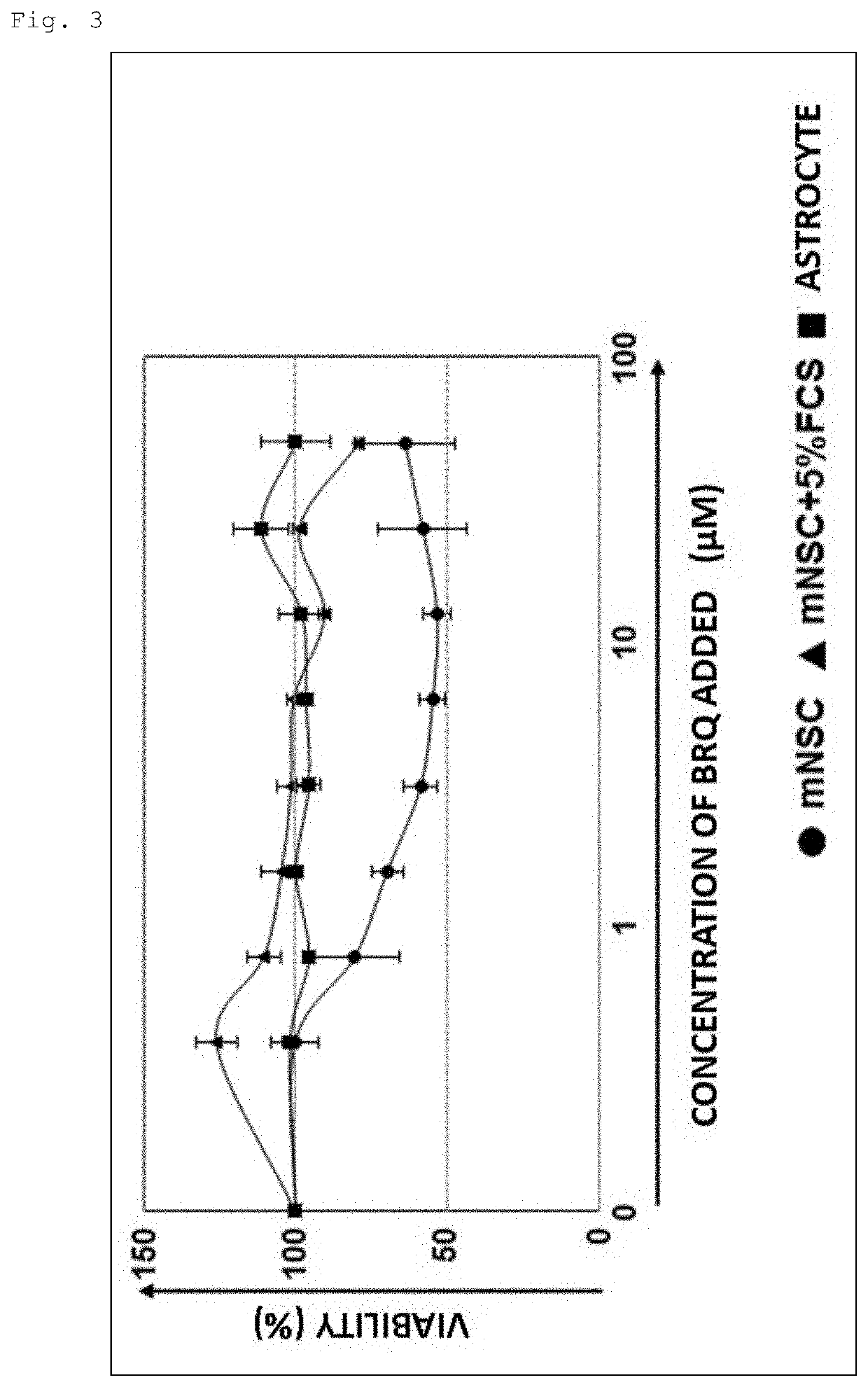
An object is to provide a composition and a method for eliminating undifferentiated pluripotent stem cells remaining in a cell group induced to differentiate from pluripotent stem cells. It has been found that while dihydroorotate dehydrogenase inhibitors exhibit cytotoxic activity against pluripotent stem cells, they do not exhibit significant cytotoxic activity against differentiated cells such as somatic stem cells.
