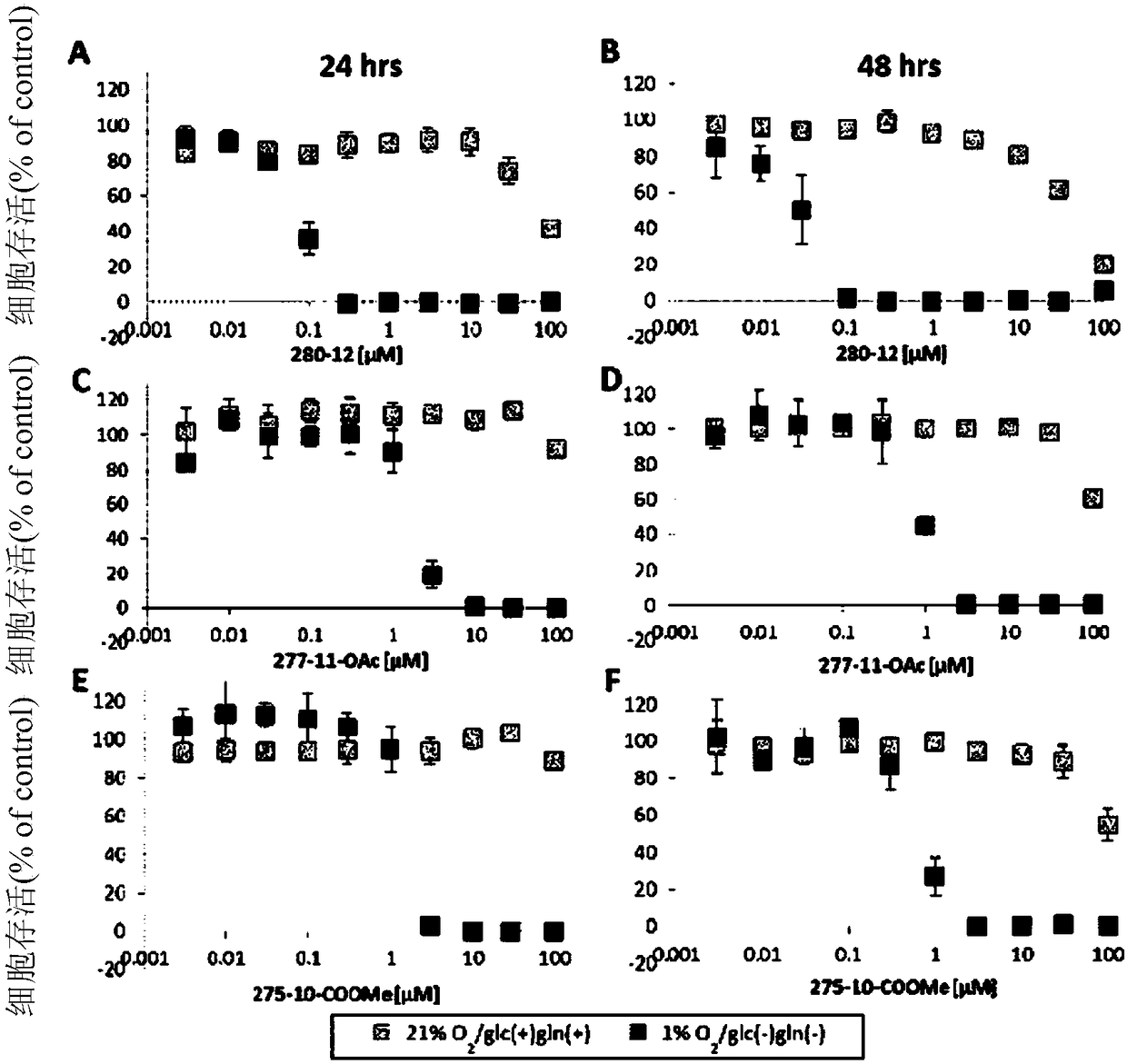
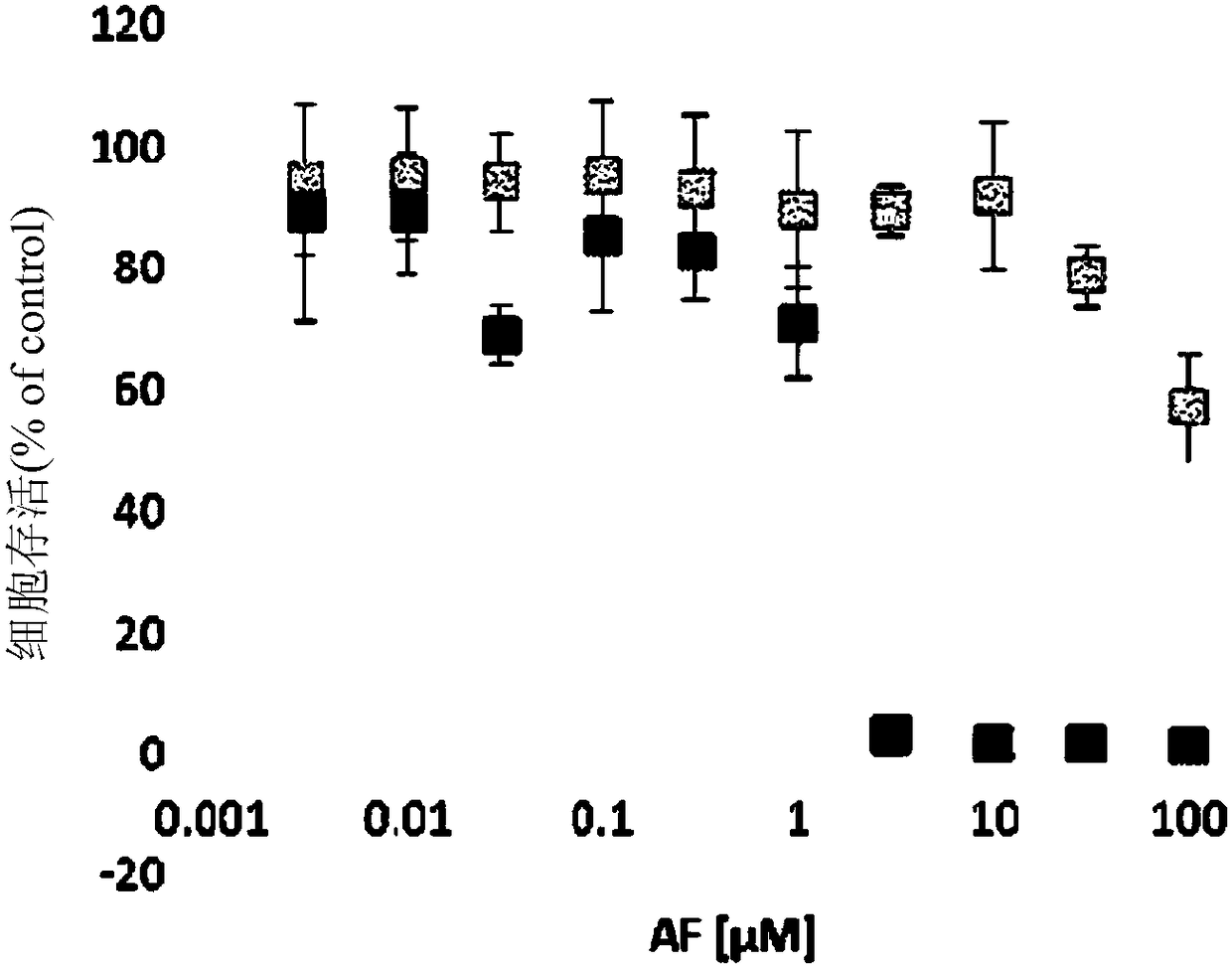
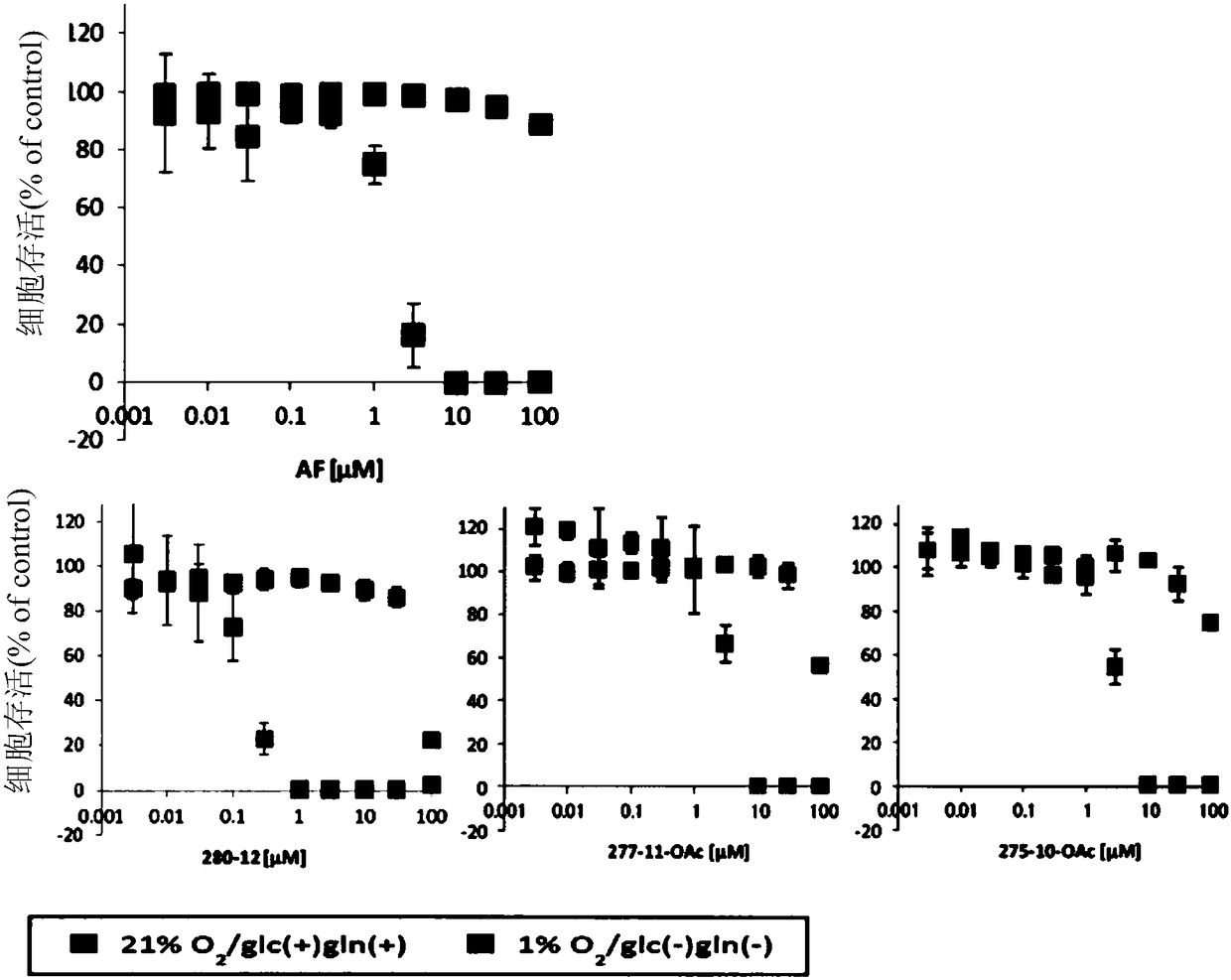
Dihydroorotate dehydrogenase inhibitors
A compound and application technology, applied in the field of dihydroorotate dehydrogenase inhibitors, can solve problems such as hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0126] The compounds used in the present invention will be specifically described below by way of examples. Furthermore, these compounds do not limit the scope of the present invention.
[0127] 1. Derivatives 215-15-COOEt, 215-15-COOIPr, 215-13-COOH
[0128] [hua 1]
[0129]
[0130] 12-(3-Chloro-5-formyl-2,6-dihydroxy-4-tolyl)dodecane Ethyl acetate
[0131] (215-15-COOEt).
[0132] CHCl in acetic anhydride (12.5 ml, 132 mmol) 3 (16 ml) solution, 30% aqueous hydrogen peroxide (10 ml, 98 mmol) was added at 0°C, and the mixture was stirred at the same temperature for 1 hour. Next, maleic anhydride (10.0 g, 102 mmol) was added as it was kept, and the mixture was stirred for 2 hours while gradually returning to room temperature. The heat generation of the reaction solution was