Ascochlorin compound and application thereof in preparation of antitumor drugs or dihydroorotate dehydrogenase inhibitor drugs
A technology of ascochlorin and anti-tumor drugs, which is applied in the field of marine natural products, can solve the problems of weak mechanism of action and anti-tumor potential to be further explored, and achieve broad-spectrum significant tumor cell proliferation inhibitory activity and broad-spectrum significant Dihydroorotate dehydrogenase inhibitory activity, effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Sclerotinia acremonium (Acremonium sclerotigenum) GXIMD02501
[0033] Acremonium sclerotigenum GXIMD 02501 was isolated from the symbiotic bacteria collected from the Beibu Bay of Guangxi, China, and preserved in the Guangdong Microbial Culture Collection Center (GDMCC). Address: 5th Floor, Building 59, Guangdong Institute of Microbiology, No. 100 Xianlie Middle Road, Guangzhou City, Guangdong Province, Guangdong Institute of Microbiology, deposit number: GDMCC No.60670.
Embodiment 2
[0034] Preparation and separation of embodiment 2 ascochlorin compound acremochlorin A
[0035] 1. Medium
[0036] 1.1. Seed culture medium: Each liter of medium contains 15g of malt extract powder, 20g of coarse sea salt, and the balance is water, pH7.5. Mix evenly according to the above-mentioned components and contents, and then sterilize at 121°C for 30 minutes for later use.
[0037] 1.2. Fermentation medium: Each 1L Erlenmeyer flask medium contains: 120g rice, 1.5g bacteriological peptone, 3g coarse sea salt, 150mL water, pH7.5. Mix evenly according to the above-mentioned components and contents, and then sterilize at 121°C for 30 minutes for later use.