Owner:HOKKAIDO UNIVERSITY
Antimalarial agents that are inhibitors of dihydroorotate dehydrogenase
Inhibitors of parasitic dihydroorotate dehydrogenase enzyme (DHOD) are candidate therapeutics for treating malaria. Illustrative of such therapeutic agents include the compound:and a triazolopyrimidine class of compounds that conform to Formula IX:and their solvates, stereoisomers, tautomers and pharmaceutically acceptable salts.
Owner:MMV MEDICINES FOR MALARIA VENTURE +2
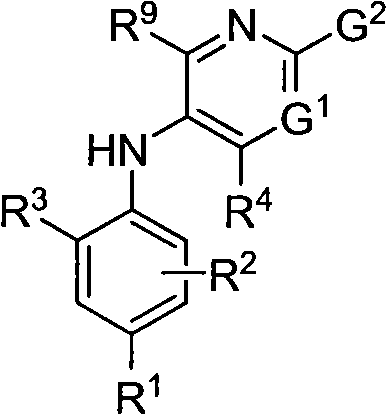
Combinations that include methotrexate and a dihydroorotate dehydrogenase inhibitor
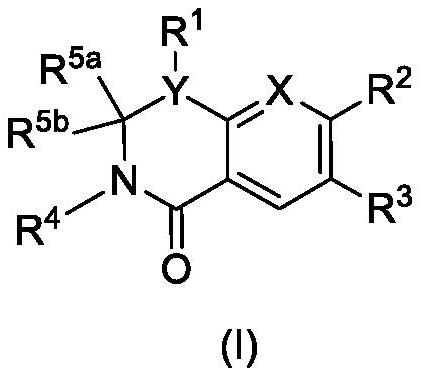
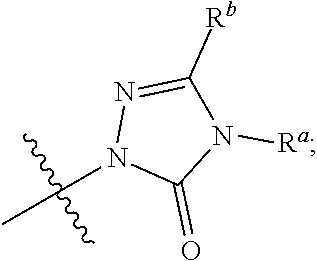
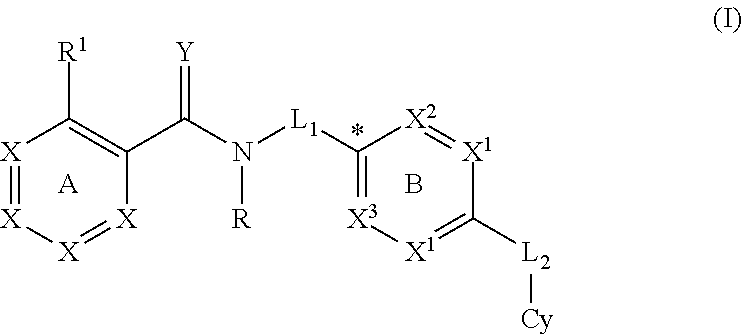
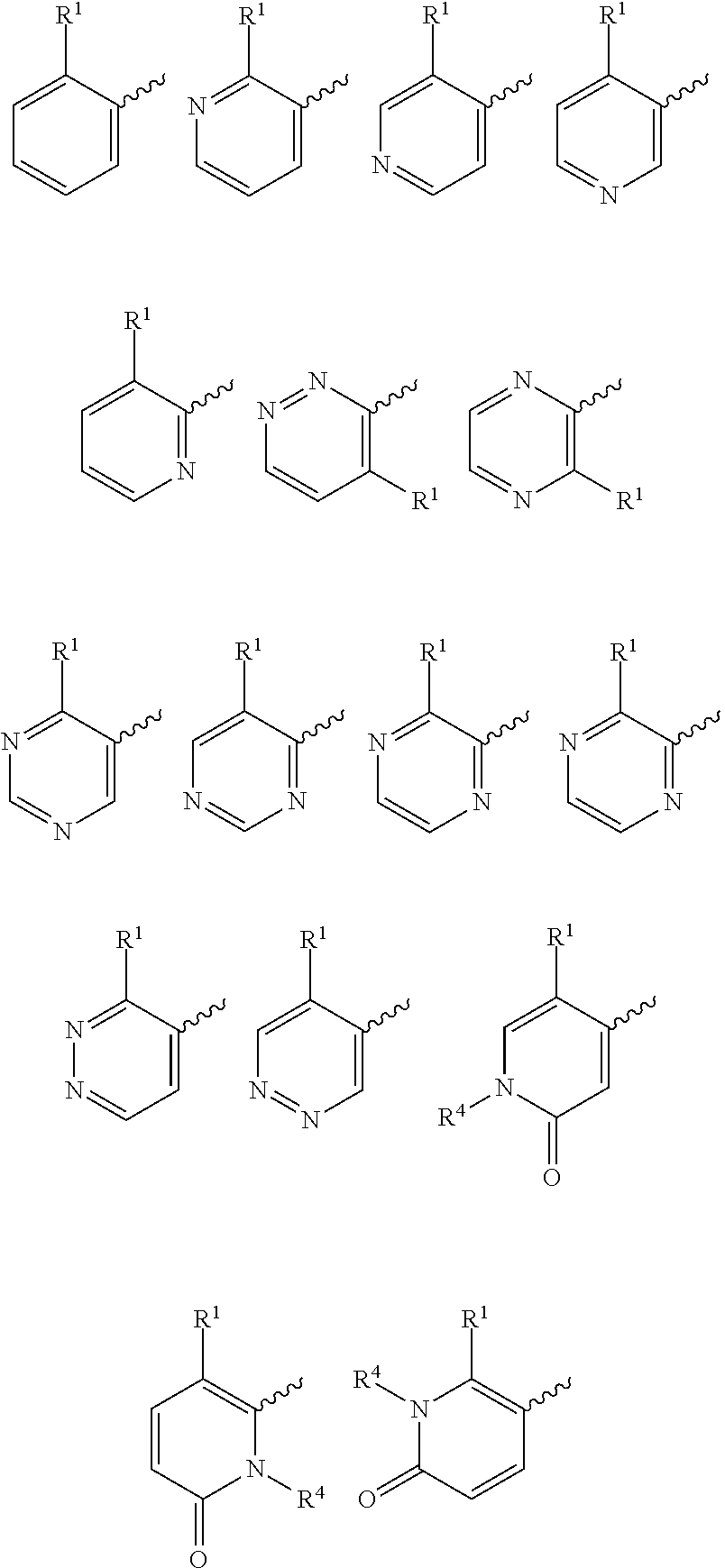
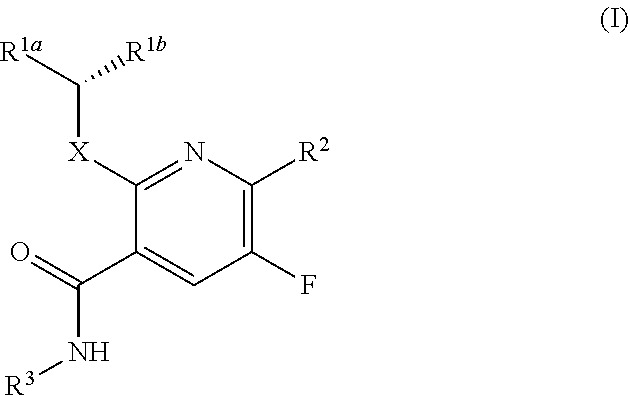
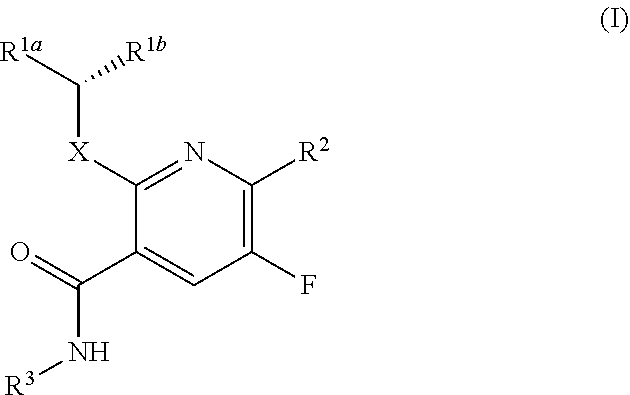
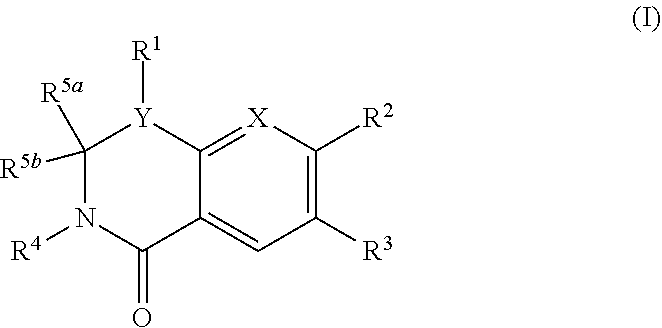
The present invention provides a combination comprising (a) methotrexate and (b) a non-hepatotoxic dihydroorotate dehydrogenase (DHODH) inhibitor of formula (I): wherein: R1 consists of a hydrogen atom, a halogen atom, C1-4 alkyl, C3-4 cycloalkyl, -CF3 and -OCF3; R2 is selected from the group consisting of hydrogen atom, halogen atom and C1-4 alkyl; R3 Selected from the group consisting of -COOR5, -CONHR5, tetrazolyl, -SO2NHR5 