confirmed, and cyclododecanone (compound 1, 2.52 g, 13.8 mmol) was added as a single unit, and the mixture was stirred at 35° C. for 16 hours. After returning the reaction solution to room temp...
Embodiment 1
[0685] [Example 1] Determination of DHOD inhibitor
[0686] DHOD inhibitor activity was measured at concentrations of 200 nM and 1000 nM using the compounds of the present invention.
[0687] Add 190 μl of assay buffer (100 mM HEPES pH8.0, 150 mM NaCl, 5% glycerol, 0.05% Triton X-100, 200 μM dihydroorotic acid, 120 μM DCIP, 11 μM decaubiquinone) and 5 μl of each inhibitor’s DMSO solution, and add 8 μg / ml of human DHOD solution 5μl, start the enzymatic reaction (end concentration 0.2μg / ml (4nM) of human DHOD), measure the reduction of DCIP at 600nm for 20 minutes, according to the change of the absorbance value at 600nm after 0 minutes and 20 minutes, The value when only the DMSO solution was added was controlled by the End Point Assay(), and the inhibition rate of human DHOD at the concentration of 200 nM and 1000 nM of each compound was measured. In addition, the concentration (nM) that inhibits 50% of the activity of human DHOD was defined as IC50 (50% inhibitory concentra...
Embodiment 2
[0690] [Example 2] Anticancer effect
[0691] Conventional anticancer agents directly act on the process of cell division, but have low specificity to tumor cells and strong damage to normal cells, so serious side effects appear, which is a serious problem. Later, molecular targeted drugs appeared, targeting molecules related to tumor cell proliferation, infiltration, and metastasis to inhibit the deterioration of tumor cells, not only inhibiting tumor cells, but also inhibiting tumor metastasis.
[0692] Therefore, screening was carried out by a cancer cell proliferation inhibition test using a panel of 39 human cancer cell lines (JFCR39) and Cancer Cell Informatics (cancer cell informatics) using information analysis (Kong D., T. Yamori; Bioorganic & Med. Chem., 20, 1947-51, 2012). The concentration (μM) that inhibits the growth of human cancer cells by 50% is referred to as GI50 (50% inhibitory concentration).
[0693] The results are shown in Table 2.
[0694] Inhibitor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com