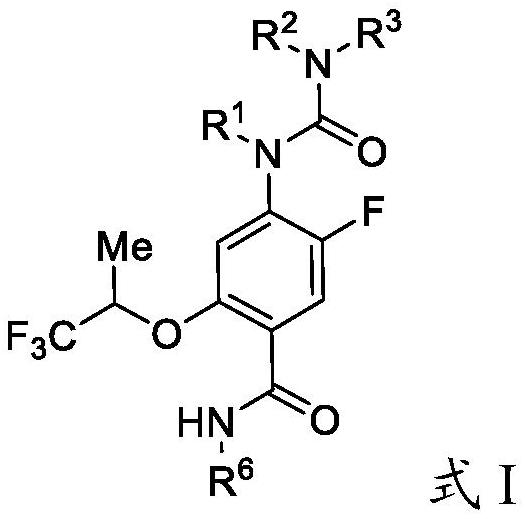
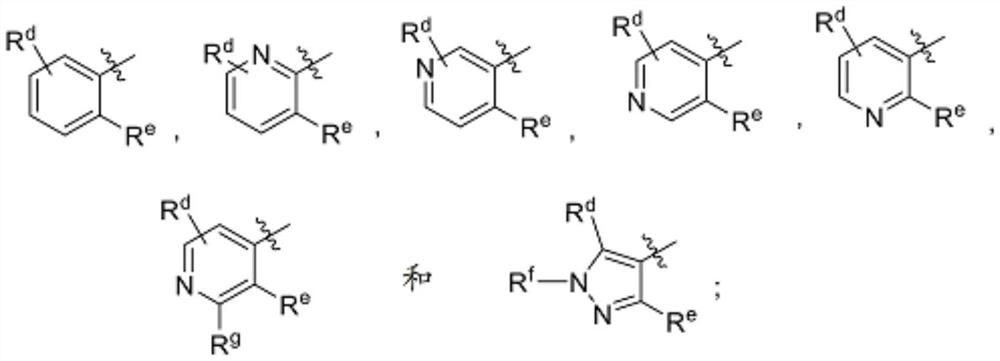
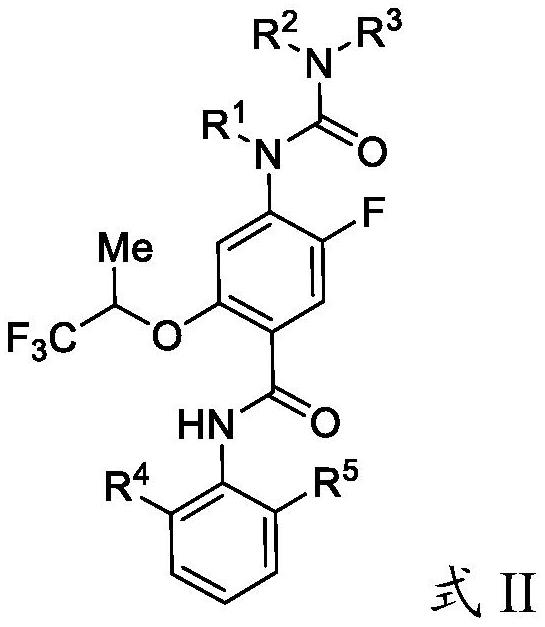
Substituted urea dihydroorotate dehydrogenase inhibitors
A technology of halogenated alkyl and alkyl, applied in the field of new compounds, can solve problems such as limited effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0258] Exemplary compounds useful in the methods of the invention will now be described with reference to exemplary synthetic schemes for their general preparation below and the specific examples that follow.
[0259] plan 1
[0260]
[0261] Compounds of formula (III) can be prepared as shown in Scheme 1, wherein R 1 for H and R 2 and R 3 as defined in formula (III). At ambient temperature, in the presence of a suitable base such as Et 3 N, NaH, etc.), in a polar aprotic solvent (such as DCM, etc.), make (S)-(4-((2-chloro-6-fluorophenyl)carbamoyl)-2-fluoro Phenyl-5-((1,1,1-trifluoropropan-2-yl)oxy)phenyl)carbamate (prepared as described in Intermediate 1 - Step F) was reacted with a secondary amine.
[0262] Scenario 2
[0263]
[0264] Compounds of formula (III) can be prepared as shown in Scheme 2, wherein R 1 and R 2 for H and R 3 as defined in formula (III). At elevated temperature (such as 100°C), in a base (such as Et 3 In the case of N, etc.), in a...
Embodiment 1
[0344] Example 1: (S)-N-(2-chloro-6-fluorophenyl)-4-(3,3-diethylureido)-5-fluoro-2-((1,1,1- Trifluoro Propan-2-yl)oxy)benzamide .
[0345]
[0346] To a mixture of N-ethylethylamine (6.82 mg, 93.23 μmol), TEA (15.72 mg, 155.39 μmol) in DCM (1 mL) was added N-[4-[(2-chloro-6-fluoro-phenyl )carbamoyl]-2-fluoro-5-[(1S)-2,2,2-trifluoro-1-methyl-ethoxy]phenyl]carbamate (40 mg, 77.70 μmol). The mixture was stirred at 25°C for 1 hour. The mixture was filtered and concentrated under reduced pressure. The residue was purified by preparative HPLC (Method A) to afford the title compound as a white solid. MS (ESI): C 21 h 21 CIF 5 N 3 o 3 The mass calculated value is 493.1; m / z measured value is 494.1 [M+H] + . 1 H NMR (400MHz, DMSO-d 6 )δ=9.00-8.72(m,1H),7.88-7.66(m,2H),7.48(m,1H),5.52-5.32(m,1H),4.78(m,1H),4.65-4.41(m, 3H), 4.26(m, 1H), 3.93(m, 1H), 3.58(m, 1H), 3.09-2.90(m, 2H), 2.86-2.65(m, 2H).
Embodiment 2
[0347] Example 2: (S)-N-(4-((2-chloro-6-fluorophenyl)carbamoyl)-2-fluoro-5-((1,1,1-trifluoropropane-2- base) oxy) phenyl) piperidine-1-carboxamide .
[0348]
[0349] The title compound was prepared in a manner analogous to Example 1, however substituting piperidine for N-ethylethylamine. MS (ESI): C 22 h 21 CIF 5 N 3 o 3 The calculated value of the mass is 505.1; the measured value of m / z is 506.1 [M+H] + . 1 H NMR (400MHz, CDCl 3 )δ=8.37-8.27(m,1H),7.60(s,1H),7.53(s,1H),7.33(m,1H),4.79-4.70(m,2H),4.50(m,1H),3.93 (m, 2H), 3.78-3.57 (m, 1H), 3.41 (s, 3H), 3.22-3.16 (m, 1H), 3.12-2.65 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com