and -CONHSO2R5 groups, wherein R5 is selected from the group consisting of hydrogen atoms and straight-chain or branched C1-4 alkyl groups; R4 is selected from the group consisting of a hydrogen atom and a C1-4 alkyl group; R9 is selected from a group consisting of a hydrogen atom and a phenyl group; G1 represents a group selected from N and CR6, wherein R6 is composed of a hydrogen atom, Halogen atom, C1-4 alkyl, C3-4 cycloalkyl, C1-4 alkoxy, -CF3, -OCF3, N-containing monocyclic C5-7 heteroaryl, N-containing monocyclic C3-7 heterocyclic And selected from the group consisting of C6-10 aryl groups, said C6-10 aryl groups may be optionally substituted by one or more substituents selected from halogen atoms and C1-4 alkyl groups; G2 means selected from the following Groups in the group: hydrogen atom, hydroxyl, halogen atom, C3-4 cycloalkyl, C1-4 alkoxy and -NRaRb, wherein Ra represents C1-4 alkyl and Rb consists of C1-4 alkyl and C1 - selected from the group consisting of 4 alkoxy-C1-4 alkyl, or Ra and Rb together with the nitrogen atom to which they are attached form a saturated 6 to 8 which may optionally contain an oxygen atom as another heteroatom membered heterocyclic ring; monocyclic or bicyclic 5 to 10 membered heteroaryl ring containing one or more nitrogen atoms, which may be optionally