[0038]2. Fermentation
[0039] 2.1. Seed culture: Inoculate activated Acremonium sclerotigenum GXIMD02501 into 1L triangular culture flasks each containing 300mL of seed culture medium, culture at 25°C and 180rpm for 72 hours to obtain seed solution.
[0040] 2.2. Fermentation culture: the seed liquid was inserted i...
Embodiment 3
[0044] Example 3 Structural identification of ascochlorin compound acremochlorin A
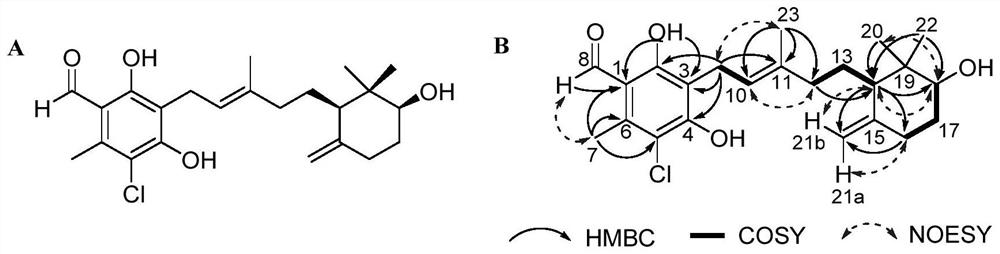
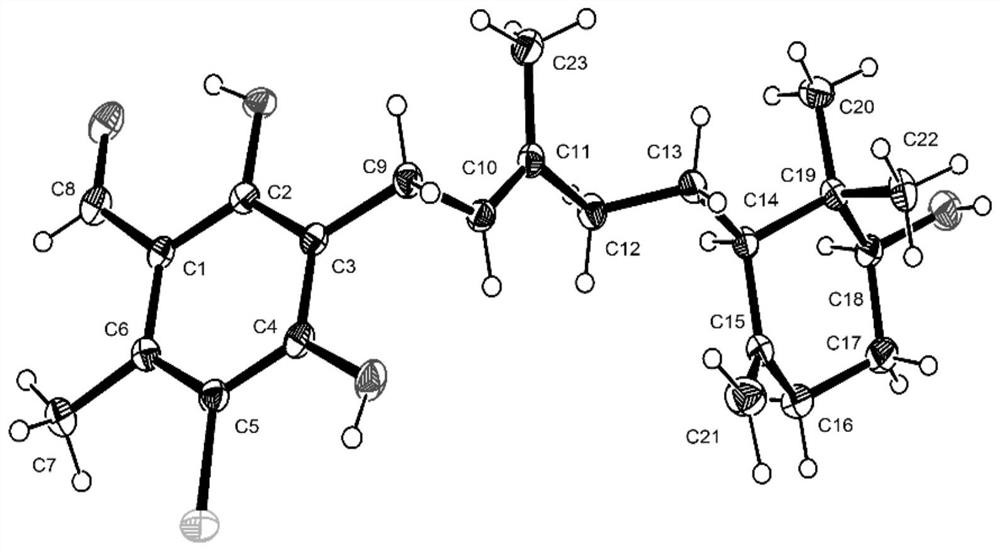
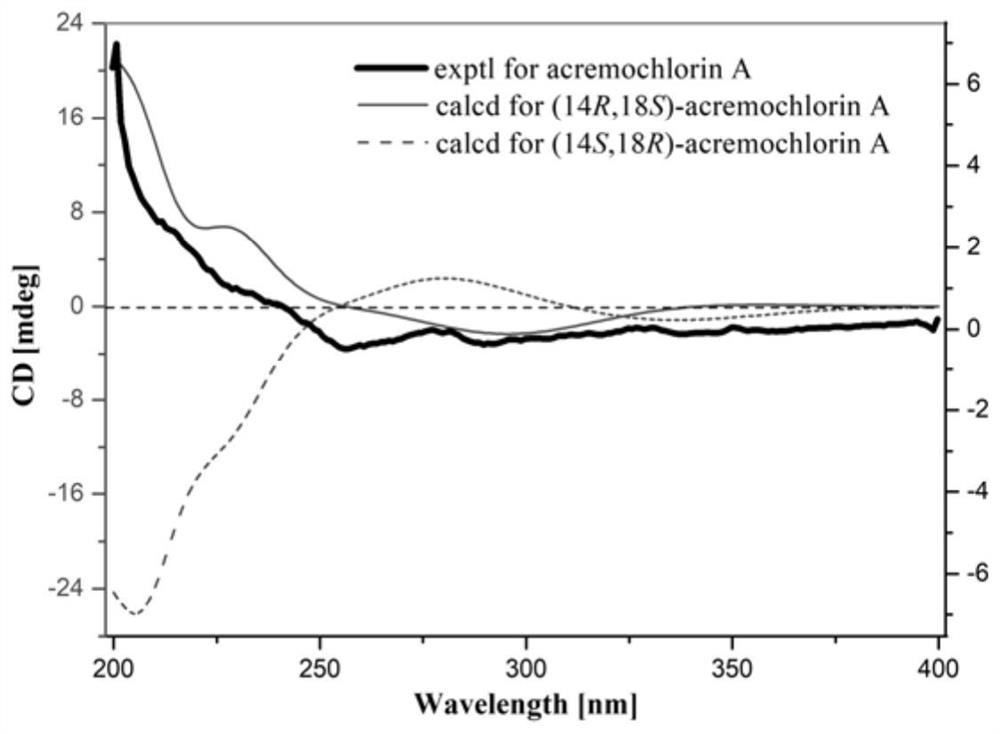
[0045] The ascochlorin compound acremochlorin A is a colorless needle-like crystal, and its NMR data assignment is shown in Table 1; its high-resolution mass spectrum HRESIMS gives a quasi-molecular ion peak m / z407.1968[M+H] + (calcd forC 23 h 32 ClO 4 , 407.1989) determined that its molecular formula is C 23 h 31 ClO 4 , containing 8 degrees of unsaturation. 1 The HNMR spectrum shows a phenolic hydroxyl group 2-OH (δ H 12.89,s), 1 aldehyde group (δ H 10.17,s), 3 alkene protons H-10(δ H 5.20,t,J=7.0Hz) and terminal methylene H 2 -21(δ H 4.79, s, H-21a; 4.53, s, H-21b), a oxymethine H-18 (δ H 3.27, overlapped) and 1 methine H-14 (δ H 1.63,m), 5 methylene H 2 -9(δ H 3.36–3.40, d, J=7.0Hz), H 2 -12(δ H 2.04,m; 1.79,m), H 2 -13(δ H 1.61, m), H 2 -16(δ H 2.25,m; 1.82,m) and H 2 -17(δ H 1.73,m; 1.44,m), and four unimodal methyl groups, H 3 -7(δ H 2.63), H 3 -20(δ H 0.96), H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com