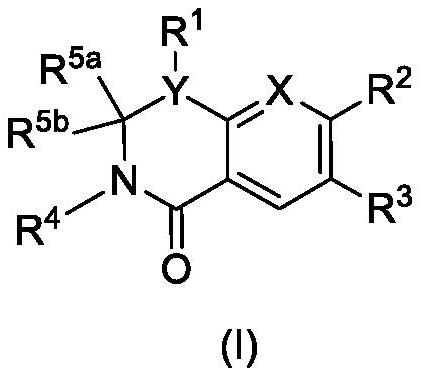
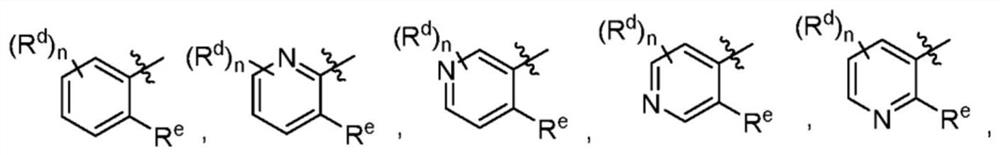
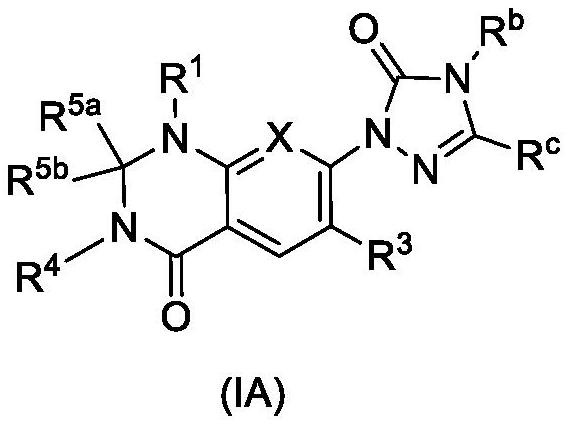
Dihydroorotate dehydrogenase inhibitors
A solvate, alkyl technology, applied in the field of new compounds, can solve problems such as limited effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0291] Exemplary compounds useful in the methods of the present invention will now be described with reference to exemplary synthetic schemes for their general preparations below and specific examples that follow.
[0292] plan 1
[0293]
[0294] According to Scheme 1, the 1,2,4-triazol-5(4H)-one compound of formula (V) (wherein PG is Bn) was prepared from ethyl 2-(benzyloxy)acetate in three steps. In the first step, 2-(benzyloxy)acetate is prepared by reacting ethyl 2-(benzyloxy)acetate with hydrazine hydrate in a suitable solvent such as EtOH, etc.; at a temperature ranging from 70°C to 85°C base) acetyl hydrazide. Make hydrazide with formula R c -Isocyanates of NCO (where R c for C 1-6 alkyl) in a suitable solvent (eg, water, etc.) to give the corresponding semicarbazides. Subsequent cyclization of semicarbazide with a suitable base such as NaOH in a suitable solvent such as water provides compounds of formula (V) (wherein PG is Bn).
[0295] Compounds of formul...
Embodiment 1
[0359] Example 1: 3-(2-Chloro-6-fluorophenyl)-7-(4-ethyl-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1, 2,4-Triazol-1-yl)-6-fluoro-1-isopropyl-2,3-dihydroquinazolin-4(1H)-one.
[0360]
[0361] Step A: 2-(Benzyloxy)acetohydrazide . To a solution of ethyl 2-(benzyloxy)acetate (55 g, 283.17 mmol) in EtOH (500 mL) was added NH 2 NH 2 ·H 2 O (28.3 g, 566 mmol, 27.5 mL). The mixture was heated to reflux at 78°C and stirred for 6 hours. The reaction mixture was concentrated under reduced pressure to give the title product as a colorless oil (52 g, crude), which was used in the next step without further purification.
[0362] Step B: 3-((benzyloxy)methyl)-4-ethyl-1H-1,2,4-triazol-5(4H)-one . To 2-(benzyloxy)acetohydrazide (52 g, 288 mmol) in H at 0 °C 2 Ethyl isocyanate (25.1 g, 346 mmol, 27.9 mL) was added dropwise to the O (500 mL) solution. After the addition, the reaction mixture was stirred at 25°C for 12 hours. Add H to the mixture 2 O (20 mL) and NaOH (57.7 g, 1.4...
Embodiment 2
[0372] Example 2: 3-(2-Chloro-6-fluorophenyl)-7-(4-ethyl-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1, 2,4-Triazol-1-yl)-6-fluoro-1-isopropyl-2-methyl-2,3-dihydroquinazolin-4(1H)-one .
[0373]
[0374] Step A: 7-(3-((benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl) - 3-(2-Chloro-6-fluorophenyl)-6-fluoro-1-isopropyl-2-methyl-2,3-dihydroquinazolin-4(1H)-one . To 4-(3-((benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)-N To a mixture of -(2-chloro-6-fluorophenyl)-5-fluoro-2-(isopropylamino)benzamide (100 mg, 179.86 μmol) in EtOH (10 mL) was added acetaldehyde (158 mg, 3.60 mmol, 202 μL), then in N 2 Under the atmosphere, the mixture was stirred at 80°C for 24 hours. Acetaldehyde (79 mg, 1.80 mmol, 101 μL) was added to the mixture, and the mixture was stirred at 80° C. for 12 hours. Acetaldehyde (79 mg, 1.80 mmol, 101 μL) was added to the mixture, and the mixture was stirred at 80° C. for 48 hours. The mixture was poured into water (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com