replaced by one or more members selected from halogen atoms, C1-4 alkyl, C1-4 alkoxy Substituents of radical, C3-4 cycloalkyl, C3-4 cycloalkoxy, -CF3, -OCF3 and -CONR7R8, wherein R7 and R8 are independently selected from hydrogen atom, straight chain or branched chain C1-4 alkane group, C3-7 cycloalkyl group, or R7 and R8, together with the nitrogen atom to which they are attached, form a group of the following formula: wherein n is an integer from 0 to 3; and phenyl, which may optionally be replaced by one or more One selected from halogen atom, C1-4 alkyl, hydroxyl, C1-4 alkoxy, C3-4 cycloalkyl, C3-4 cycloalkoxy, cyano, -CF3, -OCF3, -CONR7R8, oxadiazole Substituents of diazolyl, triazolyl, pyrazolyl and imidazolyl, said diazolyl, triazolyl, pyrazolyl and imidazolyl can be optionally replaced by C1-4 alkyl or C3-7 cycloalkyl Substituted and wherein R7 and R8 are independently selected from a hydrogen atom, a straight or branched C1-4 alkyl group, a C3-7 cycloalkyl group, or R7 and R8 together with the nitrogen atom to which they are attached form a group of the following formula: wherein n is an integer from 0 to 3; or, when G' represents CR6, G2 together with R6 forms a non-aromatic C5-10 carbocyclic group or a C6-10 aryl group, and its pharmaceutical Pharmaceutically acceptable salts and N oxides.
Owner:ALMIRALL
An ascochlorin compound and its application in the preparation of antitumor drugs or dihydroorotate dehydrogenase inhibitor drugs
ActiveCN111943828BGrowth inhibitionHigh activityFungiOrganic chemistryAscochlorinDihydroorotate Dehydrogenase Inhibitor
The invention provides a bisporin compound and its application in the preparation of antitumor drugs or dihydroorotate dehydrogenase inhibitor drugs, and relates to the field of marine natural products. An ascochlorin compound is disclosed, the structural formula of which is shown in formula (I). The cyclohexanol fragment of the compound has a rare gem-dimethyl group and an extracyclic double bond, and has a broad spectrum of significant tumor cell Proliferation inhibitory activity and dihydroorotate dehydrogenase inhibitory activity, so it is an ideal candidate compound to be developed as an anti-tumor drug or a dihydroorotate dehydrogenase inhibitor drug with novel structure and high activity.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Dihydroorotate dehydrogenase inhibitors
Owner:JANSSEN BIOTECH INC
Dihydroorotate dehydrogenase inhibitors
InactiveCN104582694BGood DHOD inhibitory activityAntibacterial agentsOrganic chemistryDiseaseAnticarcinogen
The invention provides a novel dihydroorotate dehydrogenase inhibitor, which can be applied to various diseases. The active ingredients include formula (I) (in the formula, X represents a halogen atom, R1 represents a hydrogen atom, R2 represents an alkyl group with 1 to 7 carbons, R3 represents -CHO, R4 represents -CH2-CH=C(CH3) A compound represented by -R0 (where R0 represents an alkyl group with 1 to 12 carbons that may have substituents on the terminal carbon and / or non-terminal carbon, etc.)), its optical isomers, and the compounds and The pharmaceutically acceptable salt of the optical isomer has a good inhibitory effect on dihydroorotate dehydrogenase, and can be used as an immunosuppressant, rheumatoid therapeutic agent, anticancer agent, rejection treatment drug, and antiviral drug , anti-H.pylori (Helicobacter pylori) drugs and diabetes treatment drugs, etc.
Owner:INST OF MITOCHONDRIA SCI INC
Dihydroorotate dehydrogenase inhibitors
Owner:JANSSEN BIOTECH INC
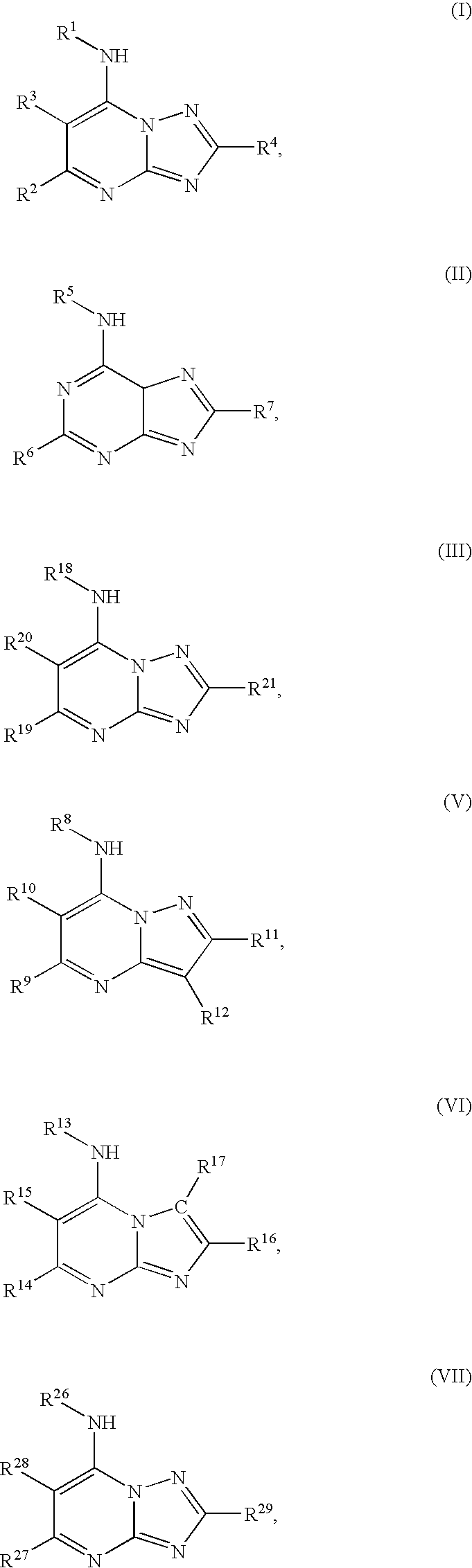
Dihydroorotate dehydrogenase inhibitors with selective anti-malarial activity
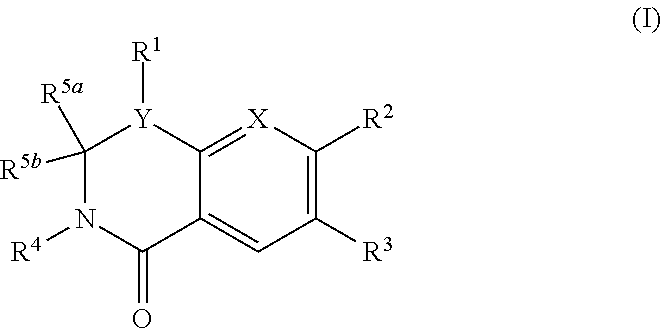
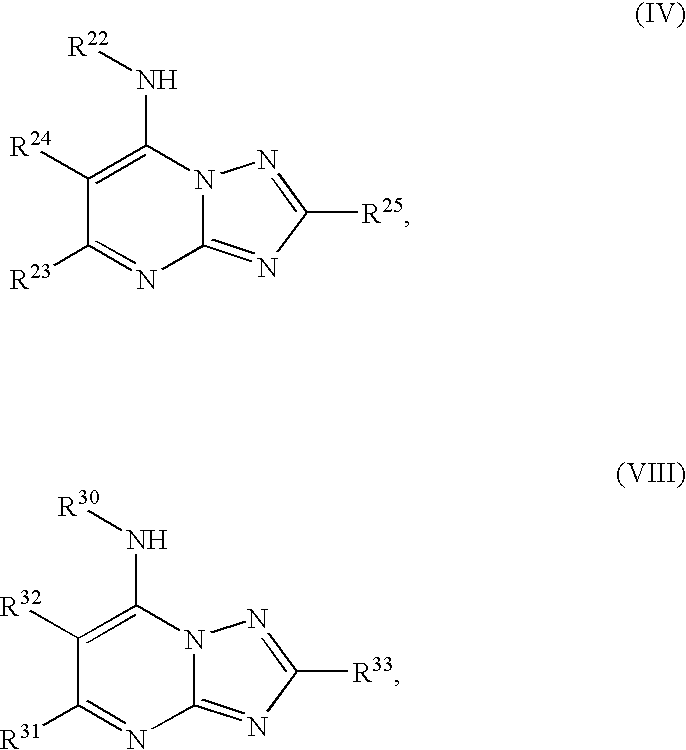
Compounds according to Formula I, Formula II, Formula III, Formula V, Formula VI, or to Formula VII,and pharmaceutical compositions of compounds that conform to Formula IV or Formula VIII:where R1 through R33 are prescribed, selectively inhibit P. falciparum dihydroorotate dehydrogenase. Accordingly, a method for preventing and treating malaria attaches to such compounds, as well as to pharmaceutically acceptable salts, solvates, stereoisomers, tautomers, and prodrugs thereof.
Owner:UNIV OF WASHINGTON +2
NOVEL HUMAN DIHYDROOROTATE DEHYDROGENASE (hDHODH) INHIBITORS AND THEIR USE IN TARGETING ONCOLOGICAL DISEASES SENSITIVE TO PYRIMIDINE STARVATION
PendingUS20210230156A1Enhanced interactionOrganic chemistryAntineoplastic agentsDiseaseDihydroorotate Dehydrogenase Inhibitor
Compounds of formulae (I) and (II)which are novel inhibitors of human dihydroorotate dehydrogenase (hDHODH) and pharmaceutical compositions containing the compounds of formulae (I) and (II) are provided. A method for treating tumor diseases, including acute myelogenous leukemia (AML), triple-negative breast cancer, PTEN-mutant tumors, and KRAS-driven tumors by administering pharmaceutical compositions containing the compounds of formulae (I) and (II) is also provided.
Owner:DRUG DISCOVERY & CLINIC SRL
Fluorinated quinoline and quinoxaline derivatives as dihydroorotate dehydrogenase (DHODH) inhibitors for treatment of cancer, autoimmune and inflammatory diseases
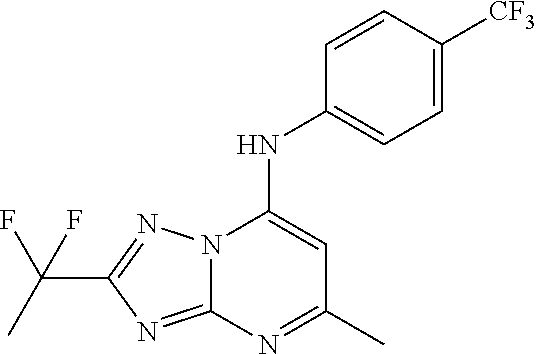
Disclosed are compounds of formula (I): (I) wherein X is CH; the compounds of formula (I) of the present invention are dihydroorotate dehydrogenase (DHODH) inhibitors and are useful in the treatment of inflammatory disorders, autoimmune disorders and cancers such as, for example, lymphoma, leukemia, cancer and sarcoma. The present specification discloses synthesis and characterization of exemplary compounds and pharmacological data thereof (e.g., pages 60 to 136; embodiments 1 to 39; table 1 and Table 2). Exemplary compounds are, for example, 4-ethyl-1-(7-fluoro-4-isopropyl-2-(2-methoxyphenyl) quinolin-6-yl)-3-(hydroxymethyl)-1H-1, 2, 4-triazol-5 (4H)-one (embodiment 1): (AA).
Owner:JANSSEN BIOTECH INC
Small molecule inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase
Inhibitors of dihydroorotate dehydrogenase (DHODH) for the Plasmodium enzyme have been identified and characterized. The inhibitors have high specificity, submicromolar efficacy against cultured parasite strains, exhibit drug-like properties, and are not overtly cytotoxic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Dihydroorotate dehydrogenase inhibitors
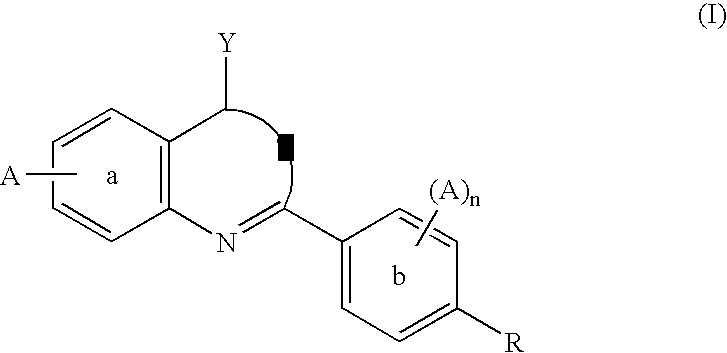
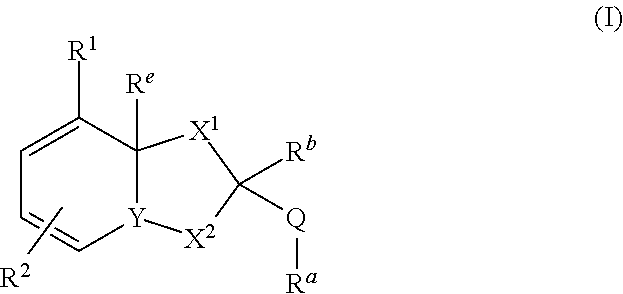
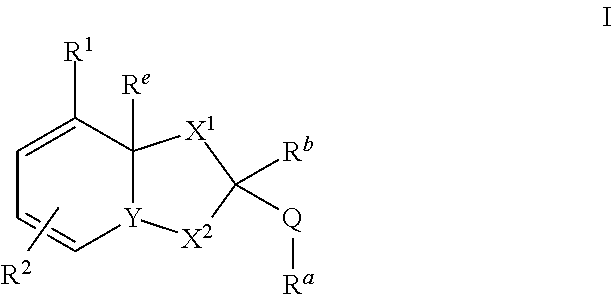
The invention relates to compounds of formula (I) wherein R1, R2, X1, X2, Y, Ra, Rb, Q have the meanings given in claim 1. The compounds are useful e.g. in the treatment of autoimmune disorders, such as multiple sclerosis and also in the treatment of cancer disorders.
Owner:MERCK SERONO SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com